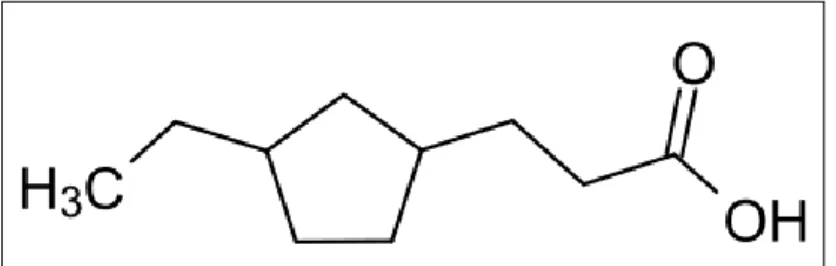
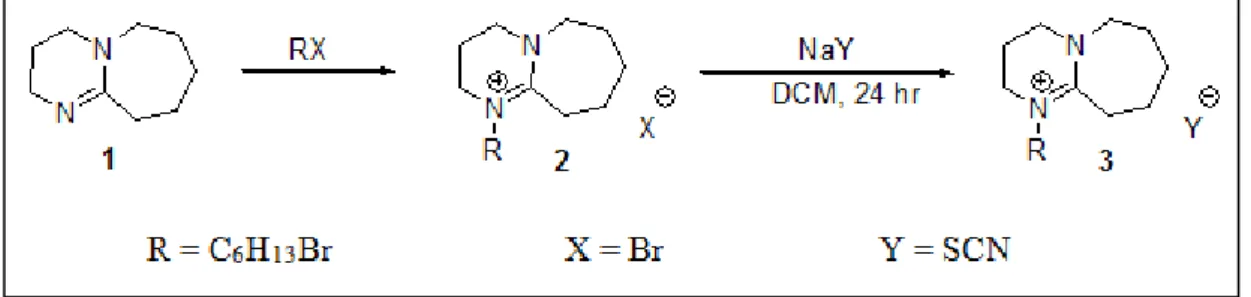
The presence of carboxylic acids in the form of naphthenic acid in crude oil can cause serious corrosion problems during oil refining. Therefore, this study aims to investigate the possibility of using an ionic liquid as a non-volatile solvent for the extraction of naphthenic acid from a model hydrocarbon liquid, which is dodecane. In this study, an ionic liquid based on 1,8-diazobicyclo[5.4.0]undec-7-ene-hexyl [DBU-Hex] cation with anionic thiocyanate [SCN] was synthesized for the extraction of naphthenic acid.
Liquid-liquid extraction for ternary system {dodecane + naphthenic acid + [DBU-Hex][SCN]} will be performed at constant temperature 25 within the specified feed compositions. Further changing the physical and molecular structure of the distillation products via cracking and reforming processes, naphthenic acid can be converted into more useful products for various applications, such as wood preservatives, paint driers, lubricants and fuel additives (Shah et al., 2014). In other cases, the presence of naphthenic acid can affect production equipment, storage, transportation facilities and also the performance of the oil.
However, there is no evidence of naphthenic acid corrosion when the temperature is above 400 because of the decomposition of naphthenic acid at high temperature (Bota et al., 2010). Considering the many problems that may arise as above, it is necessary to extract the naphthenic acid. The scope of the study is mainly focused on the liquid-liquid extraction of naphthenic acid from dodecane using the synthesized ionic liquid [DBU-Hex][SCN].
To develop a ternary phase diagram of {dodecane + naphthenic acid + [DBU-Hex][SCN]} by correlating liquid-liquid equilibrium data with NRTL or UNIQUAC model.
LITERATURE REVIEW AND THEORY
Synthesis of Ionic Liquids
The physiochemical properties of ionic liquid mostly depend on the nature of the cations and anions. For example, ionic liquids containing bis-(trifluoromethylsulfonyl)imide (Tf2N) anion will be water immiscible (hydrophobic) while acetate anion will form water soluble (hydrophilic) ionic liquids (Lethesh et al., 2014). The combination of different anions and cations thus expanded more than two thousand ionic liquids at possible low melting temperature known today.
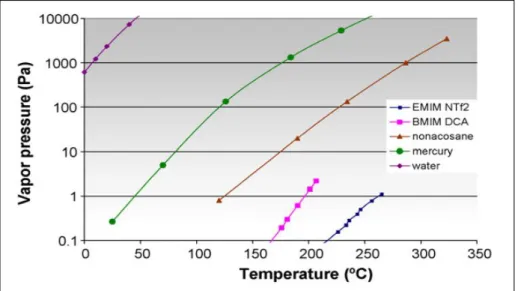
In another study by Berthod et al., vapor pressures of two ionic liquids 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide (EMIM NTfO2) and 1-butyl-3-methylimidazolium dicyanamide (BMIM DCA) were compared with three liqs. namely mercury, nonacosane and water as reference fluid. To determine parameters of the equation for the ionic liquid molar enthalpy of vaporization, thermochemical measurements were used. The calculated vapor pressures of ionic liquids EMIM NTfO2 and BMIM DCA at room temperature are 12 and 2 μPa while the corresponding vapor pressures of water, mercury and nonacosane are and 0.01 Pa.
Because ionic liquids have extremely low volatility, this green solvent has nonflammable properties (Berthod et al., 2008). The unique properties of ionic liquids make them suitable candidates for replacing organic solvent contaminants and can be used to remove unwanted impurities in a single processing step called liquid–liquid extraction (LLE).
Limitation of Liquid-Liquid Extraction
- Pressure and Temperature
- Alkyl Chain in Ionic Liquids
Technically, the extraction of dissolved chemical component X from liquid phase A is achieved by contacting the liquid solution of X with a second liquid phase, B. After partitioning of the analyte between the immiscible phases has taken place, the extracted analyte recovered from stage B. for subsequent extraction procedures. Another consideration to take into account is the density of the extraction solvent to help determine the position of the layers.
These conditions should ensure that mixtures remain in the two-phase liquid range and are soluble with each other. Nevertheless, these conditions are insignificant in isothermal and isobaric processes where heating and pressure are not required and where the goal is to achieve phase stability of the system. The liquid-liquid extraction process can also be affected by the length of the alkyl chain in ionic liquids.
The result of the experiment showed that [mmim][Tf2N] and [emim][Tf2N] ionic liquids which had shorter alkyl chain. The experimental result showed that increasing the alkyl chain length results in a large selectivity reduction due to the interaction of the aliphatic hydrocarbon with the alkyl group of cation. As the alkyl chain length increases, the electron donation of the alkyl group to the cation of ionic liquid increases, resulting in a decrease in the positive character of the cation.
Therefore, it weakened the interaction between the cation and aromatic hydrocarbons and resulted in the decrease of selectivity. Both cases above conclude that the LLE is affected by the alkyl chain of the ionic liquid. The results from the research work are expected to be useful for the extraction process in order to increase the quality of crude oil.
Project Flow Chart
- Synthesis of Halide Salts
- Synthesis of 1, 8-diazobicyclo [5.4.0] eundec-7-ene (DBU) based Ionic Liquid
- Liquid-Liquid Extraction
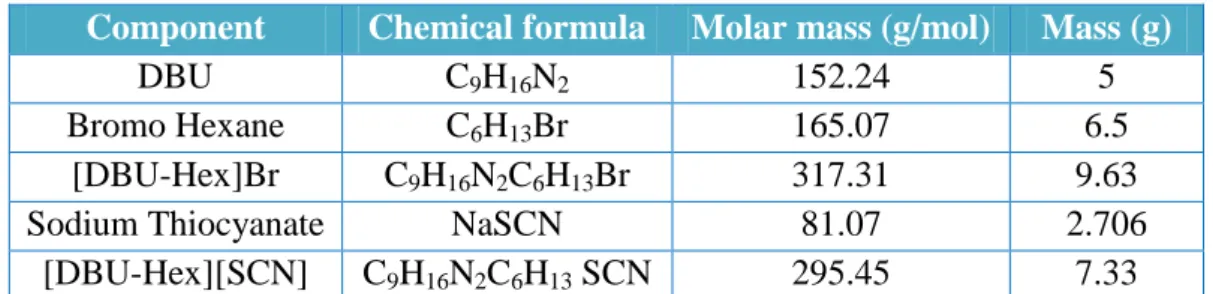
The reaction mixture was cooled using an ice bath and the acetonitrile was removed using a rotary evaporator. Cyclohexane is used to wash the resulting white solid (chloride salt) and dried in a vacuum oven at 70°C for 24 hours. After preparing the halide salts of 1-hexyl-1,8-diazobicyclo [5.4.0] undec-7-ene bromide [DBU-Hex] [Br], sodium thiocyanate in dichloromethane was added and the reaction mixture was vigorously stirred with a mechanical stirrer at 25 °C 24 hours.
The precipitate formed is filtered off and the dichloromethane layer is washed with cold water. An Anton Paar viscometer (model SVM3000) was used to measure the viscosity of ionic liquids. Mixture of known masses of dodecane, benzoic acid and ionic liquid [DBU-Hex][SCN] was transferred into tared vials.
The vials were then placed on a shaker at a shaking speed of 800 rpm for 5 h at 25 to reach thermodynamic equilibrium. After the experimental LLE data was analyzed using a gas chromatograph, the ternary systems dodecane + naphthenic acid + ionic liquid will develop as shown in the figure below with the calculated data using two non-random liquids (NRTL) or quasi-chem. universal (UNIQUAC) model. For these two models, it is assumed that each cation is fully paired with the anion and the pair is considered a single molecular species in solution.
Furthermore, reference states are considered to be pure liquids of all types at system temperature T and pressure P (Simoni et al., 2008). The NRTL model usually predicts a large heat of mixing and characteristic of solutions (Simoni et al., 2007). Most of the time, binary LLE data is used for the ionic liquid solvent or co-solvent to estimate the parameters.
However, there will be no parameter solutions for this system of equations if a model does not allow liquid–liquid phase separation (Simoni et al., 2008). For the UNIQUAC model, even if all binary interaction parameters are equal to 0, it will still be able to predict the LLE by considering only the combinatorial contribution to molar excess of Gibbs free energy. The combinatorial contribution is predicted from the values of r and q of the substances (Simoni et al., 2007).
RESULTS AND DISCUSSION
Synthesis of Ionic Liquid [DBU-Hex][SCN]
H NMR data of Ionic Liquid [DBU-Hex][SCN]
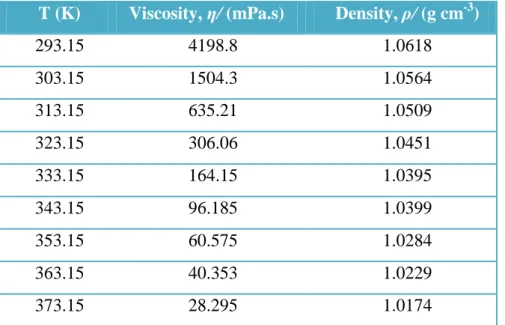
The alkyl length of ionic liquid affects the viscosity and density of ionic liquid [DBU-Hex][SCN]. Solubility test was performed prior to LLE for a known amount of naphthenic acid, namely 0.5 g, to completely dissolve in dodecane. Based on the solubility ratio, the mole fraction compositions for the dodecane, naphthenic acid and ionic liquid [DBU-Hex][SCN] with a total feed of 2 g were calculated.
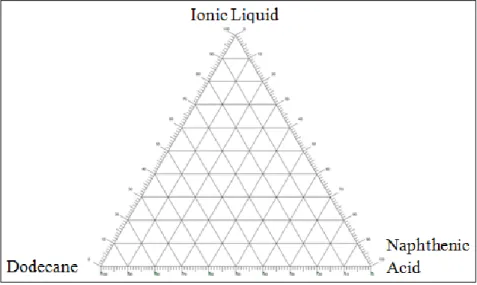
The experimental LLE data for the ternary system {dodecane + naphthenic acid + [DBU-Hex][SCN]} at T = 25 °C and atmospheric pressure are plotted in a triangular diagram in Error. The feasibility of using ionic liquid [DBU-Hex][SCN] as a solvent for liquid-liquid extraction of naphthenic acid from dodecane was evaluated using the formulas of partition ratios (Di) and separation factors (α 2, 1), where x is the mole fraction, superscript 1 and 2 refer to the hydrocarbon-rich and ionic liquid-rich phases, respectively. As shown in FIGURE 4.4, the ternary phase diagram represents the mole fractions of all three components, namely dodecane, naphthenic acid, and ionic liquid [DBU-Hex][SCN] in each hydrocarbon and ionic liquid rich phase layer.
The result showed that ionic liquid [DBU-Hex][SCN] is capable of extracting naphthenic acid from the hydrocarbon-rich phase to the ionic liquid-rich phase. This indicates that as the concentration of naphthenic acid increases, the separation will become difficult.
![TABLE 4.2 HNMR Data and CHNS details of DBU-Hex][Hex]](https://thumb-ap.123doks.com/thumbv2/azpdforg/10257296.0/27.892.160.792.115.670/table-hnmr-data-chns-details-dbu-hex-hex.webp)
CONCLUSION AND RECOMMENDATION
![FIGURE 4.1 H NMR Spectrum of 1-Hexyl-1, 8-Diazobicyclo [5.4.0] undec-7-ene Thiocyanate [DBU-Hex] [SCN]](https://thumb-ap.123doks.com/thumbv2/azpdforg/10257296.0/27.892.174.785.670.923/figure-nmr-spectrum-hexyl-diazobicyclo-undec-thiocyanate-dbu.webp)