Po ultr y N utr iti on • V in ce nz o T ufa re lli
Poultry Nutrition
Printed Edition of the Special Issue Published in Animals
www.mdpi.com/journal/animals
Vincenzo Tufarelli
Edited by
Poultry Nutrition
Poultry Nutrition
Editor
Vincenzo Tufarelli
MDPI•Basel•Beijing•Wuhan•Barcelona•Belgrade•Manchester•Tokyo•Cluj•Tianjin
Vincenzo Tufarelli
University of Bari Aldo Moro Italy
Editorial Office MDPI
St. Alban-Anlage 66 4052 Basel, Switzerland
This is a reprint of articles from the Special Issue published online in the open access journal Animals(ISSN 2076-2615) (available at: https://www.mdpi.com/journal/animals/special issues/
Poultry Nutrition).
For citation purposes, cite each article independently as indicated on the article page online and as indicated below:
LastName, A.A.; LastName, B.B.; LastName, C.C. Article Title. Journal NameYear,Volume Number, Page Range.
ISBN 978-3-03943-853-2 (Hbk) ISBN 978-3-03943-854-9 (PDF)
Cover image courtesy of Vincenzo Tufarelli.
c 2020 by the authors. Articles in this book are Open Access and distributed under the Creative Commons Attribution (CC BY) license, which allows users to download, copy and build upon published articles, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications.
The book as a whole is distributed by MDPI under the terms and conditions of the Creative Commons license CC BY-NC-ND.
Contents
About the Editor . . . xi Preface to ”Poultry Nutrition” . . . .xiii Nimra Azeem, Muhammad Nawaz, Aftab Ahmad Anjum, Shagufta Saeed, Saba Sana, Amina Mustafa and Muhammad Rizwan Yousuf
Activity and Anti-Aflatoxigenic Effect of Indigenously Characterized Probiotic Lactobacilli against Aspergillus flavus—A Common Poultry Feed Contaminant
Reprinted from:Animals2019,9, 166, doi:10.3390/ani9040166 . . . 1 Daniel Hernandez-Patlan, Bruno Sol´ıs-Cruz, Karine Patrin Pontin, Juan D. Latorre, Mikayla F. A. Baxter, Xochitl Hernandez-Velasco, Ruben Merino-Guzman, Abraham M´endez-Albores, Billy M. Hargis, Raquel Lopez-Arellano and Guillermo Tellez-Isaias Evaluation of the Dietary Supplementation of a Formulation Containing Ascorbic Acid and a Solid Dispersion of Curcumin with Boric Acid againstSalmonellaEnteritidis and Necrotic Enteritis in Broiler Chickens
Reprinted from:Animals2019,9, 184, doi:10.3390/ani9040184 . . . 11 Alaeldein M. Abudabos, Muttahar H. Ali, Mohammed A. Nassan and Ahmad A. Saleh
Ameliorative Effect ofBacillus subtilison Growth Performance and Intestinal Architecture in Broiler Infected with Salmonella
Reprinted from:Animals2019,9, 190, doi:10.3390/ani9040190 . . . 25 Mahmoud Mostafa Azzam, Shou-qun JIANG, Jia-li CHEN, Xia-jing LIN, Zhong-yong GOU, Qiu-li FAN, Yi-bing WANG, Long LI and Zong-yong JIANG
Effect of Soybean Isoflavones on Growth Performance, Immune Function, and Viral Protein 5 mRNA Expression in Broiler Chickens Challenged with Infectious Bursal Disease Virus
Reprinted from:Animals2019,9, 247, doi:10.3390/ani9050247 . . . 31 Youssef A. Attia, Nicola F. Addeo, Abd Al-Hamid E. Abd Al-Hamid and Fulvia Bovera
Effects of Phytase Supplementation to Diets with or without Zinc Addition on Growth Performance and Zinc Utilization of White Pekin Ducks
Reprinted from:Animals2019,9, 280, doi:10.3390/ani9050280 . . . 43 Moataz M. Fathi, Ibrahim Al-Homidan, Tarek A. Ebeid, Ahmed Galal and
Osama K. Abou-Emera
Assessment of Residual Feed Intake and Its Relevant Measurements in Two Varieties of Japanese Quails (Coturnixcoturnix japonica) under High Environmental Temperature
Reprinted from:Animals2019,9, 299, doi:10.3390/ani9060299 . . . 55 Ivana Prakatur, Maja Miskulin, Mirela Pavic, Ksenija Marjanovic, Valerija Blazicevic,
Ivan Miskulin and Matija Domacinovic
Intestinal Morphology in Broiler Chickens Supplemented with Propolis and Bee Pollen
Reprinted from:Animals2019,9, 301, doi:10.3390/ani9060301 . . . 65 Jason D. Keegan, Giorgio Fusconi, Mauro Morlacchini and Colm A. Moran
Whole-Life or Fattening Period Only Broiler Feeding Strategies Achieve Similar Levels of Omega-3 Fatty Acid Enrichment Using the DHA-Rich Protist,Aurantiochytrium limacinum
Reprinted from:Animals2019,9, 327, doi:10.3390/ani9060327 . . . 77
v
Haiam A. Mohammed, Ahmed Shaban Abdelaziz, Ghada I. Abd El-Rahman and Mohamed Tharwat Elabbasy
Effect of Dietary Modulation of Selenium Form and Level on Performance, Tissue Retention, Quality of Frozen Stored Meat and Gene Expression of Antioxidant Status in Ross
Broiler Chickens
Reprinted from:Animals2019,9, 342, doi:10.3390/ani9060342 . . . 91 Sivakumar Allur Subramaniyan, Da Rae Kang, Jin Ryong Park, Sharif Hasan Siddiqui, Palanisamy Ravichandiran, Dong Jin Yoo, Chong Sam Na and Kwan Seob Shim
Effect of In Ovo Injection of L-Arginine in Different Chicken Embryonic Development Stages on Post-Hatchability, Immune Response, and Myo-D and Myogenin Proteins
Reprinted from:Animals2019,9, 357, doi:10.3390/ani9060357 . . . .111 Seyed Mohammad Ghoreyshi, Besma Omri, Raja Chalghoumi, Mehrdad Bouyeh,
Alireza Seidavi, Mohammad Dadashbeiki, Massimo Lucarini, Alessandra Durazzo, Rene van den Hoven and Antonello Santini
Effects of Dietary Supplementation of L-Carnitine and Excess Lysine-Methionine on Growth Performance, Carcass Characteristics, and Immunity Markers of Broiler Chicken
Reprinted from:Animals2019,9, 362, doi:10.3390/ani9060362 . . . .129 Shad Mahfuz and Xiang Shu Piao
Application of Moringa (Moringa oleifera) as Natural Feed Supplement in Poultry Diets
Reprinted from:Animals2019,9, 431, doi:10.3390/ani9070431 . . . .147 K. F. M. Abouelezz, Y. Wang, W. Wang, X. Lin, L. Li, Z. Gou, Q. Fan and S. Jiang
Impacts of Graded Levels of Metabolizable Energy on Growth Performance and Carcass Characteristics of Slow-Growing Yellow-Feathered Male Chickens
Reprinted from:Animals2019,9, 461, doi:10.3390/ani9070461 . . . .167 Ahmed A. Saleh, Abeer A. Kirrella, Safaa E. Abdo, Mahmoud M. Mousa, Nemat A. Badwi, Tarek A. Ebeid, Ahmed L. Nada and Mahmoud A. Mohamed
Effects of Dietary Xylanase and Arabinofuranosidase Combination on the Growth
Performance, Lipid Peroxidation, Blood Constituents, and Immune Response of Broilers Fed Low-Energy Diets
Reprinted from:Animals2019,9, 467, doi:10.3390/ani9070467 . . . .181 Ayman E. Taha, Osama A. AbdAllah, Khalil M. Attia, Ragaa E. Abd El-Karim,
Mohamed E. Abd El-Hack, Mohamed A. El-Edel, Islam M. Saadeldin, Elsayed O. S. Hussein and Ayman A. Swelum
Does in Ovo Injection of Two Chicken Strains with Royal Jelly Impact Hatchability, Post-Hatch Growth Performance and Haematological and Immunological Parameters in Hatched Chicks?
Reprinted from:Animals2019,9, 486, doi:10.3390/ani9080486 . . . .193 Jun Li, Yefei Cheng, Yueping Chen, Hengman Qu, Yurui Zhao, Chao Wen and Yanmin Zhou Dietary Chitooligosaccharide Inclusion as an Alternative to Antibiotics Improves Intestinal Morphology, Barrier Function, Antioxidant Capacity, and Immunity of Broilers at Early Age Reprinted from:Animals2019,9, 493, doi:10.3390/ani9080493 . . . .207 Wenchao Liu, Yilin Yuan, Chenyu Sun, Balamuralikrishnan Balasubramanian, Zhihui Zhao and Lilong An
Effects of Dietary Betaine on Growth Performance, Digestive Function, Carcass Traits, and Meat Quality in Indigenous Yellow-Feathered Broilers under Long-Term Heat Stress
Reprinted from:Animals2019,9, 506, doi:10.3390/ani9080506 . . . .219
Enayatullah Hamdard, Zengpeng Lv, Jingle Jiang, Quanwei Wei, Zhicheng Shi, Rahmani Mohammad Malyar, Debing Yu and Fangxiong Shi
Responsiveness Expressions of Bitter Taste Receptors Against Denatonium Benzoate and Genistein in the Heart, Spleen, Lung, Kidney, and Bursa Fabricius of Chinese Fast Yellow Chicken
Reprinted from:Animals2019,9, 532, doi:10.3390/ani9080532 . . . .233 Mahmoud Alagawany, Shaaban S. Elnesr, Mayada R. Farag, Mohamed E. Abd El-Hack, Asmaa F. Khafaga, Ayman E. Taha, Ruchi Tiwari, Mohd. Iqbal Yatoo, Prakash Bhatt, Gopi Marappan and Kuldeep Dhama
Use of Licorice (Glycyrrhiza glabra) Herb as a Feed Additive in Poultry: Current Knowledge and Prospects
Reprinted from:Animals2019,9, 536, doi:10.3390/ani9080536 . . . .251 Mahmoud Alagawany, Shaaban S. Elnesr, Mayada R. Farag, Mohamed E. Abd El-Hack, Asmaa F. Khafaga, Ayman E. Taha, Ruchi Tiwari, Mohd. Iqbal Yatoo, Prakash Bhatt, Sandip Kumar Khurana and Kuldeep Dhama
Omega-3 and Omega-6 Fatty Acids in Poultry Nutrition: Effect on Production Performance and Health
Reprinted from:Animals2019,9, 573, doi:10.3390/ani9080573 . . . .263 Mahmoud Mostafa Azzam, Rashed Alhotan, Abdulaziz Al-Abdullatif, Saud Al-Mufarrej, Mohammed Mabkhot, Ibrahim Abdullah Alhidary and Chuntian Zheng
Threonine Requirements in Dietary Low Crude Protein for Laying Hens under High-Temperature Environmental Climate
Reprinted from:Animals2019,9, 586, doi:10.3390/ani9090586 . . . .283 Zaheer Ahmad, Ming Xie, Yongbao Wu and Shuisheng Hou
Effect of Supplemental Cyanocobalamin on the Growth Performance and Hematological Indicators of the White Pekin Ducks from Hatch to Day 21
Reprinted from:Animals2019,9, 633, doi:10.3390/ani9090633 . . . .295 Federica Mannelli, Sara Minieri, Giovanni Tosi, Giulia Secci, Matteo Daghio, Paola Massi, Laura Fiorentini, Ilaria Galigani, Silvano Lancini, Stefano Rapaccini, Mauro Antongiovanni, Simone Mancini and Arianna Buccioni
Effect of Chestnut Tannins and Short Chain Fatty Acids as Anti-Microbials and as Feeding Supplements in Broilers Rearing and Meat Quality
Reprinted from:Animals2019,9, 659, doi:10.3390/ani9090659 . . . .305 Mingming Shen, Zechen Xie, Minghui Jia, Anqi Li, Hongli Han, Tian Wang and Lili Zhang Effect of Bamboo Leaf Extract on Antioxidant Status and Cholesterol Metabolism in Broiler Chickens
Reprinted from:Animals2019,9, 699, doi:10.3390/ani9090699 . . . .321 Enayatullah Hamdard, Zhicheng Shi, Zengpeng Lv, Ahmadullah Zahir, Quanwei Wei, Mohammad Malyar Rahmani and Fangxiong Shi
Denatonium Benzoate-Induces Oxidative Stress in the Heart and Kidney of Chinese Fast Yellow Chickens by Regulating Apoptosis, Autophagy, Antioxidative Activities and Bitter Taste Receptor Gene Expressions
Reprinted from:Animals2019,9, 701, doi:10.3390/ani9090701 . . . .335
vii
The Effect of DietaryCamelina sativaOil or Cake in the Diets of Broiler Chickens on Growth Performance, Fatty Acid Profile, and Sensory Quality of Meat
Reprinted from:Animals2019,9, 734, doi:10.3390/ani9100734 . . . .365 Shuyun Ji, Xi Qi, Shuxue Ma, Xing Liu and Yuna Min
Effects of Dietary Threonine Levels on Intestinal Immunity and Antioxidant Capacity Based on Cecal Metabolites and Transcription Sequencing of Broiler
Reprinted from:Animals2019,9, 739, doi:10.3390/ani9100739 . . . .381 Cebisa Kumanda, Victor Mlambo and Caven Mguvane Mnisi
Valorization of Red Grape Pomace Waste Using Polyethylene Glycol and Fibrolytic Enzymes:
Physiological and Meat Quality Responses in Broilers
Reprinted from:Animals2019,9, 779, doi:10.3390/ani9100779 . . . .395 Alberto Vi ˜nado, Lorena Castillejos and Ana Cristina Barroeta
Soybean Lecithin High in Free Fatty Acids for Broiler Chicken Diets: Impact on Performance, Fatty Acid Digestibility and Saturation Degree of Adipose Tissue
Reprinted from:Animals2019,9, 802, doi:10.3390/ani9100802 . . . .407 Minyu Zhou, Yuheng Tao, Chenhuan Lai, Caoxing Huang, Yanmin Zhou and Qiang Yong Effects of Mannanoligosaccharide Supplementation on the Growth Performance, Immunity, and Oxidative Status of Partridge Shank Chickens
Reprinted from:Animals2019,9, 817, doi:10.3390/ani9100817 . . . .421 Sajid Khan Tahir, Muhammad Shahbaz Yousaf, Sohrab Ahmad, Muhammad Khurram Shahzad, Ather Farooq Khan, Mohsin Raza, Khalid Abdul Majeed, Abia Khalid, Hafsa Zaneb, Imtiaz Rabbani and Habib Rehman
Effects of Chromium-Loaded Chitosan Nanoparticles on the Intestinal Electrophysiological Indices and Glucose Transporters in Broilers
Reprinted from:Animals2019,9, 819, doi:10.3390/ani9100819 . . . .433 Tengfei He, Shenfei Long, Shad Mahfuz, Di Wu, Xi Wang, Xiaoman Wei and Xiangshu Piao Effects of Probiotics as Antibiotics Substitutes on Growth Performance, Serum Biochemical Parameters, Intestinal Morphology, and Barrier Function of Broilers
Reprinted from:Animals2019,9, 985, doi:10.3390/ani9110985 . . . .445 Shad Mahfuz and Xiangshu Piao
Use of Medicinal Mushrooms in Layer Ration
Reprinted from:Animals2019,9, 1014, doi:10.3390/ani9121014 . . . .455 Damini Kothari, Woo-Do Lee, Kai-Min Niu and Soo-Ki Kim
The GenusAlliumas Poultry Feed Additive: A Review
Reprinted from:Animals2019,9, 1032, doi:10.3390/ani9121032 . . . .469 Abd Ur Rehman, Muhammad Arif, Muhammad M. Husnain, Mahmoud Alagawany, Mohamed E. Abd El-Hack, Ayman E. Taha, Shaaban S. Elnesr, Mervat A. Abdel-Latif, Sarah I. Othman and Ahmed A. Allam
Growth Performance of Broilers as Influenced by Different Levels and Sources of Methionine Plus Cysteine
Reprinted from:Animals2019,9, 1056, doi:10.3390/ani9121056 . . . .491
Majid Shakeri, Jeremy James Cottrell, Stuart Wilkinson, Weicheng Zhao, Hieu Huu Le, Rachel McQuade, John Barton Furness and Frank Rowland Dunshea
Dietary Betaine Improves Intestinal Barrier Function and Ameliorates the Impact of Heat Stress in Multiple Vital Organs as Measured by Evans Blue Dye in Broiler Chickens
Reprinted from:Animals2020,10, 38, doi:10.3390/ani10010038 . . . .503 Silje Granstad, Anja B. Kristoffersen, Sylvie L. Benestad, Siri K. Sjurseth, Bruce David,
Line Sørensen, Arnulf Fjermedal, Dag H. Edvardsen, Gorm Sanson, Atle Løvland and Magne Kaldhusdal
Effect of Feed Additives as Alternatives to In-feed Antimicrobials on Production Performance and Intestinal Clostridium perfringens Counts in Broiler Chickens
Reprinted from:Animals2020,10, 240, doi:10.3390/ani10020240 . . . .517 Mahmoud M. Abo Ghanima, Mohamed F. Elsadek, Ayman E. Taha,
Mohamed E. Abd El-Hack, Mahmoud Alagawany, Badreldin M. Ahmed, Mona M. Elshafie and Karim El-Sabrout
Effect of Housing System and Rosemary and Cinnamon Essential Oils on Layers Performance, Egg Quality, Haematological Traits, Blood Chemistry, Immunity, and Antioxidant
Reprinted from:Animals2020,10, 245, doi:10.3390/ani10020245 . . . .537
Xingyong Chen, Kaiqin He, Congcong Wei, Wanli Yang and Zhaoyu Geng
Green Tea Powder Decreased Egg Weight Through Increased Liver Lipoprotein Lipase and Decreased Plasma Total Cholesterol in an Indigenous Chicken Breed
Reprinted from:Animals2020,10, 370, doi:10.3390/ani10030370 . . . .553 Diaa E. Abou-Kassem, Mohamed E. Abd El-Hack, Ayman E. Taha, Jamaan S. Ajarem,
Saleh N. Maodaa and Ahmed A. Allam
Detoxification Impacts of Ascorbic Acid and Clay on Laying Japanese Quail Fed Diets Polluted by Various Levels of Cadmium
Reprinted from:Animals2020,10, 372, doi:10.3390/ani10030372 . . . .563
Youssef Attia, Mahmoud El-kelawy, Mohammed Al-Harthi and Ali El-Shafey
Impact of Multienzymes Dose Supplemented Continuously or Intermittently in Drinking Water on Growth Performance, Nutrient Digestibility, and Blood Constituents of Broiler Chickens Reprinted from:Animals2020,10, 375, doi:10.3390/ani10030375 . . . .581 Ahmed A. Saleh, Khairy A. Amber, Mahmoud M. Mousa, Ahmed L. Nada, Wael Awad, Mahmoud A.O. Dawood, Abd El-Moneim E. Abd El-Moneim, Tarek A. Ebeid and
Mohamed M. Abdel-Daim
A Mixture of Exogenous Emulsifiers Increased the Acceptance of Broilers to Low Energy Diets:
Growth Performance, Blood Chemistry, and Fatty Acids Traits
Reprinted from:Animals2020,10, 437, doi:10.3390/ani10030437 . . . .595 Long Li, K.F.M. Abouelezz, Zhonggang Cheng, A.E.G. Gad-Elkareem, Qiuli Fan,
Fayuan Ding, Jun Gao, Shouqun Jiang and Zongyong Jiang
Modelling Methionine Requirements of Fast- and Slow-Growing Chinese Yellow-Feathered Chickens during the Starter Phase
Reprinted from:Animals2020,10, 443, doi:10.3390/ani10030443 . . . .605
ix
Suliman, Mohamed E. Abd El-Hack, Ayman A. Swelum and Abdullah N. Alowaimer Ameliorative Effects of Antibiotic-, Probiotic- and Phytobiotic-Supplemented Diets on the Performance, Intestinal Health, Carcass Traits, and Meat Quality of Clostridium perfringens- Infected Broilers
Reprinted from:Animals2020,10, 669, doi:10.3390/ani10040669 . . . .623 Ahmed A. Saleh, Bilal Ahamad Paray and Mahmoud A.O. Dawood
Olive Cake Meal andBacillus licheniformisImpacted the Growth Performance, Muscle Fatty Acid Content, and Health Status of Broiler Chickens
Reprinted from:Animals2020,10, 695, doi:10.3390/ani10040695 . . . .637 Fayiz M. Reda, Mohamed T. El-Saadony, Shaaban S. Elnesr, Mahmoud Alagawany and Vincenzo Tufarelli
Effect of Dietary Supplementation of Biological Curcumin Nanoparticles on Growth and Carcass Traits, Antioxidant Status, Immunity and Caecal Microbiota of Japanese Quails
Reprinted from:Animals2020,10, 754, doi:10.3390/ani10050754 . . . .653 Vera Perricone, Marcello Comi, Carlotta Giromini, Raffaella Rebucci, Alessandro Agazzi, Giovanni Savoini and Valentino Bontempo
Green Tea and Pomegranate Extract Administered During Critical Moments of the Production Cycle Improves Blood Antiradical Activity and Alters Cecal Microbial Ecology of Broiler Chickens
Reprinted from:Animals2020,10, 785, doi:10.3390/ani10050785 . . . .667 Bozena Hosnedlova, Katerina Vernerova, Rene Kizek, Riccardo Bozzi, Jaromir Kadlec, Vladislav Curn, Frantisek Kouba, Carlos Fernandez, Vlastislav Machander and Hana Horna Associations Between IGF1, IGFBP2 and TGFß3 Genes Polymorphisms and Growth
Performance of Broiler Chicken Lines
Reprinted from:Animals2020,10, 800, doi:10.3390/ani10050800 . . . .681 Roua Gabriela Popescu, Sorina Nicoleta Voicu, Gratiela Gradisteanu Pircalabioru,
Alina Ciceu, Sami Gharbia, Anca Hermenean, Sergiu Emil Georgescu, Tatiana Dumitra Panaite and Anca Dinischiotu
Effects of Dietary Inclusion of Bilberry and Walnut Leaves Powder on the Digestive Performances and Health of Tetra SL Laying Hens
Reprinted from:Animals2020,10, 823, doi:10.3390/ani10050823 . . . .705 Muhammad Abdul Basit, Arifah Abdul Kadir, Teck Chwen Loh, Saleha Abdul Aziz,
Annas Salleh, Ubedullah Kaka and Sherifat Banke Idris
Effects of Inclusion of Different Doses of Persicaria odorata Leaf Meal (POLM) in Broiler Chicken Feed on Biochemical and Haematological Blood Indicators and Liver Histomorphological Changes
Reprinted from:Animals2020,10, 1209, doi:10.3390/ani10071209 . . . .721
About the Editor
Vincenzo Tufarelli is an Associate Professor in Animal Nutrition at the Department of Emergency and Organ Transplants (DETO), Section of Veterinary Science and Animal Production of the University of Bari Aldo Moro, Italy. He has considerable experience in animal and poultry science, with a particular interest in nutrition and feed technology. He is involved in many research collaborations, even with international institutions, in the field of animal science and feed quality.
He serves as an editorial board member and peer reviewer for many indexed journals and he is the author of more than 180 scientific papers published in international journals and proceedings of national and international conferences.
xi
Preface to ”Poultry Nutrition”
Nutrition is defined in a range of ways, but is frequently inadequately understood. It is a simple concept, yet encompasses much complexity. In recent years, advances in poultry production, introduction of new and alternative products, and the development of new dietary management approaches have made it possible to increase poultry performance. However, to realize this, there must be further focus on diet quality. Producing suitable poultry products requires knowing the factors affecting quality, then exercising management accordingly. This book presents cross-discipline studies covering many aspects, ranging from poultry production and nutrition to alternative feeding systems, with the aim of disseminating information suitable for improving poultry health and products quality. Moreover, the purpose of this book is also to provide information about feed quality and alternative ingredients testing that can be used to improve poultry performance and producers’ returns.
Vincenzo Tufarelli Editor
animals
Article
Activity and Anti-Aflatoxigenic Effect of Indigenously Characterized Probiotic Lactobacilli against
Aspergillus flavus—A Common Poultry Feed Contaminant
Nimra Azeem1, Muhammad Nawaz1,*, Aftab Ahmad Anjum1, Shagufta Saeed2, Saba Sana1, Amina Mustafa1and Muhammad Rizwan Yousuf3
1 Department of Microbiology, University of Veterinary and Animal Sciences, Lahore 54000, Punjab, Pakistan;
[email protected] (N.A.); [email protected] (A.A.A.); [email protected] (S.S.);
[email protected] (A.M.)
2 Institute of Biochemistry and Biotechnology, University of Veterinary and Animal Sciences, Lahore 54000, Punjab, Pakistan; [email protected]
3 Department of Theriogenology, University of Veterinary and Animal Sciences, Lahore 54000, Punjab, Pakistan; [email protected]
* Correspondence: [email protected]; Tel.:+92-03337773240; Fax:+92-9211449-178 Received: 14 March 2019; Accepted: 11 April 2019; Published: 15 April 2019
Simple Summary:Mycotoxicosis in poultry has been seriously damaging the poultry production in Pakistan, resulting in economic losses to the country. The present study may act as a preliminary step for exploring the effect of indigenously characterized potential probiotic lactobacilli on aflatoxin production byAspergillus flavus. The present study explored anti-fungalLactobacillusstrains. Further investigations revealed their in vitro aflatoxin binding and anti-aflatoxigenic capabilities. These findings demonstratedL. gallinarumPL 149 to be an effective binder of aflatoxin B1 which may be used as a biocontrol agent againstA. flavusand aflatoxin B1 production. It may be further employed for aflatoxin binding in poultry gut after in vivo evaluations.
Abstract: Aflatoxin contamination in human food and animal feed is a threat to public safety.
Aflatoxin B1 (AFB1) can be especially damaging to poultry production and consequently economic development of Pakistan. The present study assessed the in vitro binding of AFB1 by indigenously characterized probiotic lactobacilli. Six isolates (Lactobacillus gallinarumPDP 10,Lactobacillus reuetri FYP 38,Lactobacillus fermentumPDP 24,Lactobacillus gallinarumPL 53,Lactobacillus paracaseiPL 120, andLactobacillus gallinarumPL 149) were tested for activity against toxigenicAspergillus flavusW-7.1 (AFB1 producer) by well diffusion assay. Only three isolates (PL 53, PL 120, and PL 149) had activity againstA. flavusW-7.1. The ameliorative effect of these probiotic isolates on AFB1 production was determined by co-culturing fungus with lactobacilli for 12 days, followed by aflatoxin quantification by high-performance liquid chromatography. In vitro AFB1 binding capacities of lactobacilli were determined by their incubation with a standard amount of AFB1 in phosphate buffer saline at 37◦C for 2 h. AFB1 binding capacities of isolates ranged from 28–65%. Four isolates (PDP 10, PDP 24, PL 120, and PL 149) also ceased aflatoxin production completely, whereas PL 53 showed 55% reduction in AFB1 production as compared to control. The present study demonstratedLactobacillus gallinarum PL 149 to be an effective candidate AFB1 binding agent againstAspergillus flavus. These findings further support the binding ability of lactic acid bacteria for dietary contaminants.
Keywords:Aflatoxin B1;Lactobacillus; anti-fungal;Aspergillus flavus; in vitro; poultry
Animals2019,9, 166; doi:10.3390/ani9040166 1 www.mdpi.com/journal/animals
1. Introduction
Poultry is one of the major sectors playing a role in the enhanced economic activity of Pakistan but still it faces a lot of problems, including mycotoxicosis. Mycotoxins are toxic secondary metabolites of fungal origin, which can cause various diseases and death in animals and humans. Ergot alkaloids, fumonisins, patulin, aflatoxin, citrinin, trichothecenes, ochratoxin A, and zearalenone are all examples of some different mycotoxins. Aflatoxins, produced byAspergillus parasiticus,Aspergillus flavus, and Aspergillus nomius, are of great importance because of their biological and biochemical effects on living systems [1]. Aflatoxin-producing molds are globally and can flourish on a variety of food and feed commodities during production, processing, storage, and transportation procedures [1–3]. These molds can infect crops, especially in hot and humid conditions, resulting in economic loss and adverse effects on consumers’ health.
Aflatoxin is a potent carcinogen, mutagen, contains hepatotoxic and immunosuppressant effects and inhibit several metabolic systems resulting in liver and kidney damage [1,4]. Aflatoxin and citrinin cause increased fragility of the vascular system and produce hemorrhages in body tissues. Among aflatoxins, aflatoxin B1 is the most potent, and it is categorized among class 1 human carcinogens.
Different factors including pH, temperature, water activity, available nutrients, and competitive inhibition by other microorganisms can affect aflatoxin production in feed [3]. Appropriate harvesting and storage conditions of crops and feed play important roles in aflatoxin reduction.
Various methods have been employed for the removal or inactivation of aflatoxins, including physical, biological, and chemical methods. Chemical treatments may include roasting, ammoniation, and other solvent extraction techniques. Many aflatoxin binders, like activated carbon and various mineral clays, are commercially available and act as sequestering agents and tightly bind aflatoxin; the resulting binding complex is then excreted from the animal’s body [5]. These toxin binders can restore the nutritional value of the feed, but these chemical methods are unsafe, unhealthy, and expensive [6].
Toxin removal by microorganisms is a promising and economical method for decontaminating raw materials and food [7]. Numerous investigations have reported the inhibitory effects of microbes including actinomycetes, yeast, mold, and bacteria on mold growth and aflatoxin production [3]. Thus, beneficial microorganisms may serve as an alternative therapy for mycotoxicosis.
Anti-mutagenic lactic acid bacteria can remove mutagens from food by physical means [8]. Toxin binding by bacteria occurs through cell wall components, namely polysaccharides or polypeptides.
Many researchers have studied this binding mechanism, but the exact mechanism of binding is still unknown [9].
Researchers are paying more attention towards preventing the absorption of aflatoxins in the gastrointestinal tracts of users by the aid of probiotic bacterial supplements in food and feed [10].
According to the World Health Organization (WHO), probiotics are defined as live microorganisms which when administered in adequate amounts exert healthy effects to host [11]. Lactobacillus, Bifidobacterium,Enterococcus,Saccharomyces, andBacillusmay serve as probiotics.
Lactobacilli can efficiently remove aflatoxins from contaminated broth. The toxin removal mechanism involves sequestration by binding the toxin to the cell wall instead of metabolic degradation [12]. The present study may act as a preliminary step for studying the effect of indigenously characterized potential probiotic lactobacilli on aflatoxin production byAspergillus flavus, so that lactobacilli can be used as biocontrol agents. The present study also assessed the in vitro AFB1 binding capacity ofLactobacillusspp., so that these probiotic strains can be employed as toxin binders in place of chemicals in animal feed and thereby the harmful effects of chemical toxin binders can be avoided.
Animals2019,9, 166
2. Materials and Methods 2.1. Identification of Isolates
Previously characterized probiotic lactobacilli (n=6) of poultry and fermented food origin [13]
and toxigenicAspergillus flavusW-7.1 were procured from the Department of Microbiology, University of Veterinary and Animal Sciences, Lahore, as listed in Table1. Lactobacilli were revived using De Man, Rogosa, and Sharpe (MRS) agar and identified as describe previously [14]. Fungal strain was cultured on Sabouraud Dextrose Agar (SDA) medium incubated at 37◦C for 5–6 days. Culture and microscopic characters were observed for identification as described previously [15].
Table 1.Antifungal activity of cell free supernatants of lactobacilli.
Isolates GenBank Accession # Zones of Inhibition (mm)
pH 4 pH 7
L. gallinarumPDP 10 MF980924 NZ NZ
L. reuteriPDP 24 MF980925 NZ NZ
L. fermentumFYP 38 MF980923 NZ NZ
L. gallinarumPL 53 MK182967 13 12
L. paracaseiPL 120 MK182968 16 14
L. gallinarumPL 149 MK182969 17 15
NZ: No zone of inhibition.
2.2. Antifungal Activity of Lactobacilli
Antifungal activity of lactobacilli (n=6) was determined by well diffusion assay as described elsewhere [16]. Briefly, SDA medium seeded with fungal spores (107spores/mL) was poured into sterile Petri dishes and allowed to solidify. Wells were punctured in the medium which were then sealed with sterile molten agar. Cell free supernatant (100μL) of each lactobacilli strain was added into the respective wells. After 3–4 days incubation at 28◦C aerobically, the diameter of zones of inhibition (mm) was measured.
2.3. Effect of Lactobacilli on Aflatoxin Production
The effect of lactobacilli on aflatoxin production byAspergillus flavuswas observed by inoculating 1 mL bacterial suspension (1 McFarland) in yeast extract sucrose broth (YESB) supplemented with a standard amount of fungal spores (107spores/mL), followed by incubation at 28◦C and 100 rpm for 10 days. YESB media supplemented with known fungal spores and plain YESB media without any inoculation were also incubated as positive and negative controls, respectively. After incubation, medium containing lactobacilli and fungus was filtered through Whatman filter paper no 1 and aflatoxin B1 quantity in filtrate was measured by high-performance liquid chromatography (HPLC) and compared with controls [6]. Aflatoxin B1 was detected by HPLC and quantified using the following formulae:
Quantity of Aflatoxins ng
mL
= peak area of sample
peak area of standard×100 (1)
% age reduction=1−(Peak area of AFB1 in treatment)
(Peak area of AFB1 in control) (2) 2.4. Aflatoxin B1 Extraction
For toxin extraction, a previously established protocol was used with modifications [17]. Briefly, broth culture ofAspergillus flavuswas autoclaved at 121◦C and 15 psi and then homogenized using homogenizer. Twenty-five grams of homogenate was treated with chloroform (90 mL), methanol (10 mL), NaCl (5 g), and distilled water (10 mL) and incubated at 37◦C with continuous shaking
3
(150–160 rpm) for 30 min. Filtration was carried out using Whatman filter paper #4 and filtrate was concentrated in a water bath at 50◦C. Concentrate was ground to fine powder and reconstituted in 3 mL chloroform volume and stored at 4◦C.
2.5. Toxin Binding Assay
Standard aflatoxin B1 solution was prepared by the method described elsewhere [18]. Prepared standard aflatoxin solution was then added to sterile phosphate buffer saline (PBS) containing lactobacilli culture (1 McFarland). After 2 h of incubation, cells with bound toxin were separated by centrifugation at 10,000 rpm for 5 min and unbound aflatoxin in supernatant was quantified by HPLC.
2.6. High Performance Liquid Chromatography (HPLC)
Aflatoxins were quantified by Agilent HPLC system, 1100 series (Agilent, Santa Clara, CA, USA) as described previously [19]. A mixture of acetonitrile, water, and methanol was used as mobile phase at a flow rate of 1 mL per minute. Mobile phase was firstly purified using a filtration assembly and then sonicated for 10 min at 20◦C in order to avoid gas bubbles. Next, 20μL samples were injected using a micro-syringe. After 15 min, ultra violet (UV) absorbance was recorded at 254 nm. Sample peaks were analyzed and compared with standard UV absorption data of secondary metabolites at various retention times. Limit of detection (LOD) and limit of quantification (LOQ) of standard aflatoxin were 0.01 ng/mL–100μg/mL and 0.1 ng/mL–100μg/mL, respectively.
2.7. Statistical Analysis
Mitigation of aflatoxin production and toxin binding capacity of lactobacilli was compared by one-way ANOVA (analysis of variance) followed by Turkey’s multiple comparison test using Graph pad prism 5.0 software (GraphPad Software, San Diego, CA, USA).
3. Results
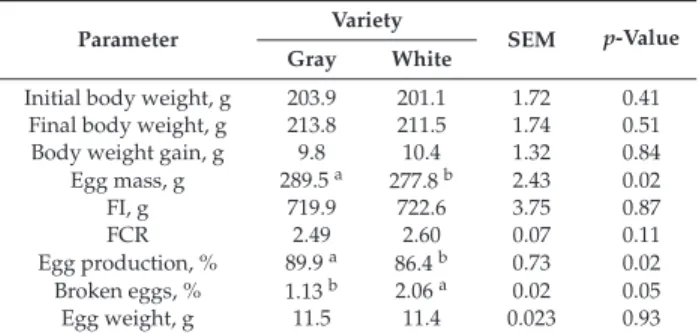
A total of six potential probiotic lactobacilli, includingLactobacillus gallinarumPDP 10,Lactobacillus reuteriPDP 24,Lactobacillus fermentumFYP 38,Lactobacillus gallinarumPL 53,Lactobacillus paracasei PL 120, andLactobacillus gallinarumPL 149, were procured from the Department of Microbiology, University of Veterinary and Animal Sciences, Lahore, Pakistan. All isolates were Gram-positive rods and catalase negative.
Only three isolates (PL 53, PL 120, and PL 149) had antifungal activity observed by well diffusion assay, as illustrated in Table1and Figure1.
Figure 1.Activity of cell free supernatant ofLactobacillus gallinarumPL 149 againstAspergillus flavus.
Animals2019,9, 166
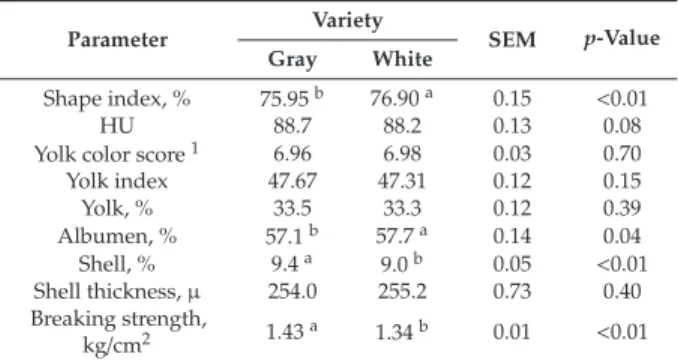
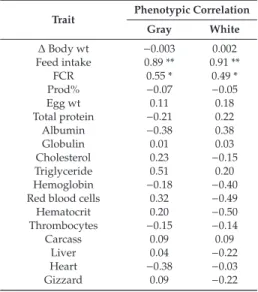
Four isolates (PDP 10, PDP 24, PL 120, and PL 149) showed 100% removal of AFB1, PL 53 caused 55.2% reduction, while FYP 38 showed an enhancing effect on aflatoxin B1 production, as described in Table2. All isolates showed a varied degree of toxin binding capacities, as described in Table3and Figure2. PL 149 was the most effective binder of aflatoxin B1, with 65% capacity.
Table 2.Effect of lactobacilli on aflatoxin B1 production.
Isolates Peak Areas Quantity of AFB1 (ng/mL) % Age Reduction
Standard 120.205 100 -
Control 0.58439 0.4 -
L. gallinarumPDP 10 ND ND 100%
L. fermentumFYP 38 0.815847 0.6 −39.6%
L. reuteriPDP 24 ND ND 100%
L. gallinarumPL 53 0.26124 0.2 55.2%
L. paracaseiPL 120 ND ND 100%
L. gallinarumPL 149 ND ND 100%
AFB1: Aflatoxin B1; ND: Not detected.
Table 3.Aflatoxin B1 binding capacity of probiotic lactobacilli.
Isolates Peak Areas Quantity of AFB1 Bound (ng/mL) % Age Reduction (Binding Capacity)
Standard 108.246 100 -
Control 927.763 857 -
L. gallinarumPDP 10 451.63 417.2 51.3%
L. fermentumFYP 38 407.553 376.5 56%
L. reuteriPDP 24 909.624 840 2%
L. gallinarumPL 53 546.523 504.8 42%
L. paracaseiPL 120 676.472 624.9 28%
L. gallinarumPL 149 326.775 301.8 65%
AFB1: Aflatoxin B1.
Figure 2.High-performance liquid chromatography chromatograms of aflatoxin B1 present in control and suspension after treatment with lactobacilli: (a) Control; (b) PDP 10; (c) FYP 38; (d) PL 149.
5
4. Discussion
Aflatoxins represent a group of fungal secondary metabolites that are of great health and economic importance. In developing countries, greater than five billion people are at risk of chronic exposure to aflatoxins, which are capable of causing liver cancer [4]. Consequently, there is an increasing demand for novel preventive and controlling strategies for aflatoxin contaminations in food and feed. Recent studies have revealed the aflatoxin binding ability of lactobacilli. Many bacteria have been reported as aflatoxin binders, includingFlavobacterium aurantiacum,L. plantarum,L. pentosus, andL. beveris[20–22].
Likewise,Lactobacillus casei psuedoplantarum371, obtained from silage inoculum, inhibited aflatoxin B1 and G1 synthesis byAspergillus flavussubsp. parasiticusNRRL 2999 in liquid medium [23]. In a previous study, a mixture of lactobacilli was found to reduce mold growth, germination of spores, and production of aflatoxins byAspergillus flavussubsp.parasiticus[24]. A large number of such studies have been reported worldwide, but few related studies have been reported in Pakistan.
The present study can act as a preliminary step in a multistep study to investigate the anti-fungal, anti-aflatoxigenic, and in vitro AFB1 binding capacities of previously characterized indigenous phytase-solubilizing probiotic lactobacilli spp. of poultry and fermented food origin [13] against toxigenicAspergillus flavus. This study identified three probiotic lactobacilli isolates (Lactobacillus gallinarumPL 53, Lactobacillus paracaseiPL 120,andLactobacillus gallinarumPL 149) as antifungal agents.
Such inhibitory effects may be a result of lactic acid production or physical interaction of lactobacilli with mold. Similar inhibitory effects ofL. acidophilusATCC 4495 andL. breviswere also demonstrated previously againstAspergillus flavusandAspergillus parasiticus, respectively [25,26].
Four isolates (PDP 10, PDP 24, PL 120, PL 149) in the present study ceased aflatoxin production completely, whereas PL 53 showed 55% reduction. On the other hand, FYP 38 showed an enhancing effect on aflatoxin B1 production. These variable results may depict different bacterial cell wall structures.
Many other investigators have reported similar results, in which various lactic acid-producing bacteria, includingLactobacillus, were capable of inhibiting aflatoxin production, whereas some lactic acid bacteria, likeLactococcus lactis, stimulated aflatoxin biosynthesis [27]. Cell wall polysaccharides and peptidoglycans have been considered bacterial tools for mycotoxin binding [28]. Extracellular metabolites ofLactobacillus caseiKC 324 has been reported to mitigate mold growth and aflatoxin production ofAspergillus flavusATCC 15517 [29]. Commercial silage was once reported to contain inhibitory lactobacilli against aflatoxin B1 and G1 production [30].L. plantarumATCC 4008, L. plantarum 12006,Lactobacillus plantarum299V,L. paracaseisubsp.paracaseiLMG 13552, andL. rhamnosusVT1 reduced aflatoxin production by 85–92% to 96.3–98.3% [31], whereas in the present study a 100% reduction in AFB1 production byL. gallinarumPDP 10 and PL 149,L. reuteriPDP 24, andL. paracaseiPL 120 was observed. It may also be a result of very low aflatoxin production in control conditions as well. Yeast can also act as an effective biocontrol agent against aflatoxins.S. boulardiiandS. cerevisiaeindividually reduced aflatoxin production by 72.8% and 65.8%, respectively, while their combinations reduced aflatoxin production from 71.1% to 96.1%. Supplementation of peanut grains with combinations of S. boulardiiplusL. delbrueckii,S. boulardiiandS. cerevisiae,L. delbrueckiiandS. cerevisiaeshowed reduction by 96.1%, 66.7%, and 71.1%, respectively [32].Lactobacillus fermentumPTCC 1744 andBifidobacterium bifidumPTCC 1644 were also reported to reduce aflatoxin production by more than 99% in comparison with controls [6], although this report is contradictory to the present research which revealed the enhancing effect ofLactobacillus fermentumon AFB1 production byA. flavus.
In the present study,Lactobacillus gallinarumPDP 10,Lactobacillus fermentumFYP 38, Lactobacillus reuteri PDP 24,Lactobacillus gallinarumPL 53,Lactobacillus paracaseiPL 120, andLactobacillus gallinarum PL 149 showed aflatoxin binding capacities of 51.3%, 56%, 2%, 42%, 28%, and 65%, respectively. These results were quite similar with that of Fazeli et al. [33]. In a previous study, the aflatoxin B1 binding capacities ofLactobacillusandBifidobacteriumstrains were assessed, which were found to range from 5.8% to 31.3% [12]. On the other hand, the present study reported up to 65% AFB1 binding abilities of probiotic lactobacilli. A previous study reported thatLactobacillus caseihad a 20% AFB1 binding capacity [34], which is less than that ofL. paracaseiPL 120 (28%), whereasLactobacillus delbrueckii
Animals2019,9, 166
subsp.lactiswas reported to have the maximum antifungal (67.43% reduction) and anti-aflatoxigenic (94.33% reduction) activity againstA. flavus[35]. Another previous study reported 43.9–64.2% aflatoxin degrading ability of lactobacilli strains [36]. Past investigations revealed similar responses of non-viable and viable cells ofEnterococcus faeciumstrains, whose binding abilities were insignificant statistically.
Hence, it was hypothesized that AFB1 detoxification ofEnterococcus faeciumis a result of aflatoxin binding to bacterial cell wall; a similar mechanism has been also described by other relevant studies [37].
An in vivo experiment revealed the neutralizing capability ofLactobacillus casei Shirotaon AFB1 toxicity on the intestine and body weight of host via binding processes [38]. Thus, lactic acid bacteria have been declared as good candidates to prevent aflatoxicosis in farm animals and poultry [9].
5. Conclusions
The present study reported the anti-fungal, anti-aflatoxigenic, and AFB1 binding capacity of six indigenously characterized probiotic strains. It was concluded thatL. gallinarumPL 149 may inhibit the AFB1 production byA. flavusand also bind AFB1.L. gallinarumPL 149 may be employed for aflatoxin binding in poultry gut after in vivo evaluations.
Author Contributions:Conceptualization: M.N. and A.A.A.; methodology: M.N., A.A.A., N.A., and S.S. (Shagufta Saeed); software: M.N.; validation: M.N., A.A.A., M.R.Y., S.S. (Shagufta Saeed), and S.S. (Saba Sana); formal analysis: M.N., N.A., M.R.Y., A.M., and S.S. (Saba Sana); investigation: N.A., A.M., and S.S. (Shagufta Saeed);
resources: M.N. and A.A.A.; data curation: N.A. and A.M.; writing—original draft preparation: A.M. and N.A.; writing—review and editing: A.M., N.A., S.S. (Saba Sana), M.R.Y., and M.N.; visualization: M.N., A.A.A., S.S. (Shagufta Saeed), and N.A.; supervision: M.N., A.A.A., S.S. (Shagufta Saeed), and S.S. (Saba Sana); project administration: M.N. and A.A.A.; funding acquisition: M.N. and A.A.A.
Funding: This project was partially supported through Higher Education Commission (HEC) project No.
4333/NRPU/R & D/HEC/14/278 and NRPU Project # 4148.
Conflicts of Interest:The authors declare no conflict of interest. The funding agency had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
1. Naseem, M.N.; Saleemi, M.K.; Abbas, R.Z.; Khan, A.; Khatoon, A.; Gul, S.T.; Imran, M.; Sindhu, Z.U.D.;
Sultan, A. Hematological and serum biochemical effects of aflatoxin B1 intoxication in broilers experimentally infected with fowl adenovirus-4 (FAdV-4).Pak. Vet. J.2018,38, 209–213. [CrossRef]
2. Elsanhoty, R.M.; Salam, S.A.; Ramadan, M.F.; Badr, F.H. Detoxification of aflatoxin M1 in yoghurt using probiotics and lactic acid bacteria.Food Control2014,43, 129–134. [CrossRef]
3. Ellis, W.O.; Smith, J.P.; Simpson, B.K.; Oldham, J.H.; Scott, P.M. Aflatoxins in food: Occurrence, biosynthesis, effects on organisms, detection, and methods of control. Crit. Rev. Food. Sci. Nutr. 1991,30, 403–439.
[CrossRef] [PubMed]
4. Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions.
Am. J. Clin. Nutr.2004,80, 1106–1122. [CrossRef] [PubMed]
5. Diaz, D.E.; Hagler, W.M.; Hopkins, B.A.; Whitlow, L.W. Aflatoxin binders I: In vitro binding assay for aflatoxin B1 by several potential sequestering agents.Mycopathologia2003,156, 223–226. [CrossRef]
6. Ghazvini, R.D.; Kouhsari, E.; Zibafar, E.; Hashemi, S.J.; Amini, A.; Niknejad, F. Antifungal activity and aflatoxin degradation of bifidobacterium bifidum and lactobacillus fermentum against toxigenic aspergillus parasiticus.Open. Microbiol. J.2016,10, 197. [CrossRef] [PubMed]
7. Zoghi, A.; Khosravi-Darani, K.; Sohrabvandi, S. Surface binding of toxins and heavy metals by probiotics.
Mini. Rev. Med. Chem.2014,14, 84–98. [CrossRef] [PubMed]
8. Corthier, G. The health benefits of probiotics.Danone Nutr.2004,29, 1–18.
9. Pizzolitto, R.P.; Bueno, D.J.; Armando, M.R.; Cavaglieri, L.; Dalcero, A.M.; Salvano, M.A. Binding of aflatoxin B1 to lactic acid bacteria andSaccharomyces cerevisiaein vitro: A useful model to determine the most efficient microorganism. InAflatoxins-Biochemistry and Molecular Biology; IntechOpen: London, UK, 2011.
7
10. Manubolu, M.; Goodla, L.; Pathakoti, K.; Malmlöf, K. Enzymes as direct decontaminating agents—mycotoxins.
InEnzymes in Human and Animal Nutrition; Academic Press: Cambridge, MA, USA, 2018; pp. 313–330.
11. Morelli, L.; Capurso, L. FAO/WHO guidelines on probiotics: 10 years later.J. Clin. Gastroenterol.2012,46, S1–S2. [CrossRef]
12. Peltonen, K.D.; El-Nezami, H.S.; Salminen, S.J.; Ahokas, J.T. Binding of aflatoxin B1 by probiotic bacteria.
J. Sci. Food Agric.2000,80, 1942–1945. [CrossRef]
13. Arif, A.; Nawaz, M.; Rabbani, M.; Iqbal, S.; Mustafa, A.; Yousuf, M.R.; Muhammad, K. Screening, characterization and physicochemical optimization of phosphorus solubilization activity of potential probioticLactobacillusspp.Pak. Vet. J.2018,38, 316–320. [CrossRef]
14. Saleem, N.; Nawaz, M.; Ghafoor, A.; Javeed, A.; Mustafa, A.; Yousuf, M.R.; Khan, I. Phenotypic and molecular analysis of antibiotic resistance inLactobacilliof poultry origin from Lahore, Pakistan.Pak. Vet. J.2018,38, 341–346. [CrossRef]
15. AL-Ruwaili, M.; Alkhalaileh, N.I.; Herzallah, S.M.; Rawashdeh, A.; Fataftah, A.; Holley, R. Reduction of aflatoxin B1 residues in meat and organs of broiler chickens by lactic acid bacteria.Pak. Vet. J.2018,38, 325–328. [CrossRef]
16. Hernández, D.; Cardell, E.; Zárate, V. Antimicrobial activity of lactic acid bacteria isolated from Tenerife cheese: Initial characterization of plantaricin TF711, a bacteriocin-like substance produced byLactobacillus plantarumTF711.J. Appl. Microbiol.2005,99, 77–84. [CrossRef]
17. Alberts, J.F.; Engelbrecht, Y.; Steyn, P.S.; Holzapfel, W.H.; van Zyl, W.H. Biological degradation of aflatoxin B1 byRhodococcus erythropoliscultures.Int. J. Food Microbiol.2006,109, 121–126. [CrossRef]
18. Zinedine, A.; Faid, M.; Benlemlih, M. In vitro reduction of aflatoxin B1 by strains of lactic acid bacteria isolated from Moroccan sourdough bread.Int. J. Agric. Biol.2005,7, 67–70.
19. Yalcin, N.F.; Avci, T.; Isik, M.K.; Oguz, H. In vitro activity of toxin binders on aflatoxin B1 in poultry gastrointestinal medium.Pak. Vet. J.2018,38, 61–65. [CrossRef]
20. Huang, L.; Duan, C.; Zhao, Y.; Gao, L.; Niu, C.; Xu, J.; Li, S. Reduction of aflatoxin B1 toxicity byLactobacillus plantarumC88: A potential probiotic strain isolated from Chinese traditional fermented food “Tofu”.
PLoS ONE2017,12, 1. [CrossRef]
21. Smiley, R.; Draughon, F. Preliminary evidence that degradation of aflatoxin B1 byFlavobacterium aurantiacum is enzymatic.J. Food. Prot.2000,63, 415–418. [CrossRef]
22. Hamidi, A.; Mirnejad, R.; Yahaghi, E.; Behnod, V.; Mirhosseini, A.; Amani, S.; Sattari, S.; Darian, E.K. The aflatoxin B1 isolating potential of two lactic acid bacteria. Asian Pac. J. Trop. Biomed. 2013,3, 732–736.
[CrossRef]
23. Gourama, H.; Bullerman, L.B. Anti-aflatoxigenic activity ofLactobacillus casei pseudoplantarum.Int. J. Food.
Microbiol.1997,34, 131–143. [CrossRef]
24. Gourama, H.; Bullerman, L.Aspergillus flavusandAspergillus parasiticus: Aflatoxigenic fungi of concern in foods and feeds: A Review.J. Food Prot.1995,58, 1395–1404. [CrossRef]
25. Onilude, A.; Fagade, O.; Bello, M.; Fadahunsi, I. Inhibition of aflatoxin-producing aspergilli by lactic acid bacteria isolates from indigenously fermented cereal gruels.Afr. J. Biotechnol.2005,4, 1404–1408.
26. Ghonaimy, G.; Yonis, A.; Abolela, M. Inhibition ofAspergillus flavusandA. Parasiticusfungal growth and its aflatoxins [B1, B2, G1 and G2] production byLactobacillus acidophillus.J. Egypt. Soc. Toxicol.2007,37, 53–60.
27. Gourama, H.; Bullerman, L.B. Antimycotic and antiaflatoxigenic effect of lactic acid bacteria: A review.
J. Food. Prot.1995,58, 1275–1280. [CrossRef]
28. Hosono, A. Desmutagenic property of cell walls of Streptococcus faecalis on the mutagenicities induced by amino acid pyrolyzates.Milchwissenschaft1988,43, 168–170.
29. Chang, I.; Kim, J.-D. Inhibition of aflatoxin production ofAspergillus flavusbyLactobacillus casei.Mycobiology.
2007,35, 76–81. [CrossRef]
30. Gourama, H.; Bullerman, L.B. Inhibition of growth and aflatoxin production ofAspergillus flavusby Lactobacillus species.J. Food. Prot.1995,58, 1249–1256. [CrossRef]
31. Gomah, N.H.; Ragab, W.; Bullerman, L. Inhibition of fungal growth and aflatoxin b1 production by some Lactobacillusstrains.Assiut. J. Agric. Sci.2009,40, 27–36.
32. Da Silva, J.F.; Peluzio, J.M.; Prado, G.; Madeira, J.E.; Silva, M.O.; de Morais, P.B.; Rosa, C.A.; Pimenta, R.S.;
Nicoli, J.R. Use of probiotics to control aflatoxin production in peanut grains.Sci. World J.2015,2015, 959138.
[CrossRef] [PubMed]
Animals2019,9, 166
33. Fazeli, M.R.; Hajimohammadali, M.; Moshkani, A.; Samadi, N.; Jamalifar, H.; Khoshayand, M.R.; Vaghari, E.;
Pouragahi, S. Aflatoxin B1 binding capacity of autochthonous strains of lactic acid bacteria.J. Food. Prot.
2009,72, 189–192. [CrossRef] [PubMed]
34. Hernandez-Mendoza, A.; Garcia, H.; Steele, J. Screening ofLactobacillus caseistrains for their ability to bind aflatoxin B 1.Food. Chem. Toxicol.2009,47, 1064–1068. [CrossRef] [PubMed]
35. Prathivadi Bayankaram, P.; Sellamuthu, P.S. Antifungal and anti-aflatoxigenic effect of probiotics against Aspergillus flavusandAspergillus parasiticus.Toxin. Rev.2016,35, 10–15. [CrossRef]
36. Roger, T.; Léopold, T.N. Effect of selected lactic acid bacteria on growth ofAspergillus flavusand aflatoxin B1 production in kutukutu.J. Microbiol. Res.2015,5, 84–94. [CrossRef]
37. El-Nezami, H.; Kankaanpaa, P.; Salminen, S.; Ahokas, J. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1.Food Chem. Toxicol.1998,36, 321–326. [CrossRef]
38. Liew, W.-P.-P.; Nurul-Adilah, Z.; Than, L.T.L.; Mohd-Redzwan, S. The binding efficiency and interaction of Lactobacillus caseiShirota toward aflatoxin B1.Front. Microbiol.2018,9, 1503. [CrossRef]
©2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
9
animals
Article
Evaluation of the Dietary Supplementation of a Formulation Containing Ascorbic Acid and a Solid Dispersion of Curcumin with Boric Acid against Salmonella Enteritidis and Necrotic Enteritis in Broiler Chickens
Daniel Hernandez-Patlan1, Bruno Solís-Cruz1, Karine Patrin Pontin2, Juan D. Latorre3, Mikayla F. A. Baxter3, Xochitl Hernandez-Velasco4, Ruben Merino-Guzman4,
Abraham Méndez-Albores5, Billy M. Hargis3, Raquel Lopez-Arellano1and Guillermo Tellez-Isaias3,*
1 Laboratorio 5: LEDEFAR, Unidad de Investigacion Multidisciplinaria, Facultad de Estudios Superiores Cuautitlan, Universidad Nacional Autonoma de México, Cuautitlan Izcalli 54714, Mexico;
[email protected] (D.H.-P.); [email protected] (B.S.-C.);
[email protected] (R.L.-A.)
2 Departamento de Medicina Veterinária Preventiva, Centro de Diagnóstico e Pesquisa em Patologia Aviária, Universidade Federal do Rio Grande do Sul, Porto Alegre RS 97105-900, Brazil; [email protected] 3 Department of Poultry Science, University of Arkansas, Fayetteville, AR 72704, USA;
[email protected] (J.D.L.); [email protected] (M.F.A.B.); [email protected] (B.M.H.) 4 Departamento de Medicina y Zootecnia de Aves, Facultad de Medicina Veterinaria y Zootecnia, Universidad
Nacional Autónoma de México, Mexico City 04510, Mexico; [email protected] (X.H.-V.);
[email protected] (R.M.-G.)
5 Laboratorio 14: Alimentos, Micotoxinas y Micotoxicosis, Unidad de Investigacion Multidisciplinaria, Facultad de Estudios Superiores Cuautitlan, Universidad Nacional Autonoma de Mexico, Cuautitlan Izcalli 54714, Mexico; [email protected]
* Correspondence: [email protected]; Tel.:+(479)-575-8495; Fax:+(479)-575-8490 Received: 3 April 2019; Accepted: 18 April 2019; Published: 22 April 2019
Simple Summary:Prophylactic or therapeutic administration of a 0.1% mixture containing ascorbic acid (AA) and a solid dispersion of curcumin (CUR) with polyvinylpyrrolidone (PVP) and boric acid (BA) (AA-CUR/PVP-BA) significantly reduced the concentration ofSalmonellaEnteritidis in broiler chickens and had a positive effect in slightly diminishing the negative impact of necrotic enteritis (NE).
Abstract:Two experiments were conducted to evaluate the effect of the prophylactic or therapeutic administration of a 0.1% mixture containing ascorbic acid and a solid dispersion of curcumin with polyvinylpyrrolidone and boric acid (AA-CUR/PVP-BA) againstSalmonellaEnteritidis (S. Enteritidis) in broiler chickens. A third experiment was conducted to evaluate the impact of the dietary administration of 0.1% AA-CUR/PVP-BA in a necrotic enteritis (NE) model in broiler chickens.
The prophylactic administration of 0.1% AA-CUR/PVP-BA significantly decreasedS. Enteritidis colonization in cecal tonsils (CT) when compared to the positive control group (PC,p<0.05).
The therapeutic administration of 0.1% AA-CUR/PVP-BA significantly reduced the concentration of S. Enteritidis by 2.05 and 2.71 log in crop and CT, respectively, when compared with the PC on day 10 post-S. Enteritidis challenge. Furthermore, the serum FITC-d concentration and total intestinal IgA levels were also significantly lower in chickens that received 0.1% AA-CUR/PVP-BA. Contrary, the PC group showed significantly higher total intestinal IgA levels compared to the negative control or AA-CUR/PVP-BA groups in the NE model. However, 0.1% AA-CUR/PVP-BA showed a better effect in reducing the concentration ofS. Enteritidis when compared to the NE model. Further studies