This paper aims to understand management and challenges in two different resource models of the clinical study. One in-house observational clinical research and another fully outsourced observational clinical study were selected as the case studies. The paper provides an example of both in-house and fully outsourced clinical studies to understand management, challenges and recommendation for choosing the resource model and improvement for a future clinical study.
A clinical study team and/or a monitoring team are functions for conducting clinical research. Today, a different model of conducting clinical studies is used in the pharmaceutical industry. A case study of two observational studies in different management models, including one in-house and one fully outsourced, will be analyzed along with experiences.
The expected benefits of this paper should be a case study of different clinical trial management models. The recommendation and limitation of each case study will be useful as lessons learned and supporting information to consider and improve clinical study activities for both internal and external management model.

LITERATURE REVIEW
Clinical Study and Drug Development Process
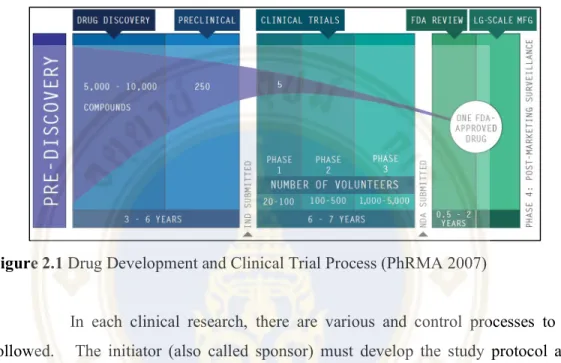
Case-control studies: This study will be conducted in patients and historically analyzed for the etiology of the disease. Of 5,000 to 10,000 potential compounds in the drug development process, only one drug has been approved for approval and commercialized (PhRMA 2007). It will be tested by researchers in the laboratory and in animal models for effectiveness and safety before the preclinical phase.
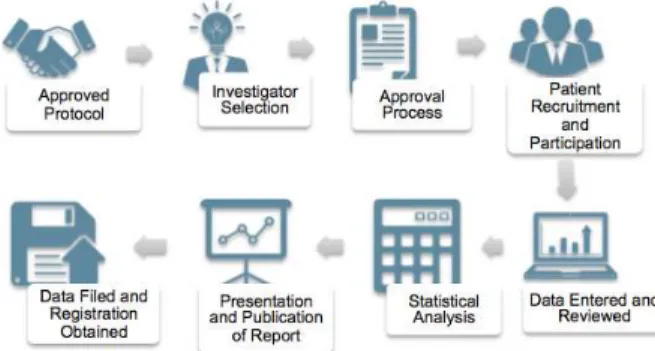
Once all preclinical testing data is complete and proven to be sufficiently safe, the drug candidate will be escalated to human trials which are called the "Clinical Trials" phase. The initiator (also called the sponsor) must develop the study protocol and related materials to be used in the clinical research. The potential physician will then be approached and invited to be an investigator in the clinical study.
All data will be recorded in the data collection form and reviewed by the clinical research team. When patients have a follow-up visit, the database is locked for statistical analysis, followed by preparation of clinical trial reports and publication of the results.
Project Management
To manage the project/survey, the following 7 steps from the beginning to closing the project are included; Establish goals, objectives and scope for the project;. Team-up; Set project budget and schedules; Set action and how to implement the project/study; Project implementation and monitoring; Respond to challenges, problems and deviations; and Post-project assessment along with lessons learned. According to the project management aspect, there are 3 traditional criteria for project success which are "Time", "Budget" and "Performance".
Nowadays, a fourth criterion which is "Customer Acceptance" has been added and has become the new Quadruple Constraint. According to Atkinson, the understanding success model was introduced and provided to more clearly define project success or failure (Pinto 2016).
Resourcing Models
There are many benefits of outsourcing to a company including; Cost reduction (change fixed costs to variable costs), Reduce internal resources/personnel, high level of expertise, faster project completion, fulfill the shortage of resources in a company and flexibility. In terms of limiting outsourcing projects and trials, they consist of one CRO that may not be strong enough to operate all activities in clinical research; Speed and quality may be at risk due to interpretation and commitment of CRO; Loss of control by sponsor; Coordination breakdown; Interpersonal conflict due to different organization; Security issues; and sometimes the investigator prefers to work with sponsor staff as CRO team. According to Good Clinical Practice (GCP) – the international guideline of ethics and procedures for conducting clinical research, although the activities are outsourced to CRO, the sponsor is responsible for the clinical studies.
The following information is an example of a clinical trial outsourcing issue; Lack of communication between a company and CRO;.
RESEARCH METHODOLOGY
Research design
Methodology
For confidentiality purposes, pseudonyms will be used to describe the general information of two example case studies. The general information of both clinical projects such as study design, resource model and resource management will be tabulated and described in Chapter 4: Analysis of findings. Study A: This clinical study is an observational study conducted in one country with more than thirty participating hospitals.
CRO is responsible for all activities from the beginning of the study to the end of the study activities, such as the research report. In order to align and understand in depth each case study selected for this article, the researcher decided to approach one person involved in each case study – Study A and Study B. The role of the interviewee would be at least project manager or should be a project coordinator because these positions not only contribute to and have information about the project activities, but also some of the strategic and tactical rationales of the clinical project.
From the criteria above, the researcher will collect data from two project managers who have experience and are involved in the case study. As per data privacy and confidentiality purposes, pseudonyms are also used to describe the background of each project manager and mentioned in this thematic paper. Adele: She is a person involved in the study preparation and study start period of Study A.
She has been the local representative to work with the company's regional study team for global clinical trials. Prior to joining the company's internal team, she has been on a clinical research team of both pharmaceutical companies and CRO. She also has experience in the fields of clinical research for more than 10 years, including clinical trials and observational studies.
Interview Questions
FINDINGS ANALYSIS
- Strategy, Objectives and Management Model
- Project Governance
- Stakeholders Engagement
- Challenges and Lesson Learned
- Experience sharing and Opinions
- Conclusion
- Recommendation
- Limitation
For Study A, the project coordinator (also known as the “monitor”) is required to visit the hospital every 6 months to review the medical record with the data on the data collection form to ensure the accuracy of the data in the study (also called the “monitoring visit”). The project manager may conduct a co-visit with the project coordinator to ensure the quality of the project. The RMP was determined from the early phase of the clinical study until the end of the study.
For the Study A – the in-house study, the quality control can be done and fully supervised by the local clinical team, while there are restrictions for the company to come into contact with all information and situation in Study B which is the fully outsourced study is . For the local medical team, the clinical study newsletter will be shared by the clinical team every month. Analysis: According to the nature of the clinical study, the stakeholders in Study A and Study B are the same.
Only one difference for the external client in Study B – the fully outsourced study is the CRO. The internal customers consist of the medical team (the project owner), the clinical team (project and operational management), the drug safety team and other functions in the company. Regarding the large scale of the clinical project, there are many challenges in Study B.
In the worst case scenario, corporate downsizing for the clinical team may occur and internal staff downsizing will be implemented. The company can push the request and continue to monitor the status of the clinical trial, which required the CRO's effort. The disadvantage of a full outsourcing model is limited access to the details of the research.
The understanding of the clinical trial process and SOP is mandatory to manage the clinical trial. The project manager must continuously follow up and track the status of the study together with the CRO performance. For a complete outsourcing model, it may require less effort from the company as all the tasks managed by the CRO.
Meanwhile, the project manager and the team should supervise the project to ensure the quality of the project. In-house clinical study should be recommended if in-house resources are available.
34; Sponsor-CRO Relationships: The Benefits and Challenges of Collaborating with Third-Party Solution Providers.” Journal for Clinical Studies 8(1):.
APPENDICES
What are the goals and rationale behind the said research regarding the resource model. In the research, between time, cost, requirements and customer acceptance, which element is most important and which limitation. What are the challenges and lessons learned from managing the research for future improvement.
34;Project management: cost, time and quality, two best guesses and a phenomenon, it's time to accept other success criteria." International Journal of Project Management. 34;The rise of outsourcing: Big pharmaceutical companies downsize, and contract research organizations are the fruits pick." Earth.