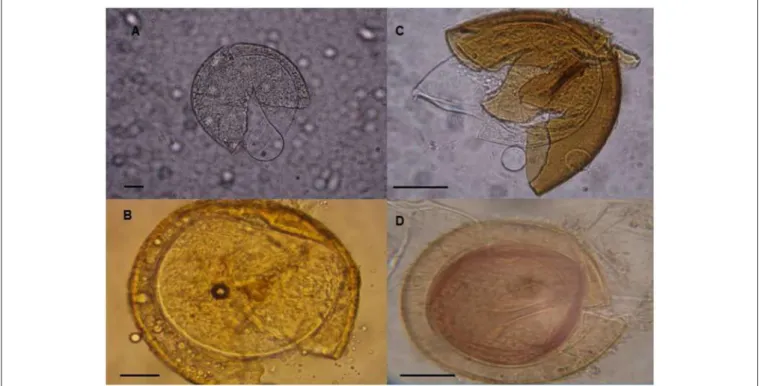
Asmelash et al. presented a review on the potential role of arbuscular mycorrhizal fungi (AMF) to significantly enhance restoration success of degraded lands where levels of infective AM abundance and diversity are often low. AMF is; (1) obligate biotrophs completely dependent on host plants for organic carbon (File et al. evolutionarily intimately associated with plants (Taylor et al multiple nucleus, and (4) asexual reproduction of eukaryotes (Schüßler et al., 2007).
WHAT IS ECOLOGICAL RESTORATION?
Therefore, designing the original species composition and ensuring their survival and establishment is an important step in ecological restoration (Angelini et al., 2011). This is the most widely accepted definition of ecological restoration (Harris et al., 2006; Higgs et al., 2014).
AMF AND THE MYCORRHIZOSPHERE ECOLOGY
It has been shown that plants preferentially allocate more carbon in favor of the more beneficial fungi (e.g. Bever et al., 2009). After many years of observation, Barea et al. 2011) concluded that the use of native AMF consortia has the maximum effect.
AMF AND MEASURABLE RESTORATION ATTRIBUTES
A recent meta-analysis on 304 papers also concluded that AMF inoculation increases the growth and productivity of plants grown alone (Lin et al., 2015). Plant-soil feedback (plant-AMF feedback) is also an important concept that explains the role of AMF in succession (Kikvidze et al., 2010).

AMF BIOTECHNOLOGY FOR THE RESTORATION OF DEGRADED LANDS
Readers are requested Adholeya et al. 2005) to understand the potential and technique of monoxenic in vitro AMF culture production for large-scale application. Readers are also referred to reading Azcón-Aguilar et al. 1999) to get the correct definitions of axenic and monoxenic cultures.
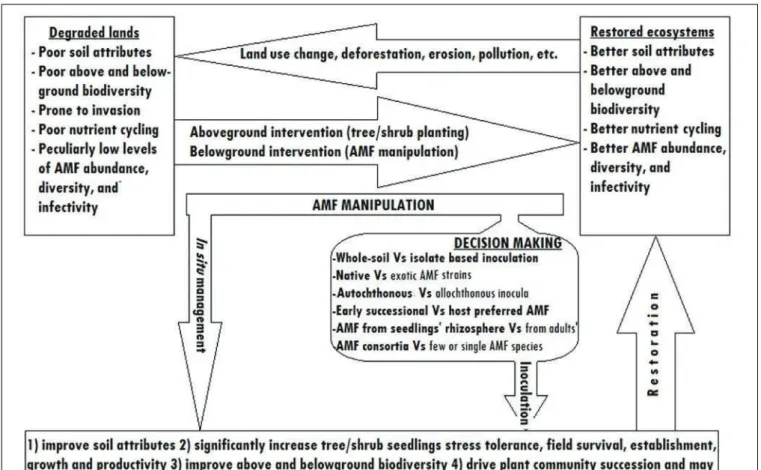
CONCLUSION
However, some studies showed that grass cover can significantly suppress individual tree/shrub seedling growth (Riginos, 2009) or have seasonally variable effects (Good et al., 2014). Nowadays, substrate-free inoculum preparation methods and in vitro production in cut plant roots are being intensively researched to make AMF inoculation less costly (Ijdo et al., 2011).
AUTHOR CONTRIBUTIONS
AMF momoxenic in vitro cultivation uses transformed [with Rhizobium rhizogenes(Riker et al.) Young et al.] hairy roots as hosts due to the fact that these hairy roots are better suited than the non-transformed hairy roots as they grow on hormone-free media and without developing shoots and leaves (P13, 20). Due to the lack of cheap and easy AMF vaccine production for large-scale application, the management of the in situ AMF is sometimes considered as an effective AMF biotechnology for the restoration of degraded lands.
ACKNOWLEDGMENTS
Therefore, investigating cover crop management options to effectively manage AMF and facilitate the growth of tree/shrub seedlings may be an important research topic. Although the pot culture inoculum preparation method is labor intensive and expensive, it can be a source of employment especially in developing countries.
The role of arbuscular mycorrhizal fungi in early-stage restoration of seasonally dry tropical rainforest in Chamela, Mexico.Rev. Alleviation of salt stress in citrus seedlings inoculated with arbuscular mycorrhizal fungi depends on rootstock salt tolerance.J.
INTRODUCTION
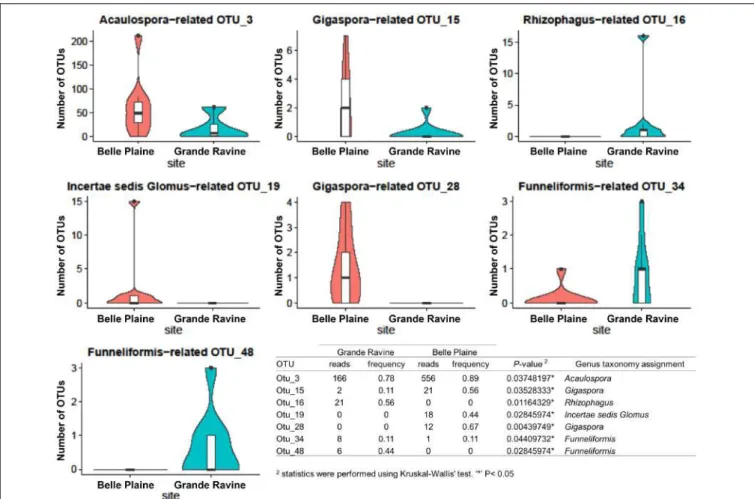
2016) showed that the microbial community structures found in the soil and root differed from those associated with the AMF spores harvested from the same samples. To the best of our knowledge, this is the first research work devoted to comparing the soil, root and microbial communities associated with AMF traces at sites contaminated with petroleum hydrocarbons.
MATERIALS AND METHODS
Sequence classifications were performed with the Mothur implementation of the RDP database using the "classify.seqs" command. Distance matrices were generated using the "dist.seqs" command and OTUs were obtained using the "cluster.split" command (method = mean, processors = 2, split method = classify, large = T).
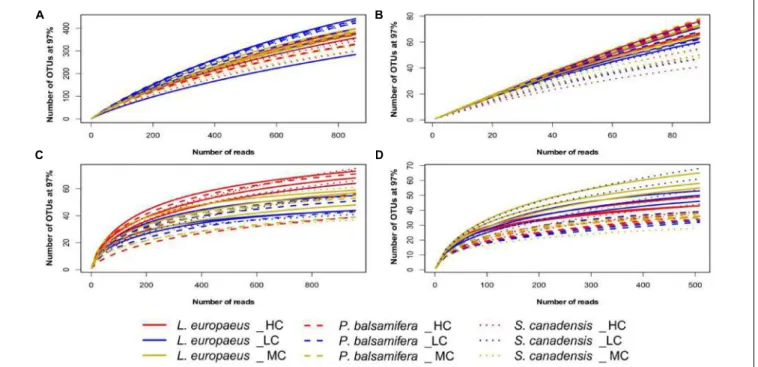
RESULTS
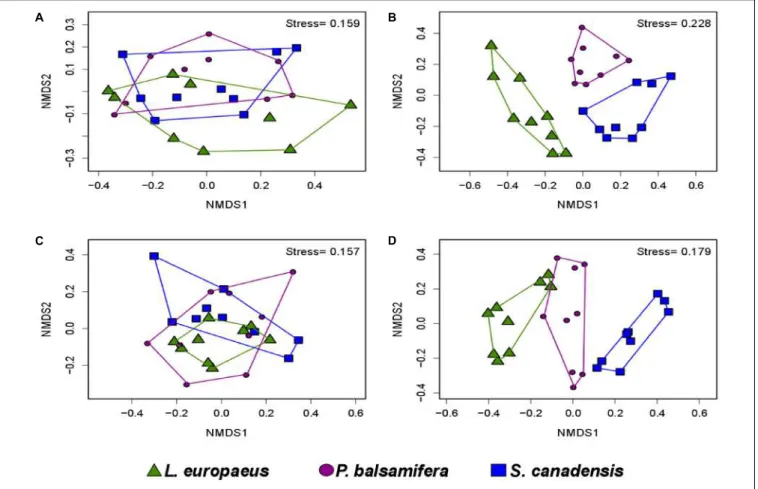
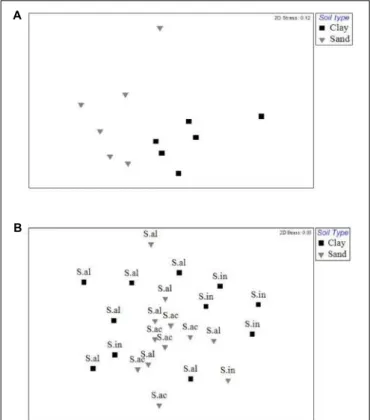
In contrast, there was no effect of plant species identity on bacterial diversity in the soil data set (Table 1 and Figure 2D). No effect of plant species identity on fungal diversity was found in soil (Table 1 and Figure 2D).
DISCUSSION
We previously found that Gammaproteobacteria and Betaproteobacteria were the most dominant classes associated with AMF spores (their abundance was 49 and 23%, respectively), while the dominant fungi belonged to unclassified fungi (55%), Pezizomycetes (13%) and Dothideomycetes (13%) (Iffis et al., 2016). In addition, comparisons between the AMF spore-associated microbiome described previously in Iffis et al. 2016) and the results of this study showed that the microbial communities living in association with AMF spores differed significantly from those in the surrounding soil and roots.
FUNDING
Similarly, Glomeromycetes are known as obligate biotrophic fungi that require host plants for their growth and reproduction (Simon et al., 1993; St-Arnaud and Vujanovic, 2007; Smith and Read, 2008). As with plant roots, AMF can release carbon sources and other signaling molecules that make the spore and mycelial surface favorable and then selective for the growth of specific microorganisms, as previously suggested (Roesti et al., 2005; Bharadwaj et al., 2011; Lecomte et al., 2011; Agnolucci et al., 2011; Agnolucci et al., 2011).
SUPPLEMENTARY MATERIAL
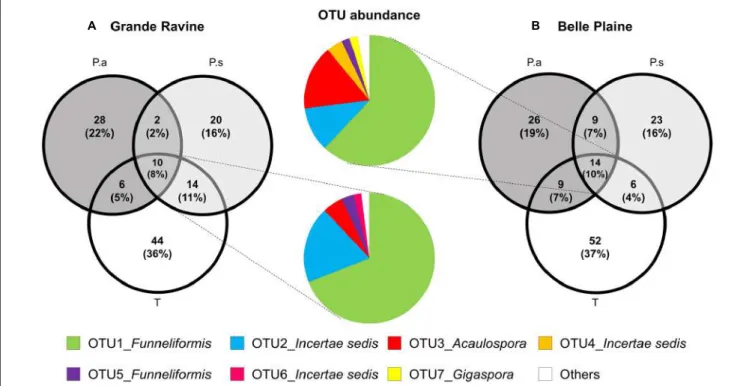
Arbuscular mycorrhizal fungi are the most common and widespread symbiosis involving 86% of land plants including many important crops (Davison et al., 2012). AM fungi are assumed to exhibit nonspecific symbiosis, but a given AM taxa may have different effects depending on the plant species ( Thioye et al., 2017 ).
MATERIALS AND METHODS Study Sites and Sampling
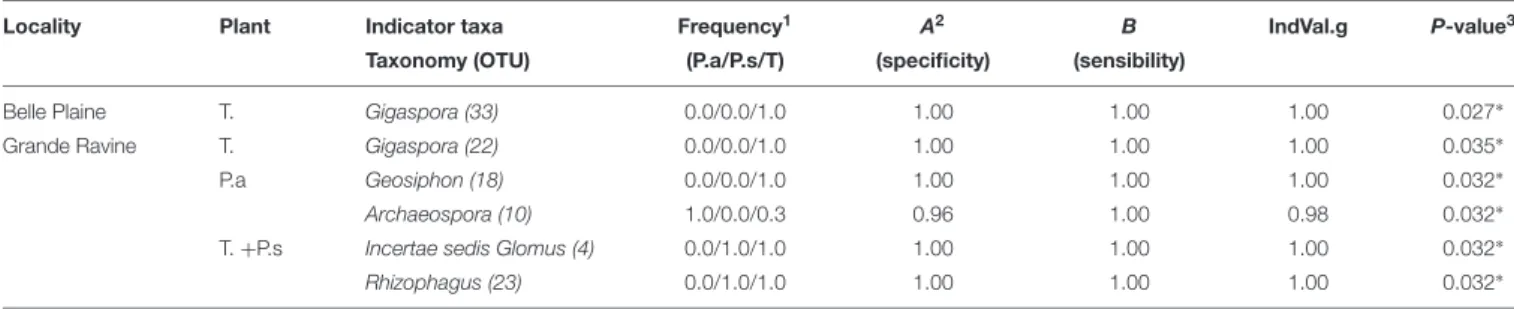
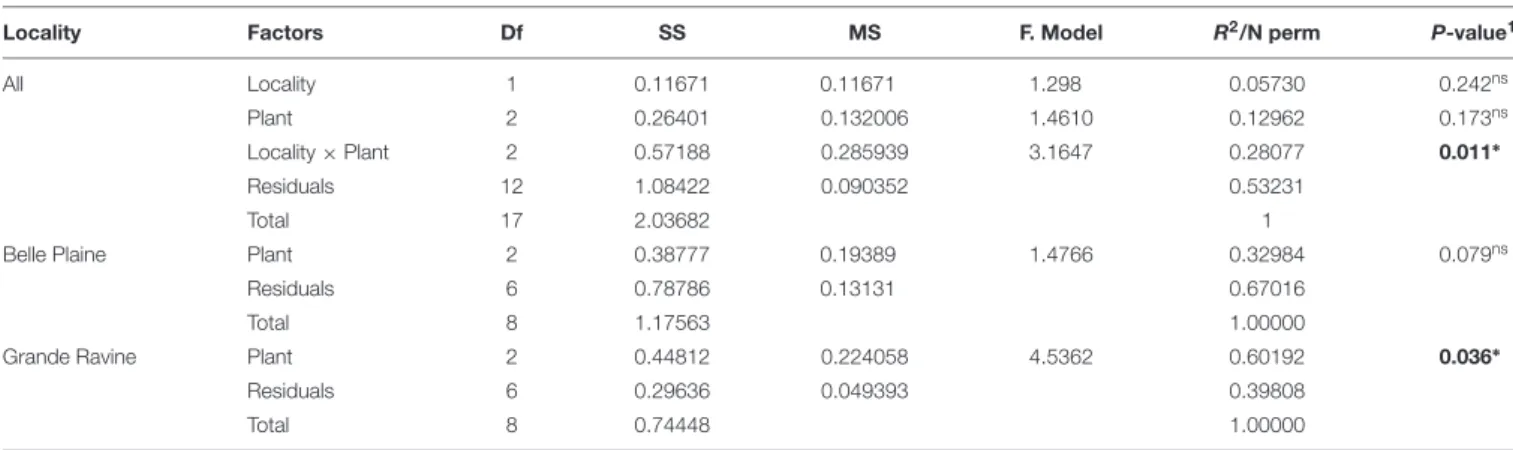
The frequency of AM fungal OTUs was determined using the strassoc() function from the R package indicspecies (De Cáceres and Legendre, 2009). AM fungal community characteristics (diversity, richness and structure) were relatively comparable in this cropping system between two forest sites (separated by 15 km).
DATA
The role that mycorrhizal associations play in the composition of long-lived tree communities is much less understood, especially in tropical forests, which have the highest tree diversity of any ecosystem (Leigh et al., 2004). These ECM associations have been suggested as one of the factors that originally facilitated and perpetuated the familial dominance of Dipterocarpaceae (Torti et al., 2001; Brearley, 2012). A recent study performed a reciprocal transplant of dipterocarp seedlings across soil types, and found no evidence for ECM fungal host specificity ( Peay et al., 2015 ).
MATERIALS AND METHODS Site Description
We identified the ECM fungal community in the soil core samples using a barcoded, high-throughput Illumina sequencing method previously described (McGuire et al., 2013). To ensure accuracy, these taxonomic assignments were compared to the best BLAST hit on GenBank (Benson et al., 2009). Black locust (Robinia pseudoacacia L.) is a leguminous tree species commonly planted in the Loess Plateau, China (Zhang et al., 2016).R.
MATERIALS AND METHODS Fungal Inoculum and Plant
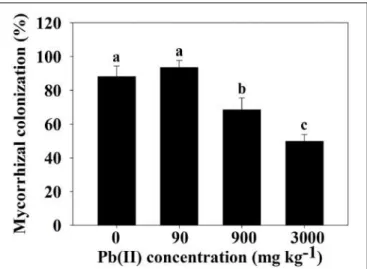
Due to natural deposits and intense human activity, Pb has become ubiquitous in soil (Fahr et al., 2013). Autoexclusion of soil Na + ions can be achieved by C4 plants in saline soils (Omoto et al., 2012). In the current scenario, a relatively small number of beneficial plant-microbe interactions are well described and exploited (Farrar et al., 2014).
ROS GENERATION AND SCAVENGING DURING PLANT-ARBUSCULAR
However, adverse conditions including abiotic and biotic stress can significantly accelerate the generation of ROS at the cellular level (Apel and Hirt, 2004; . Gill and Tuteja, 2010; Rasool et al., 2013). Compared to non-radicals, radical forms of ROS are more toxic due to their highly reactive nature (Gill and Tuteja, 2010; Sewelam et al., 2016). Important antioxidant metabolites, namely glutathione (GSH) and ascorbic acid (AsA) belong to the list of non-enzymatic components (Apel and Hirt, 2004; Gill and Tuteja, 2010; Rasool et al., 2013).
MYCORRHIZAL INTERACTION UNDER STRESS CONDITIONS
In particular, NADPH oxidases and homologs of respiratory burst oxidase are the major components of the ROS generation system in plants (Suzuki et al., 2013;Kadota et al., 2015). To alert the plants to stress adaptation, the first generation of ROS was reported to act over long distances in response to stress (Mittler et al., 2011; Sewelam et al., 2016). In plants, stress signals include redox homeostasis, antioxidant signaling and continuous production/clearance of ROS at the cellular level (Bose et al., 2014; Jajic et al., 2015).
LINK OF ROS SIGNALING WITH STRESS TOLERANCE DURING
PLANT-ARBUSCULAR MYCORRHIZAL ASSOCIATION
Enhanced activities of important antioxidant enzymes including SOD, CAT, POD, GR, and APX have been argued to improve cadmium (Cd) tolerance in tomato through AMF-mediated ROS scavenging ( Hashem et al., 2016 ). Furthermore, under ROS, H2O2 is membrane permeable and plays an important role in the signaling cascade as well as in defense response under stressful environments (Xia et al., 2009; Saxena et al., 2016). H2O2 has thus emerged as an active signaling player also involved in the regulation of specific biological responses/cellular metabolism and stress tolerance (Neill et al., 2002;Yan et al., 2007;Saxena et al., 2016).
ROS MODULATION DURING
Altered ROS signaling/metabolism, in response to biotic and abiotic stress, links to stress tolerance in mycorrhizal colonized plants consequently provides stress tolerance; while the scenario is just reversed in the case of non-colonized plants, i.e. high ROS production followed by the inhibition of plant cellular activities thus affecting the plant fitness. It was found that H2O2 induces OXI1 (Oxidative Signal Inducible1) gene which consequently triggers defense response during pathogen infection (Rentel et al., 2004; Anthony et al., 2006; Petersen et al., 2009). In Arabidopsis roots, OXI1 (a serine/threonine kinase) has been shown to be required for oxidative burst/ROS-mediated responses, including root hair elongation and disease tolerance to biotrophic pathogens ( Rentel et al., 2004 ; Petersen et al., 2009 ).
CONCLUSIONS AND PERSPECTIVES
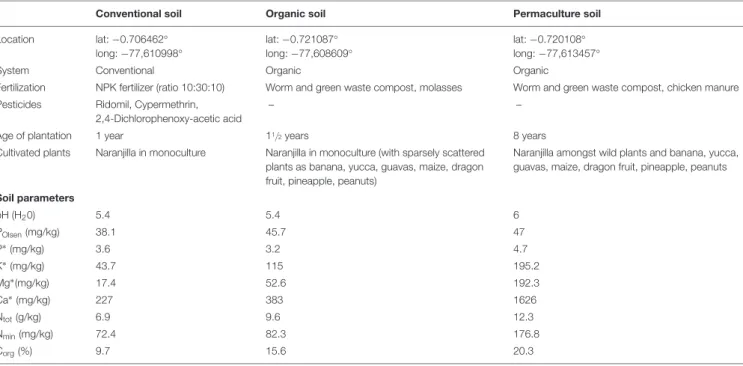
Naranjilla (Solanum quitoense, Solanaceae) is a perennial shrub and an important commercial crop in Ecuador, Colombia and Central America, where its fruits are used for a very popular drink (Acosta et al., 2009). Plantations, often located in steep areas and cultivated in monoculture, are fully exposed to soil erosion and degradation (Revelo et al., 2010). However, after 2–3 years, plantation productivity decreases due to erosion, nutrient deficiency and high prevalence of resistant pathogens in the soil (Revelo et al., 2010).
MATERIALS AND METHODS Biological Material
Taxonomic identities were assigned to the OTU representative sequences using the UNITE database (Kõljalg et al., 2013) with BLAST in the QIIME environment (Caporaso et al., 2010). Several studies have reported negative impacts of fungicides such as a decrease in hyphal growth and sporulation of AMF (Sukarno et al., 1993;. In addition, other benefits related to AMF are the stabilization of soil aggregates (Rillig, 2004), increased resistance to water stress (Garg and Chandel, 2010) and protection against pathogens (J20, et al.).
MATERIALS AND METHODS Experimental Area
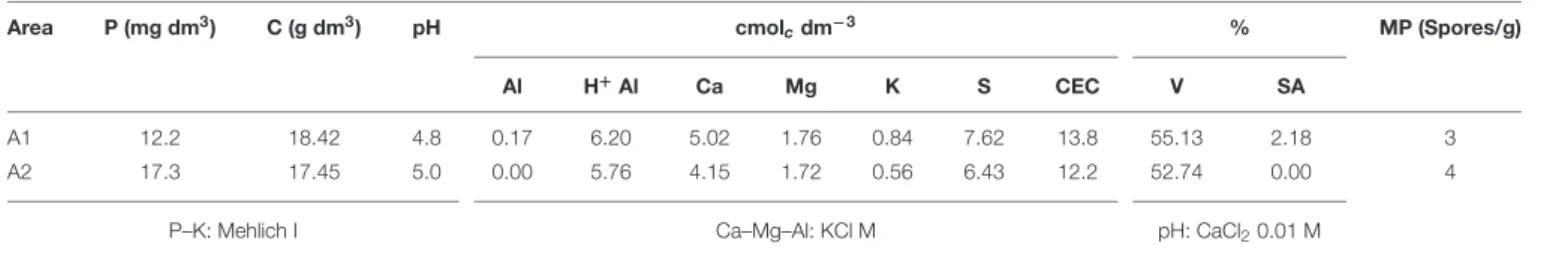
The AMF inoculation in field conditions has been evaluated by some authors such as Romero and Bago (2010), Pellegrino et al. The success of AMF inoculation in agricultural soils can be determined by many factors such as species compatibility, habitat availability for AMF and competition with native fungi (Verbruggen et al., 2013). 2014) also observed a positive linear relationship between P and yield in soybeans when inoculated with AMF. clarus on the growth and yield of cotton found here agrees with Thompson et al. 2012) who found an increase in seed cotton yield by.
ACKNOWLEDGMENT
The interaction between soil microorganisms and plants, especially in the roots, provides essential nutrients for plant growth. Since the roots directly influence the surrounding microbial populations, the microorganisms in the rhizosphere can thus also influence plant growth (Giri et al., 2005). On the other hand, it can be assumed that the Rhizobium acts as plant growth promoting bacteria in the rhizosphere and releases phytohormones (Mehboob et al., 2012).
MATERIALS AND METHODS Experimental Field
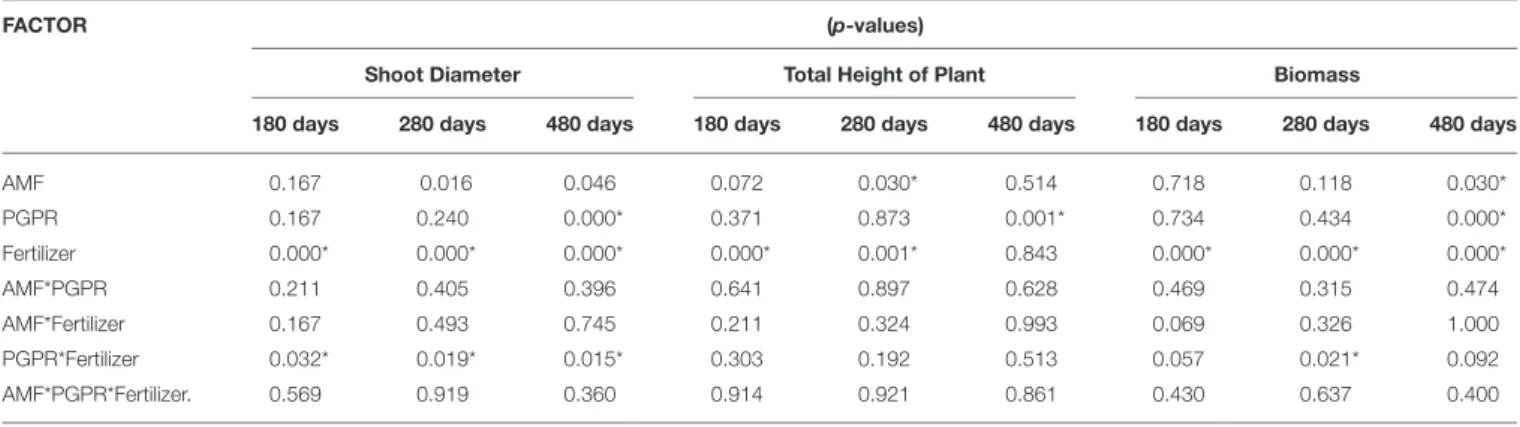
In the seedling planting method, the double inoculation of AMF (Glomus clarum) and PGPR (LEM6 or Rhi1Rhizobium strains) was more effective. The doses applied in the experiment were lower than those reported by Viégas et al. Indeed, AMF belonging to the phylum Glomeromycota (Schüßler et al., 2001) represent a multifunctional partner in the mutualistic interaction they develop with most land plants.
MATERIALS AND METHODS Plant Material
Interactions between an arbuscular mycorrhizal fungus (Scutellospora heterogama) and the root knot nematode (Meloidogyne incognita) on sweet passion fruit (Passiflora alata).Braz. Germplasm in the International Collection of Arbuscular and Vesicular Arbuscular Mycorrhizal Fungi (INVAM) and Procedures for Culture Development, Documentation and Storage. Mycotaxon. Chlorophyll fluorescence analysis to assess water deficiency and arbuscular mycorrhizal fungi (AMF) inoculation in cassava (Manihot esculentaCrantz).Adv.