In this project, a xanthone block and two alkenylated xanthone derivatives were synthesized and studied for their cytotoxic activity. Furthermore, I would like to express my appreciation to my friends, Goh Yi Fan, Bak Jor Yee and Tan Su Chin for their help and encouragement during the course of this project. Finally, I want to thank my beloved family members for their constant support, concern and encouragement in the realization of this project.
Chemicals, devices and cells used in the biological test 37 4.1. Summary of assignment of 1H-NMR and 13C-NMR spectra.

General Introduction
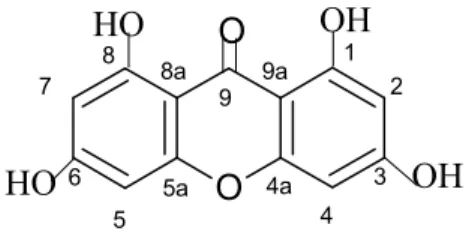
In general, natural xanthones can be classified into five groups, which are simple oxygenated xanthones, glycoside xanthones, prenylated xanthones, xantholyganoids and others. Due to the fact that xanthone derivatives extracted from natural sources were found to be limited in the types and positions of the substituent, through synthesis, various types of xanthone can be produced to allow a comprehensive study of structure activity relationships to be performed. for the identification of the cytotoxic functional group present in various xanthone derivatives. Characterization of synthetic xanthone was done by UV-Vis, 1H-NMR, 13C-NMR, 2D-NMR analysis including HMQC and HMBC, IR and mass analysis.
Objectives
Synthesis of Xanthone and Its Prenylated Derivatives
- Synthesis of Xanthonic Block
- Grover, Shah and Shah’s Method
- Synthesis via Benzophenone and Diaryl Ether Intermediate
- Prenylation of Xanthonic Block
- Improved Synthesis Method
- Further Synthesis on Prenylated Xanthone
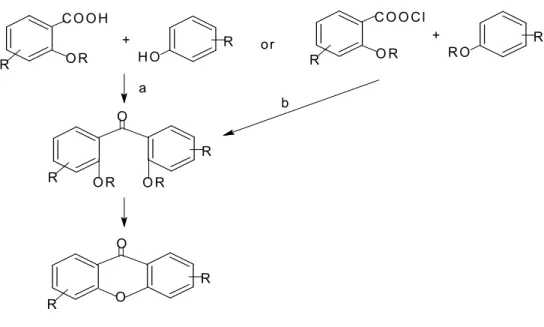
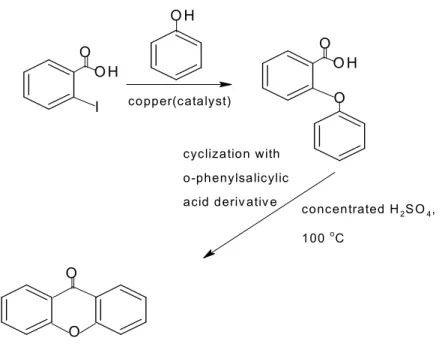
In other research, another method used in synthesizing xanthonic core was established by using benzophenone (Figure 2.2) and diaryl ether as intermediate (Figure 2.3). Part of the cell cycle is mitotic phase which can be divided into mitosis and cytokinesis. But there is other alternative using chemotherapy which involves the use of chemotherapeutic drugs to interfere with the specific stages in the cell cycle.
Synthesis of Cytotoxic Xanthone Derivative
- Bromoalkoxyxanthone
- Epoxyxanthone
- Pyranothioxanthone
- α-Mangostin
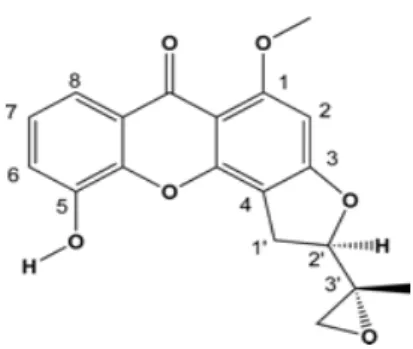
GI50 is the concentration of a compound that inhibits 50% of cell growth [Han et al., 2008] while TGI is the concentration of a compound that completely inhibits cell growth at 48 hours and LC50 is the concentration at which the tested compound 50 % killing of the cell at 48h [Han et al., 2008]. Epoxyxanthone compounds and the ring-opened halohydrinxanthones were tested for their cytotoxic activity. Two epoxy groups attached to 3, 5-position of xanthone can dramatically increase the cytotoxic activity [Na, 2009].
25 Furthermore, from the research carried out, it has been shown that thioxanthone with the presence of epoxy group has been found to possess great cytotoxic activity [Na, 2009]. From the research conducted by Ioannis Kostakis et al. was performed, pyranothioxanthones 7, 8b, 9a, 10a and 12b were found to possess good cytotoxic properties. This suggested that angular orientation of D-ring is important for biological activity [Kostakis et al., 2001].
Lemieux-Johnson oxidation of compound 4, followed by Wittig reaction afforded bromobenzene 4 [Iikubo et al., 2001]. The reaction was then followed by exchange of the TBS group with the benzyl group to produce compound 12 [Iikubo et al., 2001]. Xanthonecarboxylic acid can be obtained by oxidation of the methyl group of 3a-f to 3i-q, with potassium permanganate in alkaline solution followed by intramolecular Friedel-Craft acylation with polyphosphoric acid [Pickert et al., 1998].
Under the same reaction conditions as described in the literature, which involved xanthone prenylation [Castanheiro et al., 2009].
Chemicals
Instruments
- Nuclear Magnetic Resonance Spectrometer (NMR)
- Infrared Spectrophotometer (IR)
- Ultraviolet-Visible Spectrophotometer (UV-Vis)
- Melting Point Instrument
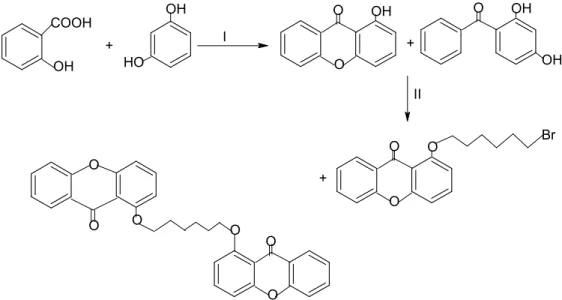
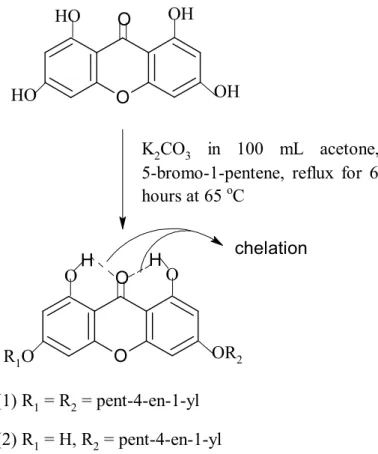
- Synthesis of 1, 3, 6, 8-Tetrahydroxyxanthone
- Alkenylation of Xanthonic Block in Organic Solvent
Then, the sample mixture with KBr powder was compressed under a high pressure to form the KBr sample pellet. The UV-Vis spectrum was used to determine the qualitative information and the position of the hydroxyl group on the xanthone block. Perkin-Elmer Lambda UV-Vis spectrophotometer was used in this project and samples were prepared using absolute ethanol to dissolve the sample.
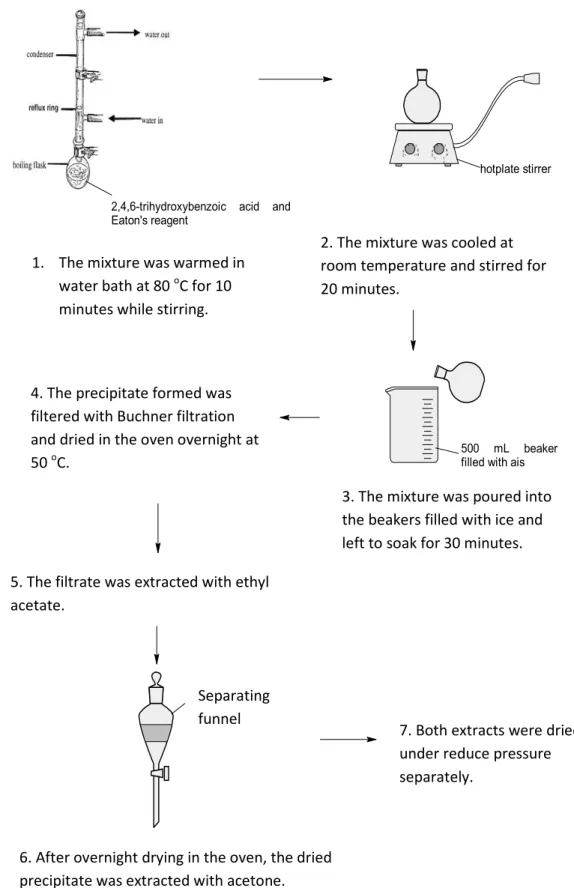
Mass spectrometry was used to provide information on the molecular mass, molecular formula and structure of the molecule. The mixture is poured into a 500 ml beaker initially filled with ice and left to soak in an ice bath for 30 minutes. Then, the precipitate is filtered with Buchner filtration and the precipitate is placed in an oven at 50 oC overnight, while the filtrate is extracted with ethyl acetate.
The mixture was poured into the ash-filled beakers and soaked for 30 minutes. The mixture was poured into the ice-filled beakers and soaked for 30 minutes. To the mixture was added 5-bromo-1-pentene and the mixture was refluxed at 65°C for 6 hours.
5-bromo-1-pentene was added to the mixture and the mixture was refluxed for 6 h at 65 oC.
Chromatography Method
Thin Layer Chromatography (TLC)
Thus, gradient elution was applied to elute and separate different components from the column. First, the sample solution was added dropwise and mixed with a small amount of silica gel in a beaker. The silica gel was then gently swirled to allow the remaining silica gel to cover the forming droplets.
48 When the column is packed, the column is first filled to 1/3 of the column's height with hexane. Then a small amount of sea sand was introduced into the column to form a thin layer of sand above the plugged column with hexane continuing to run down the column and collected in a conical flask. The suspension of silica gel was then poured into the column and the stopcock of the column was opened to allow hexane to flow down the column into the conical flask.
This should allow the silica gel to be evenly distributed in the column and prevent the formation of bubbles. Before introducing the dry packed sample into the column, the column should be run several times to firmly compress the silica gel into the column. Before loading the sample onto the column, the hexane level was controlled and maintained at 5 cm above the silica gel surface.
The hexane collected before loading sample can be reused by pouring back into the column.
Bioassay
The 1H-NMR spectrum (Figure 4.10) exhibited singlet signal at δ 11.91 for the presence of chelated hydroxyl group. The difference was the presence of additional proton signals due to the o-alkenylation at the OH group on carbon atoms C-3 and C-6 of the xanthone block. 62 The 13C-NMR spectrum (Figure 4.11) showed signals for 7 magnetically non-equivalent carbon atoms in the xanthone skeleton.
A total of 6 characteristic signals appeared in the 1H-NMR spectrum (Figure 4.10), which were due to the presence of the pent-4-en-1-yl group. The H-1' proton was unprotected due to the electron withdrawing effect caused by the electronegative oxygen atom attached to the C-1' carbons. 63 The 13C-NMR spectrum (Figure 4.11) showed signals for 5 magnetically nonequivalent carbons on the pent-4-en-1-yl group.
65 The IR spectrum (Figure 4.12), showing a broad absorption band at 3449 cm-1, indicated the presence of O-H in the compound. The 13 C-NMR spectrum (Figure 4.16) showed a signal for 13 magnetically non-equivalent carbon atoms in the xanthone skeleton. A total of 6 resonances appeared in the 1 H-NMR spectrum (Figure 4.14) due to the presence of a pent-4-en-1-yl group.
The H-1' proton was directly attached to the C-1' carbons, unprotected by the electron-withdrawing effect caused by the electronegative oxygen atoms. The 13C-NMR spectrum (Figure 4.15) showed 5 signals for 5 magnetically nonequivalent carbons present in the pent-4-en-1-yl group. The IR spectrum (Figure 4.17) showed a broad absorption band at 3431 cm-1, indicating the presence of O-H in the compound.
This resulted in the loss of the carboxyl group from the expected xanthone block of tetrahydroxyxanthone-2-carboxylic acid or tetrahydroxyxanthone-4-carboxylic acid to form tetrahydroxyxanthone.
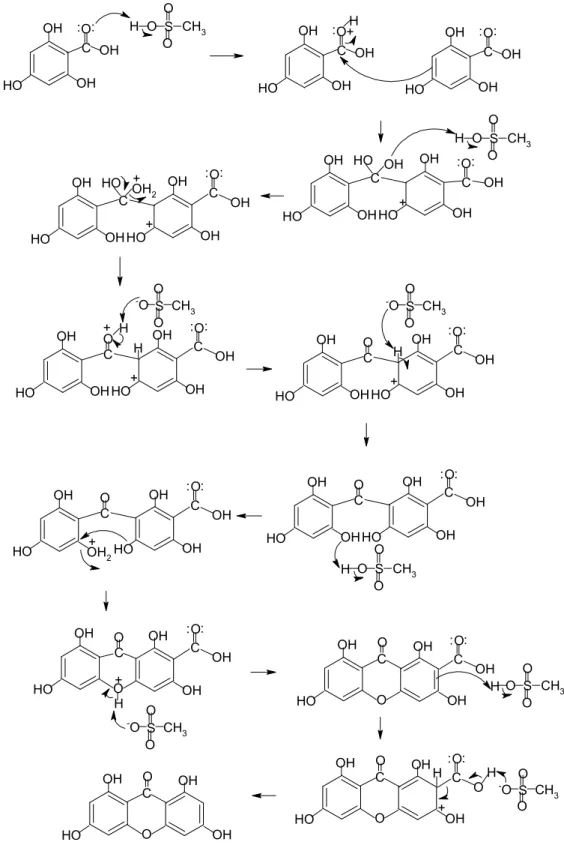
Mechanism of o-Alkenylation
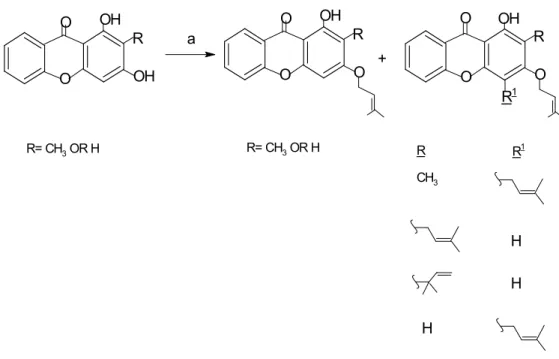
80 Based on the mechanism in Figure 4.19, two reactions took place, namely dehydration and decarboxylation. Thus, one monosubstituted o-alkenylated product, 1,3,8-trihydroxy-6-(pent-4-enyloxy)-9H-xanthen-9-one, and one di-substituted o-alkenylated product, 1,8-dihydroxy- 3,6-bis(pent-4-enyloxy)-9H-xanthen-9-one. The only difference was that 1,3,8-trihydroxy-6-(pent-4-enyloxy)-9H-xanthen-9-one was monosubstituted and 1,8-dihydroxy-3,6-bis(pent-4 -enyloxy)-9H-xanthen-9-one was di-substituted with the same substituent.
84 Although potassium carbonate K2CO3 was used as a catalyst in this reaction, acetone prevented the ionization of K2CO3 and promoted o-alkenylation of tetrahydroxyxanthone instead of c-alkenylation of tetrahydroxyxanthone.
Bioassay
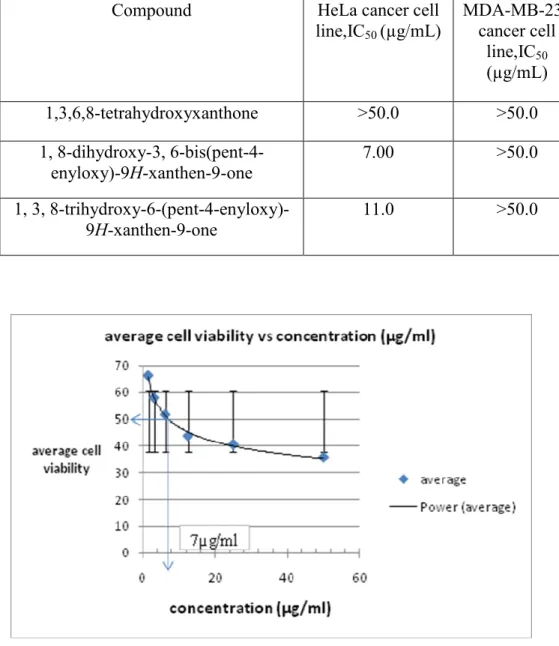
From the results of bioassay it was found that only 1, 8-dihydroxy-3, 6-bis(pent-4-enyloxy)-9H-xanthen-9-one and 1, 3, 8-trihydroxy-6-(pent) - 4-enyloxy)-9H-xanthen-9-one showed cytotoxic activity towards HeLa cancer cell line. However, all the compounds tested showed insignificant cytotoxic activity towards MDA-MB-231 cancer cell line. The smaller the IC50, the more cytotoxic the compound is to the cancer cell line.
Compound with IC50 more than 50 µg/mL was considered insignificant in their cytotoxic activity. Thus, 1, 8-dihydroxy-3, 6-bis(pent-4-enyloxy)-9H-xanthen-9-one with IC50 value of 7.00 μg/mL was found to be the most cytotoxic against HeLa cancer cell line compared with others synthesized. From the results, it also showed that the more o-substituted xanthonic block exerted more cytotoxic effect on HeLa cancer cell line.
The xanthonic block tetrahydroxyxanthone showed insignificant cytotoxic activity towards HeLa cancer cell lines and the cytotoxic effect increases when it is mono-substituted with 5-bromo-1-pentene at the –OH group of C-3. Further increase in the cytotoxic effect when tetrahydroxyxanthone was di-substituted with 5-bromo-1-pentene at –OH groups of C-3 and C-6. Thus, the higher the number of o-alkenylated group attached to xanthone, the more cytotoxic the compound towards HeLa cancer cell line.
Conclusions
However, the xanthonic blocked tetrahydroxyxanthone showed negligible cytotoxic activity with IC50 greater than 50 µg/ml against the same cancer cell line. It can thus be concluded that the more o-alkenylation on the xanthone compound, the more cytotoxic the compound is towards the HeLa cancer cell line.
Suggestion for Further Studies
90 In addition, extended pharmacological tests should be performed to identify other pharmacological potentials present in xanthone derivatives. Pharmacological activities to be studied are anti-inflammatory, antibacterial, antifungal, antioxidant, anti-allergic and antiviral. Finally, further synthesis on the xanthone block is proposed to produce a wider selection of xanthone species for a more comprehensive structure-reactivity relationship (SAR) study to examine the potential structure of the xanthone to be developed into drugs.
Further synthesis may involve cyclization of the alkenyl group to add additional ring to the structure or introduction of other functional groups into the xanthonic nucleus. COMFA and CoMSIA studies on a new series of xanthone derivatives against human oral squamous cell carcinoma (KB) cell line.
![Figure 2.7: Preparation of 3, 7-dihydroxyxanthone via Ketimino intermediate [Atkinson and Heilbron, 1926]](https://thumb-ap.123doks.com/thumbv2/azpdforg/10240729.0/33.892.240.763.128.387/figure-preparation-dihydroxyxanthone-ketimino-intermediate-atkinson-heilbron.webp)
![Figure 2.11: c-Prenylation with prenyl bromide in the presence of a strong base [Anand and Jain, 1973]](https://thumb-ap.123doks.com/thumbv2/azpdforg/10240729.0/35.892.215.765.619.897/figure-prenylation-prenyl-bromide-presence-strong-anand-jain.webp)
![Figure 2.13: Synthesis of c-prenylated 1, 1-dimethylally- and 3, 3- dimethylallyl derivatives of xanthone [Castanheiro et al., 2009]](https://thumb-ap.123doks.com/thumbv2/azpdforg/10240729.0/36.892.221.805.597.783/figure-synthesis-prenylated-dimethylally-dimethylallyl-derivatives-xanthone-castanheiro.webp)
![Figure 2.19: Synthesis pathways for epoxyxanthone derivatives [Woo et al., 2006]](https://thumb-ap.123doks.com/thumbv2/azpdforg/10240729.0/45.892.214.756.254.904/figure-synthesis-pathways-epoxyxanthone-derivatives-woo-et-al.webp)
![Figure 2.21: (a)Methanesulfonic acid, P 2 O 5 , ∆; (b)3-chloro-3-methyl-1- butyne, CuI, K 2 CO 3 , NaI, DMF; (c)N,N-DEA, ∆ [Kostakis et al., 2001]](https://thumb-ap.123doks.com/thumbv2/azpdforg/10240729.0/46.892.267.714.775.1024/figure-methanesulfonic-acid-chloro-methyl-butyne-cui-kostakis.webp)