A thesis submitted in fulfillment of the requirements for the Doctorate of Philosophy in Chemistry. Theoretical calculation and biological activities of Ru(II) and Ir(III) complexes bearing the organophosphorus auxiliary ligands. This thesis synthesizes Ru(II) and Ir(III) complexes based on four organophosphorus auxiliary ligands and studies their chemical and biological properties.
The maximum absorption wavelengths of all Ru(III) complexes increase as the number of carbon atoms in the supporting ligands increases. In addition, I would like to thank her very much for giving me the opportunity to do research at other organizations at home and abroad to study and use the various sources for conducting the research. I would also like to thank the Faculty of Science and Technology, Thammasat University, Rangsit Campus, Bangkok for letting me do the calculations there.
List of Tables
List of Abbreviations and Symbols
List of Publications
Reprints were made with the permission of publishers
The antiproliferative effects of the complexes on human breast cancer cells and their antimicrobial activity were investigated. The structures of the synthesized complexes were determined by single crystal XRD analysis, elemental analysis, spectroscopic techniques and DFT calculations. Complex2 therefore had a greater ability to penetrate the lipid layer of testa cell membranes.
The cytotoxicity of the studied complexes was determined by the tetrazolium salt (MTT) reduction test. The antibacterial activities of all complexes were evaluated in dimethyl sulfoxide using a modification of the Clinical and Laboratory Standards Institute (CLSI) microdilution method M07-A9[79]. The minimum fungicidal concentrations (MFC) of the active complexes were measured by the blotting method on Sabouraud's dextrose agar.
Wavenumber (cm -1 )
Synthesis, crystal structure and Hirshfeld surface analysis of [bis(diphenylphosphanyl)methane- κ P ]chloridobis[2-(pyridin-
Ekkapong Klaimanee, Peerapong Sangwisut, Saowanit Saithong and Nararak Leesakul
IUCr Journals
CRYSTALLOGRAPHY JOURNALS ONLINE
Synthesis, crystal structure and Hirshfeld surface analysis of [bis(diphenylphosphanyl)methane-jP]-chlorobis[2-(pyridin-2-yl)phenyl-j2N, C1]-. The title IrIII complex, [Ir(C11H8N)2Cl(C25H22P2)], was synthesized from the substitution reaction between the (ppy)2Ir(-Cl)2Ir(ppy)2(ppy = deprotonated 2-phenylpyridine, C11H8N) dimer and 1 ,1-bis(diphenylphosphanyl)methane (dppm, C25H22P2) under an argon gas atmosphere for 20 hours. The N donor atoms of the ppy ligands are mutually trans while the C atoms are cis.
In this current work, we report the synthesis and characterization of the title photoactive complex, (I), obtained by the reaction between (ppy)2Ir(-Cl)2Ir(ppy)2) dimer (ppy. = deprotonated 2-phenylpyridine, C11H8N ) with 1,1-bis(diphenylphosphanyl)methane under an atmosphere of inert gas. The IrIII atom is linked to two C,N-bidentate 2-phenylpyridine (ppy) anions via five-membered chelate rings where the N1 and N2 atoms of the pyridine rings exist in atransorientation to each other [N1—Ir1—N and C11 and C22 are in cis. As expected, the average Ir-C and Ir-N bond lengths are much shorter than the Ir-Cl and Ir-P bonds, based on the size of the different species.
In addition, pairwise intermolecular hydrogen bonds are observed between C14–H14 of the pyridine ring of the ppy ring and Cl1 (Table 2). Additional insight into the weak intermolecular contacts in the crystal packing (I) was obtained by Hirshfeld surface analysis and two-dimensional fingerprint graphics (McKinnon et al. Spackman & Jayatilaka, 2009) generated using Crystal Explorer 17.5 (Turneret al., 2017). . . A pair of intermolecular contacts is shown as red spots on the Hirshfeld surface near the Cl1 atom of the neighboring molecule and the H14 atom of the linked pyridine ring.
The relative contributions of different contact types to the total intermolecular interactions across the Hirshfeld surface are represented in two-dimensional fingerprint plots. A search of the SciFinder database (SciFinder, 2020) for phosphorescent complexes of ppy with iridium(III) diphosphine (dpp) reveals eight structures closely related to the title compound. Haoet al.(2019) reports the crystal structure of an ionic complex of [Ir(ppy)2(dppm)]+; dppm = bis(diphenylphosphanyl)methane bidentate ligand.
However, none of the remaining publications describe a monomeric IrIII complex similar to the title compound.
Introduction
Cisplatin, cis-Pt(NH3)2Cl2 and other molecules in the platinum family have been shown to be effective anticancer drugs (Lyngdoh et al., 2019; Ronconi et al., 2008). However, Pt(II) in the platinum family causes adverse effects on normal cells, including leukopenia, anemia, hepatotoxicity, cardiotoxicity, vomiting, diarrhea, stomatitis, and pain (Oun et al., 2018), etc. Many works have reported that ruthenium(II) and iridium(III) metal complexes are promising anticancer agents with higher efficacy and negligible toxicity than cisplatin (Hearn et al., 2013; Kar et al., 2020; Lin et al., 2018; Pluim et al.).
[trans-RuCl4(DMSO)Im][ImH], NAMI-A (Bergamo et al., 2000) is known as the most successful ruthenium-based anticancer compound that does not cause toxicity to normal cells (Huxham et al., 2003) and has been approved for use in clinical trials. RAPTA derivatives (RAPTA-T and RARTA-B; (Chelopo et al., 2013) containing two chloride ligands were susceptible to hydrolysis in a low chloride environment. Various complexes containing iridium(III) or (IV) ions as a metal Center has been extensively investigated; the complexes have a wide range of possible applications due to their biological activity (Pérez-Arnaiz et al., 2018), the ability of photoactive compounds in LEDs (Chi et al., 2010) and their sensing units in optical.
Phosphorescent iridium(III) complexes can achieve high quantum efficiency, leading to the promising materials for light-emitting diodes. You et al., 2009) Cyclometalated iridium(III) complexes with 2-phenylpyridine and other auxiliary groups typically have a distorted octahedral molecular geometry (Chao et al., 2017; Liu et al., 2017). Many cyclometalated iridium(III) complexes have been investigated for their anticancer activities (Du et al., 2019; Song et al., 2017; Xiao et al., 2018; Yang et al., 2019) with respect to the hydrolysis mechanism on a chloride ligand created a monohydrate complex.
Bacac et al., 2004) Iridium(III) complexes primarily target apoptosis leading to cancer cell death (Hearn et al., 2013). Due to the strong interaction of the border acidic metal and the primary ligand, most of them are N-donating ligands (Chi et al., 2010; Goldsmith et al., 2005; Lin et al., 2011). As explained earlier, phosphine derivatives (Biancalana et al., 2017), especially in the RAPTA family, have been qualified to inhibit the growth of breast cancer cells.
Diphosphine derivatives have been shown to be effective ligands in anticancer drug design for more than a decade (Herry et al., 2019; Li et al., 2018).
Literature reviews Literature reviews
- Literature reviews
The starting material [RuCl(p-cymene)(µ-Cl)]2 dimers was reacted with dppm ligand (1:1.8 ratio) in THF solution. The results revealed that the binding between Ru(II) metal center and the pyrimidine ligand strongly inhibits cell proliferation. In the first stage, cyclometalated Ir(III)-chlorine-bridged dimers with the general formula C^N2Ir(µ-Cl)2IrC^N2 were synthesized using the Nonoyama method (Nonoyama, 1974) which involves the reaction between IrCl3 .nH2O with an excess of cyclometalating ligand (2-2.5 Equiv) in a 3:1 molar ratio of 2-ethoxyethanol and water under the reflux condition.
For the second step, the chlorine-bridged dimer complex was reacted with acetylacetone in the molar ratio of 1:2. After cooling to ambient temperature, a colorful precipitate was filtered out and washed with water, hexane and ether. The precursor dimer complexes were prepared from the reaction between IrCl3.3H2O and related C^N ligands in (1:2 molar ratio) under the reflux condition at 120oC for 24 hours in a mixture of 2-ethoxyethanol and water.
After cooling to room temperature, the product was isolated with water and washed with methanol. We further reacted with the pymH ligand (molar ratio 1:3) in the presence of sodium methoxide, added silver trifluoroacetate and 1,2-dichloroethane and refluxed for 16 hours at 83 °C. The solvent was removed in vacuo after cooling to room temperature, and the crude yellow product was washed with methanol to remove unreacted pymH. Scheme 2) The complexes were characterized mainly by X-ray crystallography and their photophysical properties, cyclic voltammetry and ground state electronic calculation were studied using the B3LYP DFT hybrid functional.
![Figure 3 ORTEP view of the complex [(ɳ 6 -C 6 H 6 )Ru–(µ-Cl) 3 –RuCl(dppb)]. C 2 H 4 Cl 2 , showing the atom labelling and the 50% probability ellipsoids](https://thumb-ap.123doks.com/thumbv2/filepdfco/9586961.143969/66.892.355.601.173.356/figure-ortep-complex-rucl-showing-labelling-probability-ellipsoids.webp)
Synthetic routes of iridium complexes
- Instruments
- Synthesis of the Ru(II) complexes (publication I)
- Synthesis of the Ir(III) complexes (publication II)
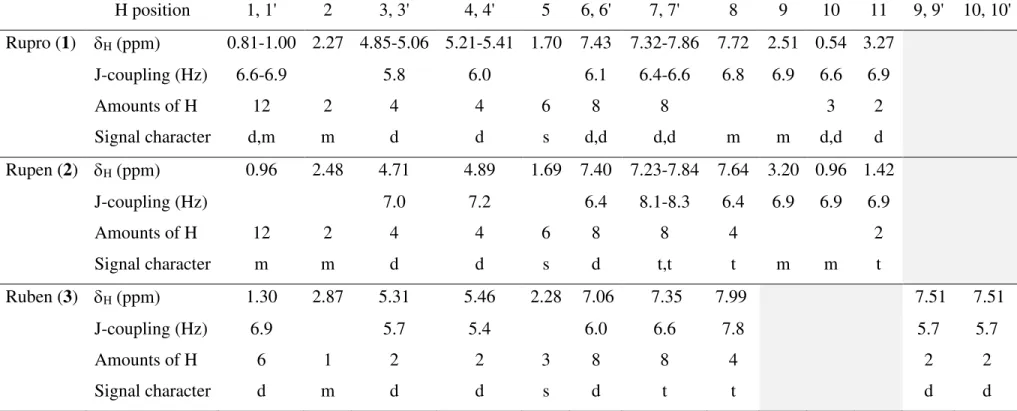
- X-ray Crystal structures of Ru(II) and Ir(III) complexes .1 Ru(II) complexes; 1-3
- Cell culture
- Antibacterial and Antifungal assay
The structures of all complexes were solved by SHELXT software (Sheldrick, 2015a) and refined by SHELXL (Sheldrick, 2015b). The SQUEEZE method was applied to an efficient and effective alternative that corrected the data for the joint structure determinations with the problematic solvent perturbation. The dark brown crystals of Ru(II) complexes were obtained and washed with diethyl ether.
The synthesized complexes were completely soluble in dichloromethane and DMSO (1 mg in 1 ml of tested solvent). Subsequently, the photophysical properties and antiproliferative activity against 3 breast cancer cell lines of these four complexes were investigated. In publication II, we mainly focus on the X-ray structure determination alone, which can be summarized as follows: the asymmetric moiety of the Ir(III) complex was a distorted octahedral molecular structure (Figure 12).
Gas-phase DFT was used to optimize the geometry of the ground state of the Ru(II) complexes. The test details and results for complexes 1-3 were mentioned in publication 1, and complex 4 was indicated in the draft manuscript III. To compare the types of structures between monometallic and bimetallic complexes, the bimetallic complex showed a greater activity than Ru(p-cymene)(dppm)Cl2.
Higher numbers of phenyl rings in 1, 2 and 3 may increase the possibility of intercalation of the complex into the DNA helix, cell penetration and cellular uptake. Nevertheless, we still have to worry about the optimal size of the molecule for cell penetration as well. According to publication I, all complexes 1-3 were also tested for antimicrobial activity in DMSO using a colorimetric broth microdilution method regarding the modified versions as defined in publication 1.
For the antifungal activity, the minimal fungicidal concentrations (MFCs) of complexes 1–3 were performed and compared results with positive inhibitory controls of amphotericin B and clotrimazole, as shown in Table 3. 31 The polarizability of the metal was reduced as described by Tweedy's. Chelation theory (Muthukumar et al., 2009). Although the antibacterial and antifungal activities of all studied complexes were weaker than the commercial drugs, it is interesting to note that the bimetallic complexes studied in this work gave better results compared to a monometallic complex of Ru(p-cymene) (dppm) ) Cl2 in our previous work (Chuklin et al., 2017).
; ppy = 2-phenylpyridine and L are](https://thumb-ap.123doks.com/thumbv2/filepdfco/9586961.143969/72.892.178.773.177.802/structure-complexes-coworkers-published-anticancer-phosphorescent-complexes-phenylpyridine.webp)
Conclusion
VITAE
![Fig 3. Normalized absorption spectra of [Ru 2 (p-cymene) 2 (dppp)Cl 4 ] (1), [Ru 2 (p- (p-cymene) 2 (dpppe)Cl 4 ] (2), and [Ru 2 (p-cymene)(dppbe)Cl 4 ] (3) complexes.](https://thumb-ap.123doks.com/thumbv2/filepdfco/9586961.143969/26.892.464.827.99.388/normalized-absorption-spectra-cymene-cymene-dpppe-cymene-complexes.webp)
![Figure S1. IR spectrum of [Ru 2 (p-cymene) 2 (dppp)Cl 4 ] (1), Rupro complex.](https://thumb-ap.123doks.com/thumbv2/filepdfco/9586961.143969/33.1263.241.1030.131.718/figure-ir-spectrum-ru-cymene-dppp-rupro-complex.webp)
![Figure S2. IR spectrum of [Ru 2 (p-cymene) 2 (dpppe)Cl 4 ] (2), Rupen complex.](https://thumb-ap.123doks.com/thumbv2/filepdfco/9586961.143969/34.1263.233.1037.124.744/figure-ir-spectrum-ru-cymene-dpppe-rupen-complex.webp)
![Figure S3. IR spectrum of [Ru 2 (p-cymene)(dppbe)Cl 4 ] (3), Ruben complex.](https://thumb-ap.123doks.com/thumbv2/filepdfco/9586961.143969/35.1263.223.1036.123.742/figure-ir-spectrum-ru-cymene-dppbe-ruben-complex.webp)
![Table S2. Selected hydrogen bonds for complexes 1-3 [Å and o ]](https://thumb-ap.123doks.com/thumbv2/filepdfco/9586961.143969/37.892.110.776.155.979/table-s-selected-hydrogen-bonds-complexes-å-o.webp)
![Figure S4. 1 H-NMR spectrum expanded of [Ru 2 (p-cymene) 2 (dppp)Cl 4 ] (1), Rupro complex in CDCl 3 (300 MHz)](https://thumb-ap.123doks.com/thumbv2/filepdfco/9586961.143969/38.1263.179.1079.97.730/figure-nmr-spectrum-expanded-cymene-rupro-complex-cdcl.webp)
![Figure S5. 1 H- NMR spectrum expanded of [Ru 2 (p-cymene) 2 (dpppe)Cl 4 ] (2), Rupen complex in CDCl 3 (300 MHz)](https://thumb-ap.123doks.com/thumbv2/filepdfco/9586961.143969/39.1263.183.1076.99.734/figure-spectrum-expanded-cymene-dpppe-rupen-complex-cdcl.webp)
![Figure S6. 1 H- NMR spectrum expanded of [Ru 2 (p-cymene) 2 (dppp)Cl 4 ] (3), Ruben complex in CDCl 3 (300 MHz)](https://thumb-ap.123doks.com/thumbv2/filepdfco/9586961.143969/40.1263.195.1086.97.731/figure-nmr-spectrum-expanded-cymene-ruben-complex-cdcl.webp)