This thesis is submitted in partial fulfillment of the requirements for the degree of Master of Science in Chemistry. I would like to thank the chairman and all members of the Department of Chemistry at the United Arab Emirates University, especially Prof.
Introduction
Overview
Statement of the Problem
There is a need to provide a cost-effective treatment process for the removal of organic pollutants in water and wastewater. The scope of work was increased to include the removal of two reactive textile dyes.
Relevant Literature
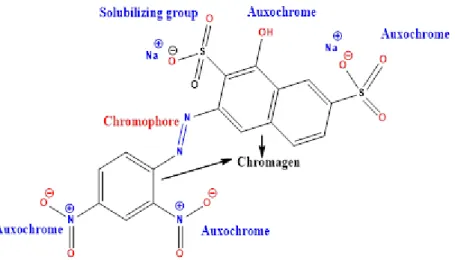
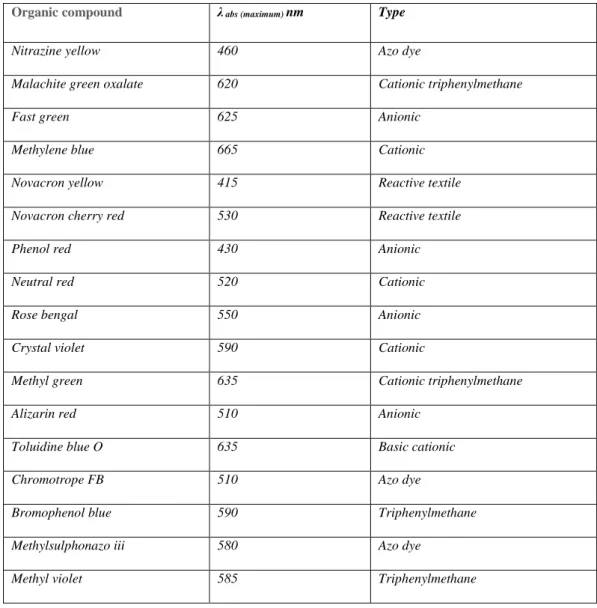
A chromophore is the active site of a dye responsible for light absorption in the visible spectrum and consists of various atomic groups/functionalities such as nitro (-NO2), azo (-N=N), nitroso (- N= O), thiocarbonyl (-C=S), carbonyl (-C=O) and alkenyl (C=C) functions. Disperse dyes, reactive dyes, direct dyes, basic dyes, and acid dyes are water-soluble dyes, while water-insoluble dyes are bath dyes, sulfur dyes, and caustic dyes.
Impact of Organic Pollutants on the Environment and Human Health
Some azo dyes have been linked to human bladder cancer, splenic sarcoma, hepatocarcinoma, and genetic defects in animal studies, as well as chromosomal abnormalities in mammalian cells.
Removal of Organic Pollutant in Wastewater
Nevertheless, these are significant advantages that make the electrochemical oxidation of the dye waste-laden water a suitable process for wastewater classification (Nidheesh et al., 2013; Xiaochao et al., 2018). Adsorption has long been considered one of the most effective traditional ways of removing dye from wastewater (Salleh et al., 2011).
Adsorption Phenomenon
- Adsorption Isotherms and Models
- Adsorption Kinetic
- Types of Adsorbents
Solute adsorption between the solution and the adsorbent reaches equilibrium as the adsorption phenomena progresses. Most of the adsorbents discussed so far, especially inorganic adsorbents, are cost effective.
Methods
- Adsorbent
- Adsorbates
- Activation of Coffee Grounds
- Preparation of Dye Stock Solutions
- Characterization of the Adsorbent Materials
- Batch Adsorption Experiments
- Adsorption Kinetics Studies
- Adsorption Isotherm Study
- Experiments on Desorption and Regeneration of Sorbent Materials
Coffee grounds were initially dried for 7 hours to remove moisture, and 100 g of the dried coffee grounds were mixed in the ratio of 1 to 3 to 10 M solution of phosphoric acid. Enough distilled water was used to wash the coffee grounds to remove residual acid. 50 ppm of each of the dye stock solutions was prepared by dissolving 50 mg of the appropriate dye in distilled water and making up to 1000 ml in a 1000 ml volumetric flask.
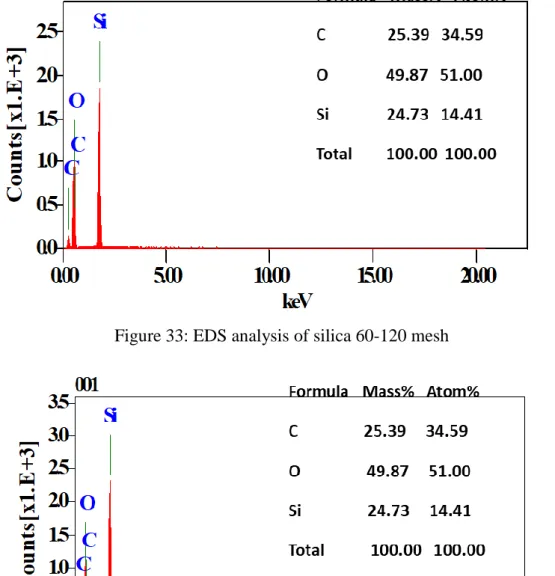
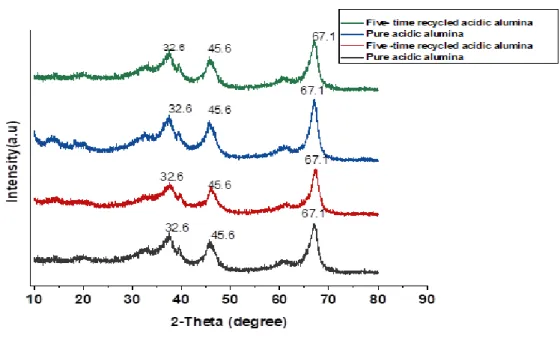
The crystal structure of the adsorbent material was examined with a SHIMADZU Lab X-XRD-6100 powder X-ray diffractometer unit. Microstructural features of the samples were monitored by JEOL JSM-6010LA scanning electron microscope (SEM, Akishima, Tokyo, Japan). Fields of the sample were inspected under a high vacuum (ULVAC KIKO lnc, model: G-100DB, Miyazaki, Japan) and photomicrographs of the sample were captured using InTouchScope JSM software.
The surface area, pore sizes and pore volume of the materials are all determined by the BET analysis. The adsorption capacities of the different sorbents and different dye solutions were measured as a function of time to obtain adsorption kinetic data.
Results and Discussions
Characterization of the Adsorbent Materials
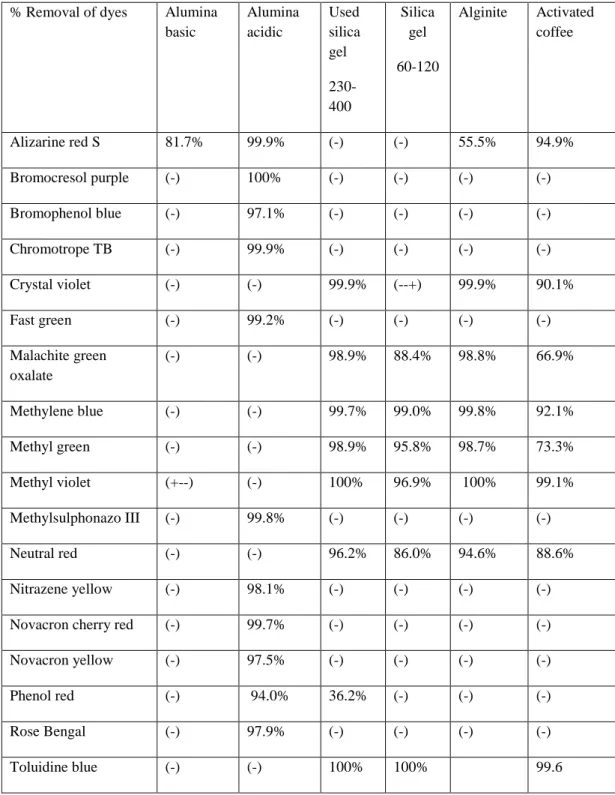
To further confirm that there were no changes in the sorbent materials that could affect their adsorption capacity after thermal treatment, scanning electron microscopy was used to take micrographs of the adsorbent materials before the first adsorption and after five regenerations (see Appendix B for pictures ). This explains why there is little or no difference in the adsorption capacity of the pristine inorganic silica and (acidic and basic) alumina materials used. However, the alginite morphology was disturbed after the first regeneration, a difference in surface morphology was visible, as shown in Figures 45 and 46.
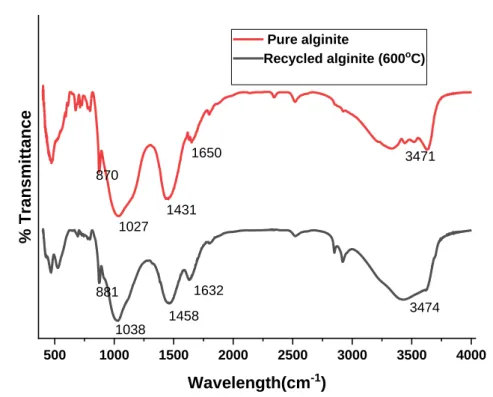
The changes observed in the alginite regeneration experiment at 600oC are shown in Figure 8. Alginite regeneration was experimentally thermally v. This result is supported by what we observed in the SEM image of pure alginite and after the first regeneration of alginite. The alginite surface morphology was disrupted and the difference in appearance of pure and regenerated alginite was visible, as shown in Figures 45 and 46 in Appendix B, respectively.
There is little or no significant difference in the weight percent and atoms of the elements present in the adsorbent material before adsorption and after the five-fold regeneration. For the alginite, the loss of carbon due to combustion correlates with the percentage increase in mass of the non-volatile elements, such as Al, Si and Fe, present in the material.
Adsorption Studies
- Optimization Studies on Dye Removal by Different
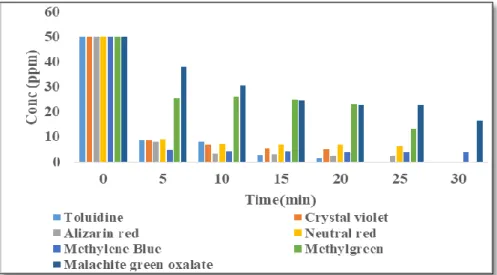
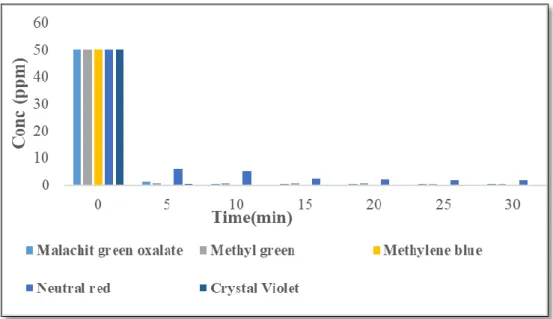
Methyl violet is expected to easily access the ACCG inner pore while the nitrogen and sulfur in one of the aromatic rings of toluidine blue O will block the occupied active site and prevent contact interaction with other adsorbates with the active site. The pH of the dye solutions was adjusted by the addition of 0.1 N HCl or KOH, respectively. The effect of the initial concentration of Toluidine Blue O from 15 ppm to 50 ppm on ACCG was observed at a contact time of 10 min and adsorbent dose of 0.5.
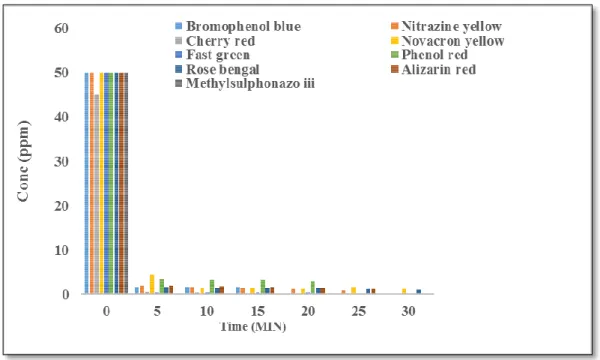
As shown in Figure 15 below, the percent removal of Toluidine blue O depends on the initial concentration of the dye sample. The effect of sorbent dose was tested by adsorption of Nitrazine yellow dye on acidic alumina. However, the same adsorption loading was observed with 0.1 g of acidic alumina after a contact time of 10 min.
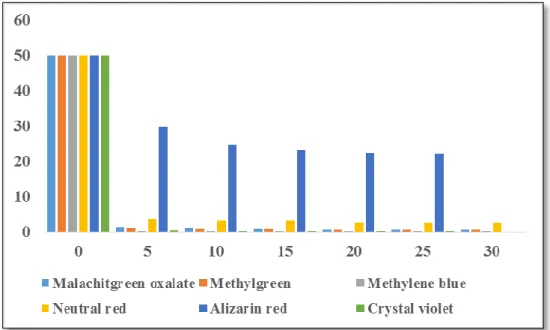
Herein the effect of temperature on Neutral red dye is illustrated as shown in Figure 18 below. Moreover, a higher ambient temperature tends to enhance the deprotonation of the adsorption functional group leading to more adsorption sites for the binding of the N+(CH3)2 (T.-T. Li et al., 2013).
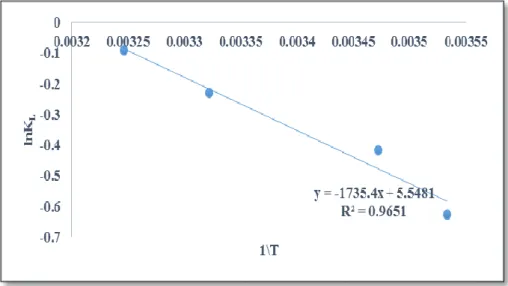
Analysis of the Thermodynamics of the Adsorption Process
As listed in Table 4, the negative value of ΔG at all temperature indicates that adsorption process was spontaneous. The ΔG absolute value increases as the temperature increases, this indicates that the degree of spontaneity of the reaction increases as the temperature increases. This implies that the overall adsorption process of the Neutral red dye on the surface of the adsorbent is endothermic.
However, the solubility of the autochromic group (N+(CH3)2) of neutral red in water explains the endothermic character of the reaction, because adsorption occurs in aqueous environments consisting of two dyes and water molecules. However, because water molecules have a lower molar volume than dye molecules, a significant number of water molecules are desorbed from the sorbent surface, resulting in a significant amount of energy required for this process. Also, there is an increase in the randomness at the liquid-solid interface during the fixation of the adsorbate in the active site of the adsorbent.
Thus, in general, the adsorption process of neutral red on rough silica can be described by the structural change of the adsorbate and the adsorbent during the adsorption process. Adsorption of neutral red is unfavorable in terms of enthalpy, but favorable in terms of entropy change.
Adsorption Isotherm
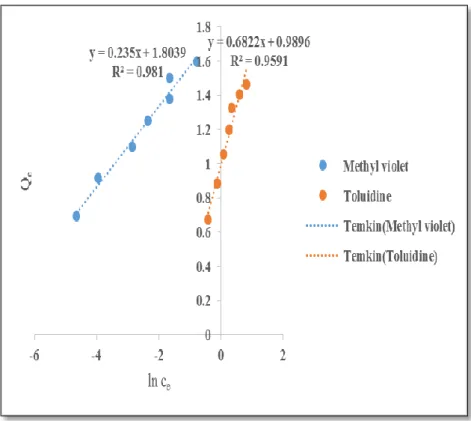
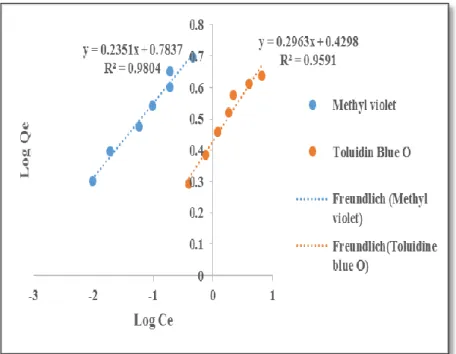
Using the Freundlich model to fit the adsorption of methyl violet and toluidine blue onto ACCG, the plots give a correlation coefficient of 0.9804 and 0.9591 and a determinant value (nF) of 4.25 and 3.37, respectively. Using the Temkin isotherm to stimulate the adsorption data, the correlation coefficients for methyl violet and toluidine blue are 0.9619 and 0.9747, respectively. Adsorption is also considered exothermic because B is positive for both methyl violet and toluidine blue.
Using the Elovich isotherm to fit the experimental data for the absorption of methyl violet and toluidine blue in ACCG, the correlation coefficients are 0.9478 and 0.9447. As a result, the Elovich isotherm is not suitable for the adsorption of methyl violet and toluidine blue on ACCG. Using the Flory-Huggins isotherm to fit the experimental data of the adsorption of methyl violet and toluidine blue on ACCG, the high correlation coefficients of 0.9619 and 0.9747 for methyl violet and toluidine blue suggest that the Flory-Huggins model is a good fit good. for the data.
Using the Harkin - Jura model to fit the experimental data, the R2 value is 0.920 and 0.8716 for methyl violet and toluidine blue, respectively. This suggests that Harkin-Jura is not a good fit for the adsorption of methyl violet and toluidine blue on ACCG.
Adsorption Kinetics
- Intra-particle Diffusion Model
- Activation Energy Studies
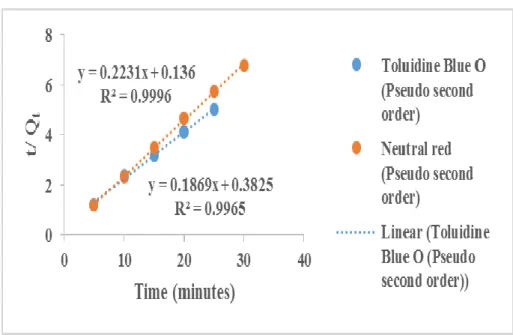
On the other hand, as highlighted in Table 6, the Qe values calculated for the adsorption of neutral red and toluidine blue on ACCG are in good agreement with the experimental Qe value. This result is supported by the finding of other researchers, where the adsorption of neutral red on different. On the other hand, using a pseudo-second-order kinetic model, the Qe value calculated for the adsorption of neutral red (on alginite, activated silica and coarse silica) and toluidine blue (on alginite) are in good agreement with the experimental Qe values.
These results suggest that the pseudo-second-order kinetic model fits well with the experimental data; the adsorption mechanism is chemisorption. Similar findings were deduced from the adsorption of neutral red on holloysite nanotubes (Luo et al., 2010). The adsorption process of Nitrazine Yellow fits well with the pseudo-second order kinetics with an R2 close to 1.
Our findings are consistent with the adsorption of Nitrazin Yellow on chitosan-montmorillonite hydrogel (Fahanwi et al., 2016). Where Qt is the adsorption capacity at time t (mg/g), kp is the intra-particle diffusion rate constant (mg/gmin1/2).
Recycling of the Adsorbents
Risk Assessment
Conclusion
Adsorption kinetics and removal of cationic dye, Toluidine Blue O, from aqueous solution with Turkish zeolite. Adsorption characteristics of aluminum nanoparticles for the removal of hazardous paint, Orange G from aqueous solutions. Improving the One-Step Extraction Performance of Biphasic Aqueous Systems Using New Symmetrical Ionic Liquids for Decolorization of Toxic Dye Effluents.
Synergistic removal of Cu(II) and nitrazin yellow dye using an environmentally friendly chitosan-montmorillonite. Removal of lead(II) from aqueous solution by ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: Kinetic and equilibrium modeling. Removal of Pb(II) ions from aqueous solutions by sulfuric acid-treated palm leaves.
Functional groups grafted aluminum nanofibers: A new design in efficient sorbents for the removal of toxic pollutants from water. Preparation of composite fibrous chitosan/sodium alginate foams for the adsorption of cationic and anionic dyes.