The thesis is submitted as partial fulfillment of the requirements for the PhD in Chemistry. Cover: Plausible mechanism for CO2 cycloaddition of epoxides to cyclic carbonates (photo: Fatmah Rashed Ali Alkindi). I further declare that there is no potential conflict of interest in relation to the research, data collection, authorship, presentation and/or publication of this dissertation.
The optical property of the prepared photocatalyst was investigated using UV-Vis diffuse reflectance spectroscopy (UV-Vis DRS), powder X-ray diffraction (PXRD) for phase determination, scanning electron microscopy (SEM) for morphology and structural analysis, Brunauer-Emmett −Teller (BET ) for specific surface area and porosity determination, and energy dispersive X-ray spectroscopy (EDX) for elemental chemical identification. The photocatalytic activities of the prepared TiO2 were evaluated using the cycloaddition reaction of various epoxides to CO2. As prepared, TiO2 (anatase) quantitatively achieved the formation of cyclic carbonates over a series of epoxides used for the CO2 cycloaddition reaction.
Alzamly, for giving me the chance to research and providing vital assistance during this process. I am eternally grateful to my beloved father Rashed Alkindi, who supported and believed in me, as well as my incredible, exceptional and loving mother, for their unwavering support. I would like to express my sincere gratitude to my sister Shama, who has provided me with invaluable support and entertainment over the years.
I owe a lot to my friends Reem and Lamia who supported me and stood by me during this journey.
Introduction
When exposed to UV radiation, Ti-oxo cluster has been shown to have a remarkable capacity for UV absorption and significant activity for CO2 binding.17 This makes it one of the many molecular metal-oxo cluster materials that be used as Second, because of these materials' intrinsic discreteness and solubility, they are ideally suited for processing techniques involving either solids or solutions. The high propensity of early transition metals such as titanium (IV) and oxo-bridged multinuclear clusters produced from these is encouraging by the sense of the discovery of new molecular solids with even more fascinating characteristics.18.
Due to its abundance, lack of toxicity and flammability, carbon dioxide (CO2) can be used as a C1 feedstock despite being both cheap and renewable.19. Due to its efficiency as an atom economy process, CO2 cycloaddition to epoxides is one of the most profitable methods in sustainable progress, viz. For more than 50 years, it has been known that cyclic carbonates (CCs) can be made by inserting carbon dioxide (CO2) into epoxides.
An important example is propylene carbonate (PC), which serves as a feedstock along with unsaturated cyclocarbonates and as an alternative green dipolar aprotic solvent for organic synthesis. In this thesis, we describe the controlled synthesis of an anatase TiO2 polymorph from a hexanuclear titanium oxo cluster. In addition, a titanium(IV)-oxo carboxylate group was prepared in 2-propanol using titanium(IV) isopropoxide and 4-aminobenzoic acid.
Photocatalytic Cyclic Carbonates Formation over Hexanuclear
- Introduction
- Experimental
- Materials
- Hexanuclear Titanium Oxo Cluster Synthesis
- Characterization
- Powder X-Ray Diffraction (PXRD)
- UV–Vis Diffuse Reflectance Spectroscopy (UV–Vis DRS)
- Thermogravimetric Analysis (TGA)
- Scanning Electron Microscopy (SEM)
- Nuclear Magnetic Resonance (NMR)
- Photocatalytic Activity
- Results and Discussion
- PXRD of Hexanuclear Titanium Oxo Cluster
- UV-Vis DRS of Hexanuclear Titanium Oxo Cluster
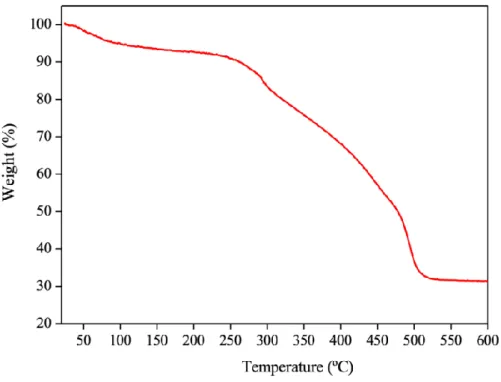
- Thermogravimetric Analysis (TGA) of Hexanuclear Titanium Oxo
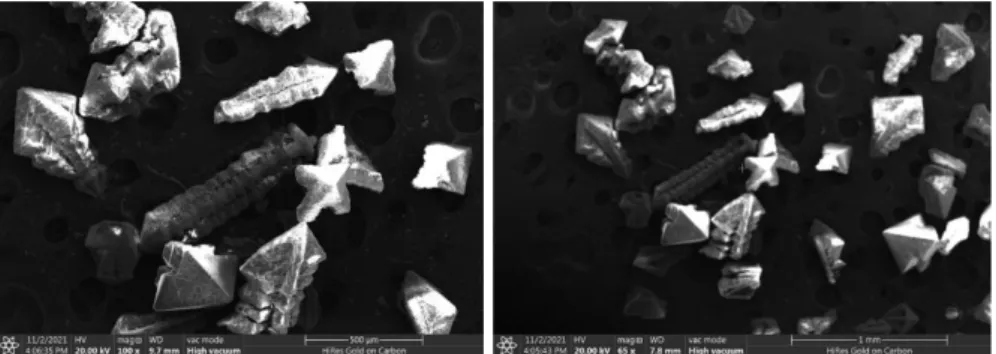
- Scanning Electron Microscopy
- Photocatalytic Activities of Hexanuclear Titanium Oxo Cluster
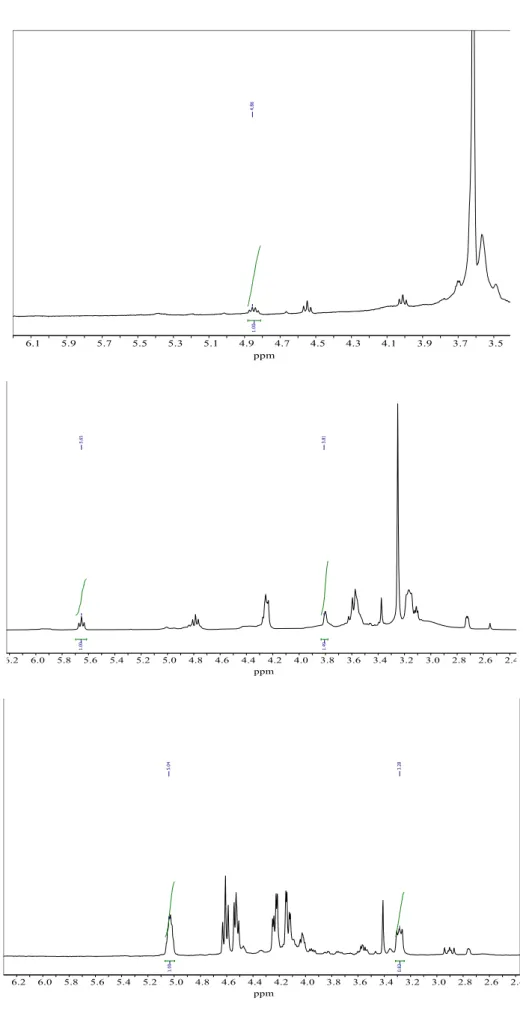
- NMR Spectra
- Conclusion
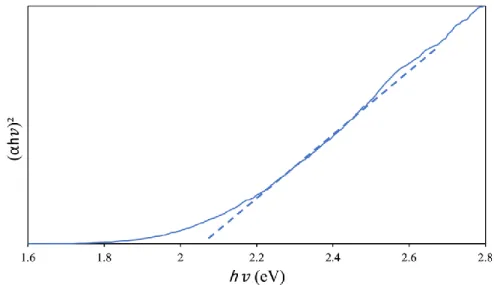
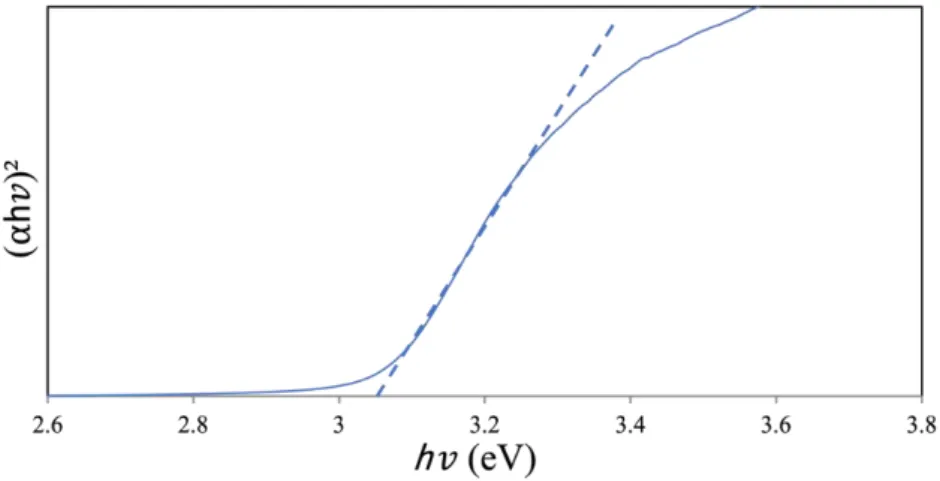
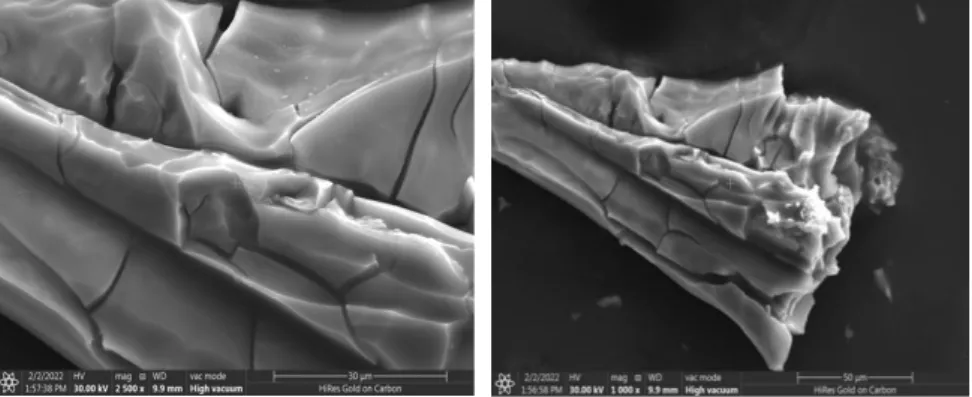
The UV-DRS analysis of the photocatalyst of the titanium oxocluster as prepared is shown by the Tuac plot in Figure 2. Based on a direct transition allowed, the bandgap in the purely prepared titanium oxocluster was calculated to be 2.1 eV. The surface morphology and particle shape of Titanium-oxo Cluster are shown in Figure 4 on two different scale bars.
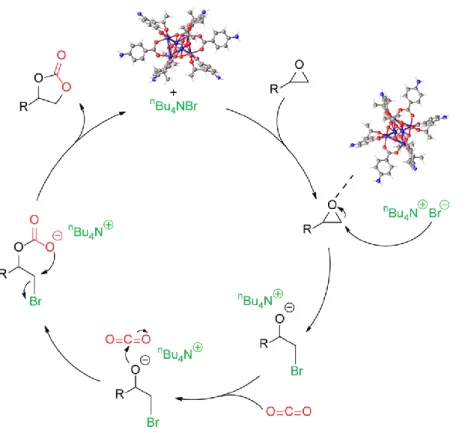
The CO2 cycloaddition reaction was initiated when the Lewis acid site (Ti3+ nodes) on the oxo group of the hexanuclear titanium coordinated to the oxygen atom of the epoxides, which resulted in the weakening of the carbon-oxygen bond. The Ti active sites on the oxo group of titanium were a crucial role in the activation of the cycloaddition reaction.
Anatase TiO 2 Derived from Hexanuclear Titanium-Oxo Cluster
- Introduction
- Experimental
- Materials
- Synthesis of Hexanuclear Titanium Oxo Cluster and Anatase TiO 2
- Characterization
- Powder X-Ray Diffraction (PXRD)
- UV–Vis Diffuse Reflectance Spectroscopy (UV–Vis DRS)
- N 2 Adsorption–Desorption Analysis
- Scanning Electron Microscopy (SEM) and Energy-Dispersive X-
- Nuclear Magnetic Resonance (NMR)
- Photocatalytic Activity
- Results and Discussion
- PXRD Analysis of Pure Anatase-Phase TiO 2
- UV-Vis DRS of Pure Anatase-Phase TiO 2
- N 2 Adsorption–Desorption Analyses of Pure Anatase-Phase TiO 2
- Scanning Electron Microscopy (SEM) and Energy-Dispersive X-
- Photocatalytic Activities Pure Anatase-Phase TiO 2
- NMR Spectra
- Conclusion
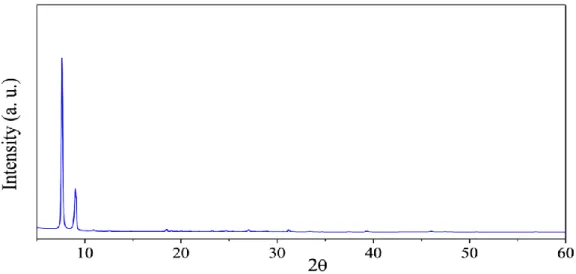
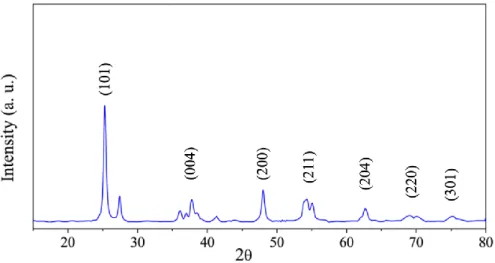
As a result of changes in the crystal size and textural quality of nanostructured TiO2, extensive research has recently been reported for the synthesis of nanostructured TiO2 with different properties for specific applications. The performance of the photocatalyst was investigated using the cycloaddition reaction of epoxide to CO2 as a photocatalytic model reaction. As shown below, the PXRD of the prepared titanium oxo cluster is in perfect agreement with the PXRD of the previously reported one.24 Moreover, the PXRD database (JCPDS file no.) showed that all diffraction peaks were correctly indexed to those of the pure tetragonal phases of TiO2 (anatase).
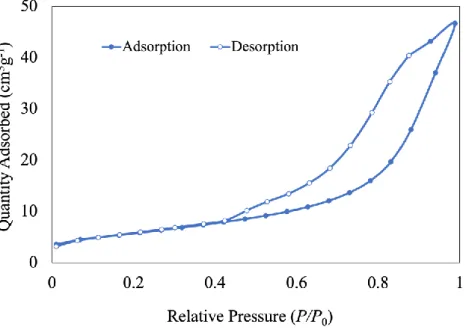
No extra peaks associated with starting materials or any impurities were observed, indicating successful preparation of pure anatase phase TiO2. The UV-DRS analysis of the prepared TiO2 photocatalyst was calculated using the Tauc plot shown in Figure 8. The surface area of pure anatase phase TiO2 was calculated using the Brunnauer-Emmett-Teller method (BET) with a value of 20 m2/g.
In addition, a photocatalyst made of pure TiO2 in the anatase phase shows a mesopore size distribution of 38 to 40.7 nm. In Equation 1, the OCH protons in the starting material (1Ha) were compared to their respective counterparts in the product (1Hb) to calculate the conversion percentage (Table 3). As shown in Figure 12, the NMR spectra of the product show the expected peaks without any impurities or starting material, except when 1,2-epoxy-3-phenoxypropane was used, indicating that the reaction is quantitatively complete.
Consequently, we speculate that the reaction may occur within the mesopore of TiO2 and not in the surface. To confirm the role of the pure anatase phase TiO2 photocatalyst, three control experiments were performed (Table 4): (1) photocatalytic reaction without light at room temperature (entry I), (II) photocatalytic reaction without pure anatase phase TiO2. Cyclic propylene carbonate conversion was only 10% when using nBu4NBr without the use of pure anatase-phase TiO2 photocatalyst, and only 3% when using 353 K without light, according to the results.
PXRD pattern of calcined hexanuclear titanium oxo cluster confirms the presence of pure anatase phase TiO2. Prepared TiO2 was used as an effective photocatalyst for CO2 cycloaddition to epoxides producing cyclic carbonates quantitatively, except when bulky 1,2-epoxy-3-phenoxypropane was used. In conclusion, calcination of titanium oxo cluster provides a facile and affordable way to fabricate photoactive catalysts.
Summary and Future Work
Efficient inhibition of photogenerated electron-hole recombination through persulfate activation and dual pathway degradation of micropollutants on iron molybdate. Influence of Nitrogen Content Levels on Structural Properties and Photocatalytic Activities of N-Doped TiO2 as Nanorices with Different Calcination Temperatures. Nanostructured TiO2-CeO2 Mixed Oxides by an Aqueous Sol-Gel Process: Effect of Ce:Ti Molar Ratio on Physical and Sensory Properties.
From isolated Ti-Oxo clusters to infinite Ti-Oxo chains and plates: recent developments in photoactive Ti-based MOFs. Highly selective synthesis of cyclic carbonates via solvent-free cycloaddition of CO2 and epoxides using ionic liquid grafted onto rice husk derivative MCM-41. One-pot conversion of CO2 and glycerol into value-added products, using propylene oxide as a coupling agent.
A combination of heterogeneous catalysis and photocatalysis for the olefination of quinoxalin-2(1H)-ones with ketones in water: A green and efficient route to (Z)-enaminones. Effect of crystallization methods on morphology and photocatalytic activity of anodized TiO2 nanotube array films. Mediation of both valence and conduction bands of TiO2 by anionic dopants for visible and infrared light photocatalysis.
Enhancing the photoconversion efficiency of TiO2 nanotubes using UV radiation-assisted anodization as a prospective method: an efficient photocatalyst for the elimination of recalcitrant organic pollutants. Optimization of thermal conditions of Sol-Gel method for synthesis of TiO2 using RSM and its influence on photodegradation of tartrazine yellow dye.