The nucleoside reverse transcriptase inhibitors promote mitochondrial (mt) dysfunction by strongly inhibiting mt polymerase gamma (Pol-y) and subsequently mtDNA replication. In contrast, the non-nucleoside reverse transcriptase inhibitors efavirenz (EFV) and nevirapine (NVP) do not inhibit Pol-y although EFV has been shown to induce mt depolarization (Av|/mlow) in vitro at supra-therapeutic concentrations. Both parameters for each cohort were significantly lower (P <. 0.05) than those of the HAART-naïve patients.
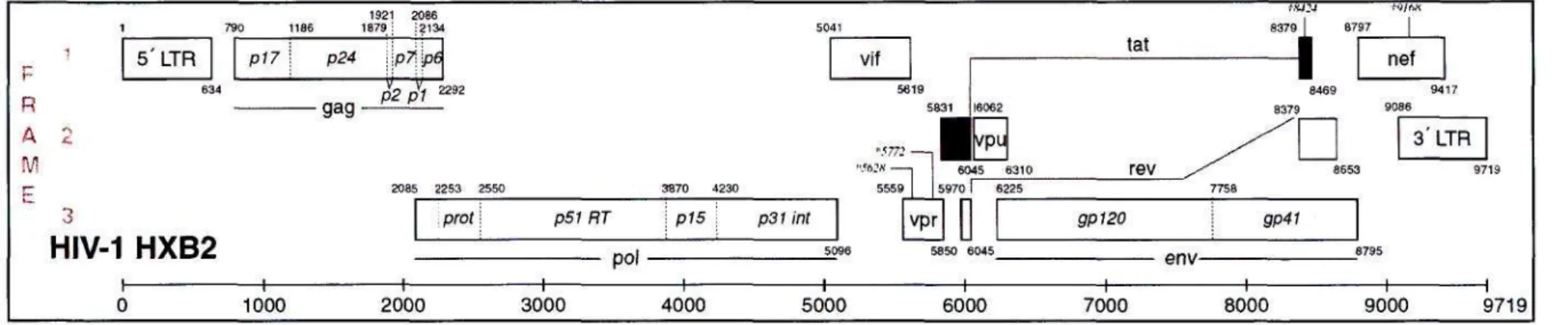
The nursing staff at the King Edward VIII Family Clinic, for their assistance in recruiting patients for my study. The reading frame of each gene is indicated on the left side of the diagram.
LIST OF TABLES
INTRODUCTION
However, despite a dramatic improvement in the prognosis of patients infected with HIV-1, HAART remains ineffective in eradicating the virus from an individual once infected. Of the estimated 5.5 million South Africans already infected with HIV-1, about 1 million individuals are currently in need of ARV treatment, of which only about 300,000 are receiving it. The high cost of ARV drugs, poor health care infrastructure and lack of trained doctors and nurses pose major obstacles to the successful implementation of HAART in rural South Africa.
Furthermore, the need for HIV-1-infected patients to receive lifelong HAART, together with the narrow therapeutic range of ARV drugs, predisposes these patients to the risk of developing short- and long-term adverse toxic side effects. This highlights the need for ARV drug rollout programs to be accompanied by effective mechanisms to ensure patient adherence to HAART regimens and to closely monitor their development of adverse drug side effects.
LITERATURE REVIEW
THE HUMAN IMMUNODEFICIENCY VIRUS-1 (HIV-1)
- THE HIV-1 GENOME
HIV-1 HXB2
GENETIC VARIANTS OF HIV-1
The subtypes are classified and distinguished from each other based on phylogenetic analysis of full-length viral genomes, with each subtype represented by its own consensus sequence [ 13 ]. The CRFs are viruses whose genomic sequences are recombinants (mosaics) of more than one phylogenetic subtype and are common in geographic regions with a high prevalence of more than one viral subtype. Inter-subtype recombination occurs when a host cell is productively super-infected with two distinct viral subtypes, with the gRNA strands of each subtype compatible to form heterodimers during viral packaging [ 16 ].
VIRAL ULTRASTRUCTURE
After infection, the viral genomic ssRNA is reverse transcribed into dsDNA, with HIV-1 RT frequently switching between the two packaged copies of viral gRNA templates [ 17 ], a process known as copy selection [ 18 ]. GP41 contains the HR1 and HR2 helices and hydrophobic fusion peptide that penetrates and anchors the virion in the host cell membrane [ 27 , 28 ]. Host cell receptors such as Human Leukocyte Antigen (HLA) class I and II are also incorporated into the viral envelope during budding [29].
The capsid core contains two copies of viral ssRNA in complex with NC protein and RT and IN enzymes [32] (Fig. 1.2B). The virion matures and is rendered infectious by PR-mediated autocatalytic cleavage of Gag and GagPol polyproteins.
![Figure 1.2. Schematic diagrams of the immature (A) and mature (B) HIV-1 virion [38]. The](https://thumb-ap.123doks.com/thumbv2/pubpdfnet/10637880.0/22.893.188.680.150.788/figure-schematic-diagrams-immature-mature-b-hiv-virion.webp)
HOST CELLS
VIRAL LIFE CYCLE
The shift in viral tropism is determined by amino acid changes primarily in the V3 loop of gp120 [59–61]. Gag and GagPol are synthesized in a ratio of approx. 20:1, the latter being produced after a -1 ribosomal frameshift during translation [78]. Viral particles are assembled from the oligomerization of Gag and GagPol polyproteins under the host cell membrane [31, 79] (Fig. 1.2A).
The N-myristylated terminal in the MA (M) domain of p55 Gag facilitates the insertion of Gag into the host plasma membrane [ 80 , 81 ]. The nascent viral particle consisting of Gag and GagPol precursors (Fig. 1.2A) is immature and non-infectious.
THE PATHOGENESIS OF HIV-1
- APOPTOSIS
- CASPASES
- THE EXTRINSIC PATHWAY
- THE INTRINSIC (MITOCHONDRIAL) PATHWAY
However, autocatalytic activation of initiator caspases occurs only upon recruitment and oligomerization of several molecules of procaspase-2/8/10 and procaspase-9 either to cell surface death receptors (extrinsic pathway) or to apoptosis (intrinsic pathway). Death effector domains (DEDs and caspase recruitment domains (CARDs) [118] are located in the long N-terminal peptides of procaspase-8/-10 and procaspase-2/-9, respectively. These domains mediate the targeting and association of intrinsic and extrinsic initiator caspases with by complementary DED of the death receptor or CARD of the apoptosome [108, 119].
Death receptor-induced T-cell apoptosis in HIV-1-infected patients is primarily mediated by Fas binding (CD95/APO!). In Fas type-II cells, such as the Jurkat cell line, DISC formation is restricted, leading to mitochondrial permeability and subsequent activation of caspases-8 and -3 downstream of mitochondria [166] (Fig. 1.4). Consequently, only type II Fas-mediated apoptosis can be blocked by overexpression of the antiapoptotic proteins Bcl-2 and BC1-XL, which inhibit mitochondrial permeability [ 166 ].
Signals from all pathways converge especially at the mitochondrion, culminating in the activation of the proteolytic caspase cascade. In type II cells, DISC formation is limited and is characterized by mitochondrial PT followed by release of cytochrome c into the cytoplasm and caspase activation. Opening of PTPC is regulated by the pro- and anti-apoptotic BCL-2 (B-cell CLL/Lymphoma 2) family of proteins (Fig. 1.3).
BCL-XL inhibits PTPC opening by integrating into the OMM and directly binding to VDAC [ 180 ], while Bax, Bak and BIM promote PTPC opening by binding to VDAC [ 181 , 182 ]. Cytochrome c release is accompanied by a collapse of the mitochondrial internal transmembrane potential (Ai|/m) [196], known as permeability transition (PT) or depolarization (Av|/mlow), which irreversibly commits the cell to apoptosis [196]. 197, 198]. Apoptosome formation induces autocatalytic activation of procaspase-9, which then recruits and activates procaspase-3 [202] (Figure 1.3).
![Figure 1.3. Schematic diagram of the intrinsic and extrinsic apoptosis induction pathways [175]](https://thumb-ap.123doks.com/thumbv2/pubpdfnet/10637880.0/31.901.105.766.157.906/figure-schematic-diagram-intrinsic-extrinsic-apoptosis-induction-pathways.webp)
ANTI-RETRO VIRAL DRUGS
- THE NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS
- MITOCHONDRIAL TOXICITY OF THE NRTIs
- THE NON-NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS
- MITOCHONDRIAL TOXICITY OF THE NNRTIs
- THE PROTEASE INHIBITORS
- MITOCHONDRIAL PROTECTIVE EFFECT OF Pis
In addition to their antiviral properties, NRTIs, NNRTIs and Pis modulate lymphocyte apoptosis at the mitochondrial level, which will be discussed further. Chemical structures of NRTIs (nucleoside derivatives) [214] commonly prescribed in South Africa for the treatment of HIV-1 infection. Incorporation of an NRTI-TP into the 3v end of the extended viral DNA strand results in premature termination of reverse transcription [ 216 , 220 ].
Chemical structures of NNRTIs [214] commonly prescribed in South Africa for the treatment of HIV-1 infection. This is mediated by the binding of EFV to RT embedded in the GagPol polyprotein thereby promoting oligomerization of the GagPol polyproteins. This causes the premature activation of the PR enzyme embedded within GagPol, and the subsequent cleavage of the polyproteins into their structural and enzyme constituent subunits.
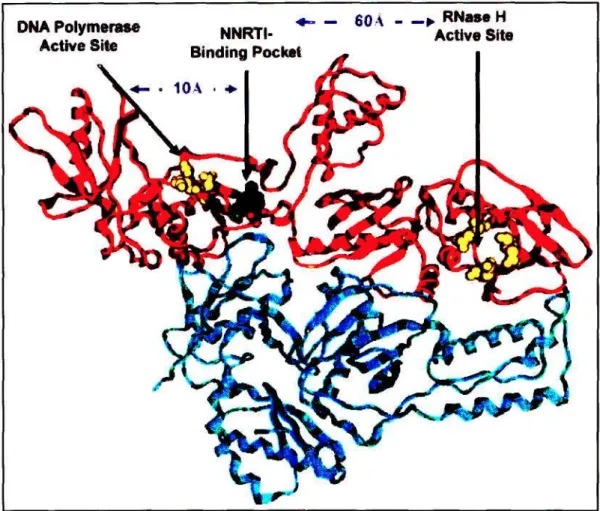
Residues located in the DNA polymerase and RNase H active sites are indicated with yellow spheres [255]. In contrast to the NRTIs, much less is known about the apoptosis-inducing potential of the NNRTIs and their mechanism/s. UCPs are proton transporters, present in the IMM, that catalyze a regulated discharge of the proton gradient across the IMM, i.e.
Inhibition of HIV-1 PR results in the production of immature, non-infectious viral particles. NFV blocked PTPC opening at the level of ANT and thus Ai|/m collapse and cytochrome c release in the presence of apoptotic stimuli, but did not inhibit the proteolytic activity of active caspase or -8 [265]. Thus Pis maintains the integrity of the mitochondrial lymphocyte Aym by preventing PTPC opening and UCP-mediated unloading of Av|/m.
![Figure 1.5. Chemical structures of the NRTIs (nucleoside derivatives) [214] commonly prescribed in South Africa for the treatment of HIV-1 infection](https://thumb-ap.123doks.com/thumbv2/pubpdfnet/10637880.0/35.903.85.827.638.921/chemical-structures-nucleoside-derivatives-commonly-prescribed-treatment-infection.webp)
SCIENTIFIC PAPER PUBLICATION
PREFACE
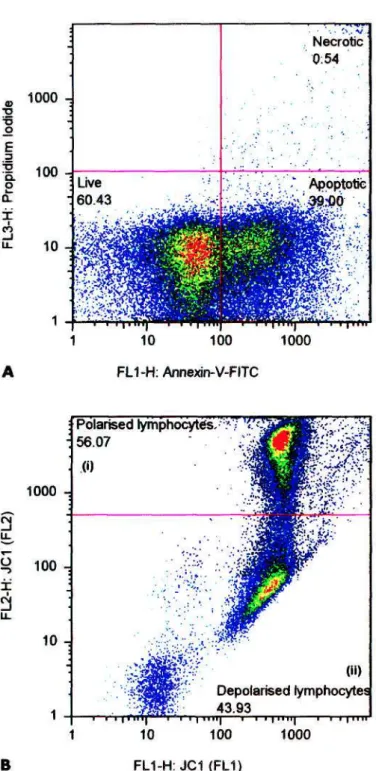
Correlations between lymphocyte apoptosis and Ai}ow and with duration of HAART were analyzed with Spearman tests. The mean total lymphocyte apoptosis of the HAART-naïve cohort was higher than in both regimen 1 a and lb cohorts, with only the latter comparison being significant (P = 0.037). Statistical differences between each cohort for the corresponding subcategories regarding lymphocyte apoptosis and Ai|rnikm were tested.
Notably, total lymphocyte apoptosis was consistently higher than TH apoptosis in all subgroups of both regimens, a finding similar to comparison of the means of these parameters (Table 1). A significant decrease in TH apoptosis below total lymphocyte apoptosis and a decrease in HAART-naïve TH. However, the lack of a significant correlation between lymphocyte apoptosis and Ai|jmlow lymphocytes in the HAART-naïve cohort seemed paradoxical.
Lymphocyte apoptosis and Ai|»mlow data for HAART-naïve and HAART-treated HIV-1 infected patients.
CONCLUSION
Dual role of the putative human immunodeficiency virus type 1 RNA dimerization initiation site in genomic RNA packaging and proviral DNA synthesis. A novel long-range base pair interaction in human immunodeficiency virus type 1 RNA shuts down the Gag start codon. Association of human immunodeficiency virus type 1 integrase, matrix, and reverse transcriptase antigens with viral nucleic acids after acute infection.
The CCR5 and CXCR4 coreceptors are both used by primary isolates of human immunodeficiency virus type 1 of subtype C. Molecular determinants of the V3 loop of the glycoprotein gpl20 of human immunodeficiency virus type 1, responsible for controlling cell tropism. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule.
Human immunodeficiency virus type 1 Vpr protein affects nuclear localization of viral nucleic acids in nondividing host cells. Furin convertase and PCI can cleave the human immunodeficiency virus (HIV)-1 envelope glycoprotein gp160 into gp120 (HIV-1 SU) and gp41 (HIV-I TM). Role of capsid precursor processing and myristoylation in human immunodeficiency virus type 1 morphogenesis and infectivity.
Cis elements and transacting factors involved in human immunodeficiency virus RNA dimerization HIV-1. The human immunodeficiency virus type 1 p6gag domain is sufficient for Vpr incorporation into heterologous viral particles. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer through activation of nuclear factor kappa B.
Tumor necrosis factor alpha functions in an autocrine manner in inducing human immunodeficiency virus expression. Synergistic stimulatory effects of tumor necrosis factor alpha and interferon gamma on human immunodeficiency virus type 1 replication and apoptosis of HIV-1-infected host cells.
![Figure 1.4. Schematic overview of the Fas type I (A) and type II (B) pathways [166]. In type I](https://thumb-ap.123doks.com/thumbv2/pubpdfnet/10637880.0/32.900.106.770.239.790/figure-schematic-overview-fas-type-type-pathways-type.webp)
![Figure 1.6. Chemical structures of the NNRTIs [214] commonly prescribed in South Africa for the treatment of HIV-1 infection](https://thumb-ap.123doks.com/thumbv2/pubpdfnet/10637880.0/37.905.118.735.519.805/figure-chemical-structures-nnrtis-commonly-prescribed-treatment-infection.webp)
![Figure 1.8. Chemical structures of the Pis [214] commonly prescribed in South Africa for the](https://thumb-ap.123doks.com/thumbv2/pubpdfnet/10637880.0/41.903.196.705.198.645/figure-chemical-structures-pis-commonly-prescribed-south-africa.webp)
