LIST OF TABLES
Abstract
The vast majority of rutile grains from the electrostatic fractions were relatively pure Ti02 and contained low concentrations of 'other' oxides. The blue color of the rutile grains appears to be affected by a combination of Si02, AI20 3 and Nb20 5 substitutions. Apart from Fe3+, no single element; appears to be solely responsible for the observed changes in the physical characteristics (magnetic susceptibility, electrostatic conductivity, and color) of homogeneous rutile grains from the Sibaya Formation.
Acknowledgements
Introduction
- Titanium Deposits
- South Africa and the World Titanium Market
- South African Titanium Deposits
The main applications of titanium pigments are in the production of paints, lacquers, enamels (57%), plastics (17%) and paper (15%), with rubber, coated fabrics and textiles, printing inks, ceramics and cosmetic industries are responsible for the production of titanium pigments. virtually the entire remainder (Wipplinger, 1998). In the non-pigment sector (titanium metal and alloys), production fell significantly in 1999 due to reduced demand caused by lower consumption in the commercial aircraft industry (Murphy and Taylor, 1999). Heavy mineral deposits along the west coast were explored by the Geological Survey of South Africa in the 1950s.
REPUBLIC OF
SOUTH AFRICA
NAMIBIA
Titanium Mineral Recovery from Placer Deposits
The sand is then passed to a gravity circuit where a series of Humphries spirals separate the heavy from the light minerals. The light minerals are discharged as residues and the heavy minerals undergo further processing (Fig. 1.4.1) in the main mineral separation plant. However, before the heavy minerals leave the floating magnetite of the concentrator, it is removed and discharged to tailings using low-intensity wet magnets.
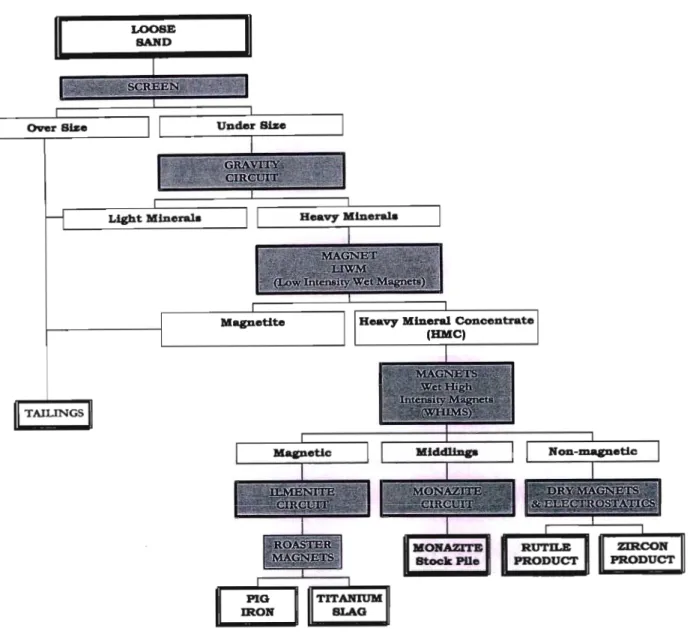
Rutile recovery problems
Regional Geology
- Overview
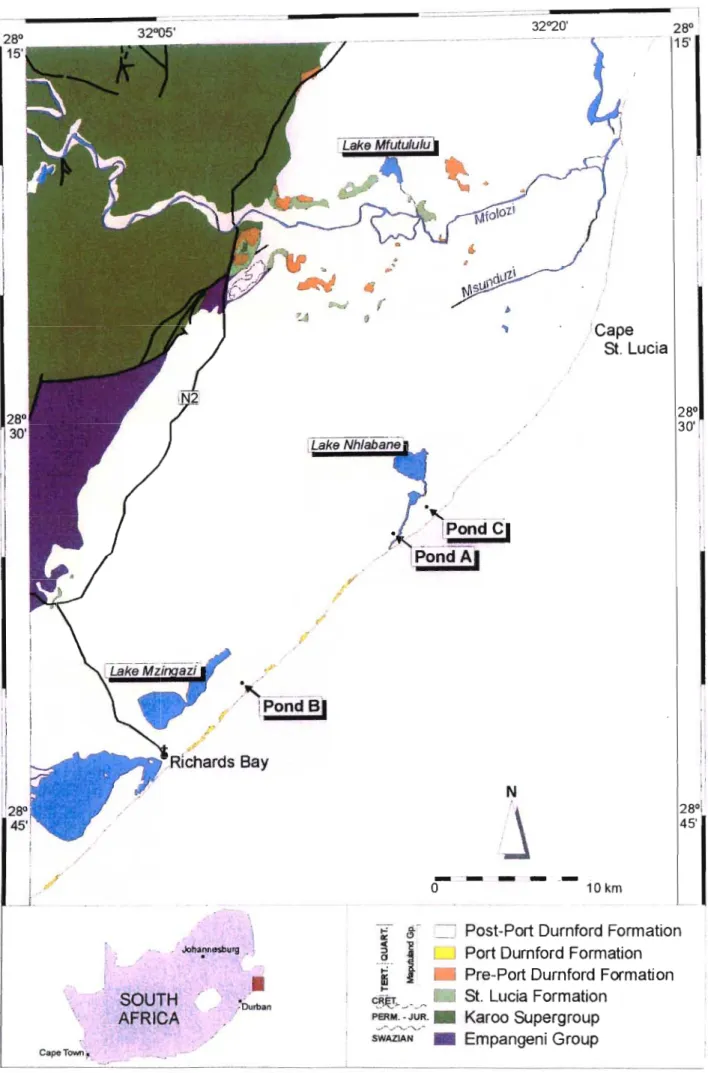
- Study Area
- Stratigraphy of KwaZulu-Natal
The samples collected consisted of the heavy mineral concentrate (HMC) produced by mine ponds A, B and C. The Natal Group rests unconformably on the older rocks of the Natal Structural and Metamorphic Province with a hiatus of approximately 500 Ma. The Dwyka Group is conformably overlain by marine sandstones and shales of the Permian Ecca Group (Johnson, 1994).
GROUPS AND FORMATIONS AGE LITHOLOGY
Cainozoic Sediments
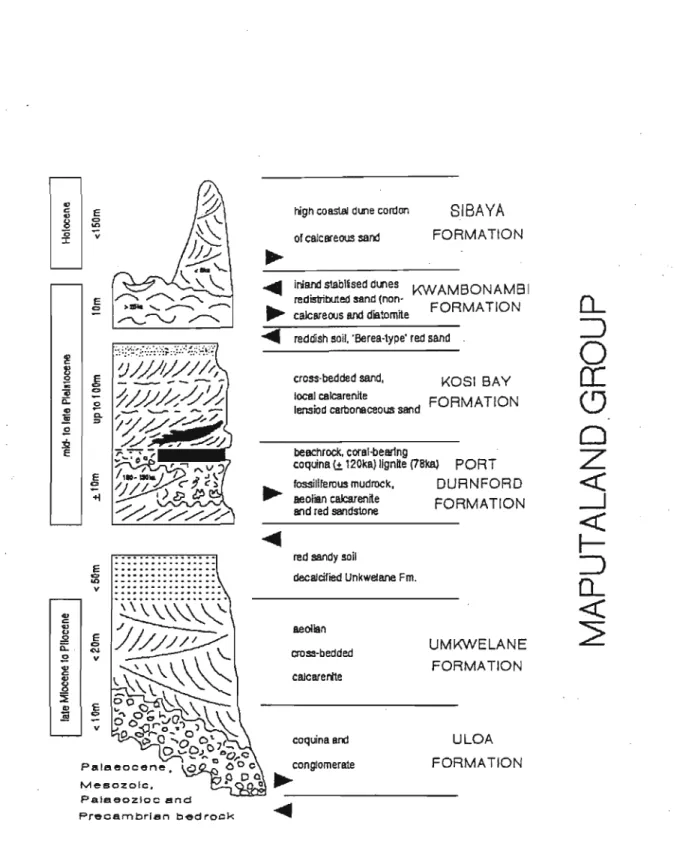
Both Hobday and Orme, (1974); and Hobday and Jackson (1979) described the stratigraphy of the Zululand Coastal Plain, "as units of Recent/Holocene eolian sediments; overlying a Cainozoic Port Durnford Formation; having a base consisting of Cretaceous sediments" . However, this nomenclature ignored the marked unconformities within the Port Durnford Formation, as well as the unconformity between the Port Durnford Formation and the overlying eolian sediments. The term Kosi Bay Formation was introduced for the red, brown and white sands formerly called the Upper Argilaceous Member of the Port Durnford Formation (Singh 1995; G.
Revised Stratigraphy
- Uloa Formation
- Umkwelane Formation
- Port Durnford Formation
- Kosi Bay Formation
- Kwambonambi Formation
- Sibaya Formation
The top of the Umkwelane Formation has been reworked to form a red, sandy paleo-soil without fossils (Singh, 1995). The dune sand in the Sibaya Formation is still accumulating as the strong onshore winds carry beach sand inland. The depositional environment of the Sibaya Formation is considered to have been very similar to that of the Kwambonambi Formation.
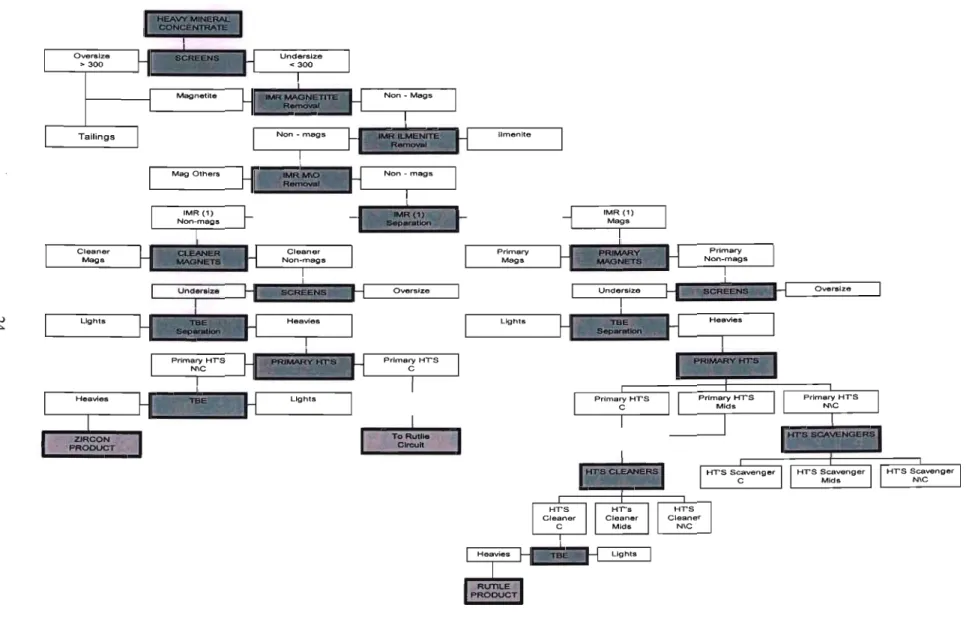
Mineral Processing
- Overview of mineral processing
- Heavy Mineral Extraction at Richards Bay
- Dune Rehabilitation
- Mineral Separation
- Separation Techniques
- Magnetic Separation
- Electrostatic Separation
- Laboratory Separation Results
- Rutile Distribution in the Heavy Mineral Concentrate
- IImenite sub-fractions
- Mineralogy
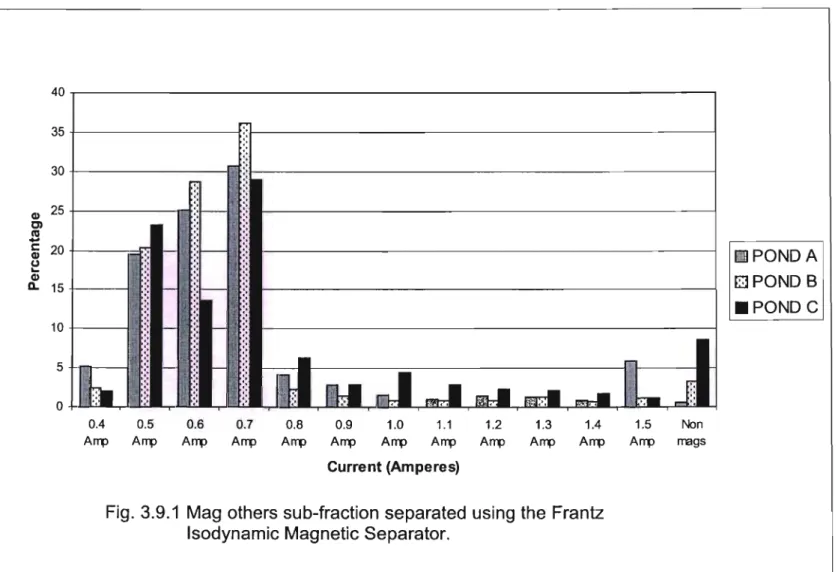
- Mag Others sub-fractions
- Mineralogy
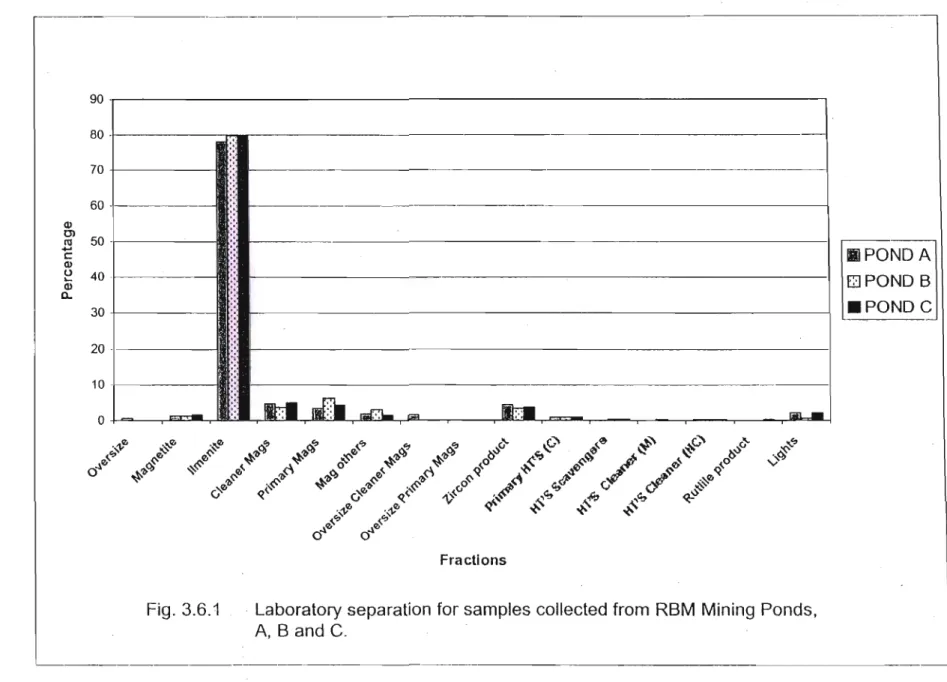
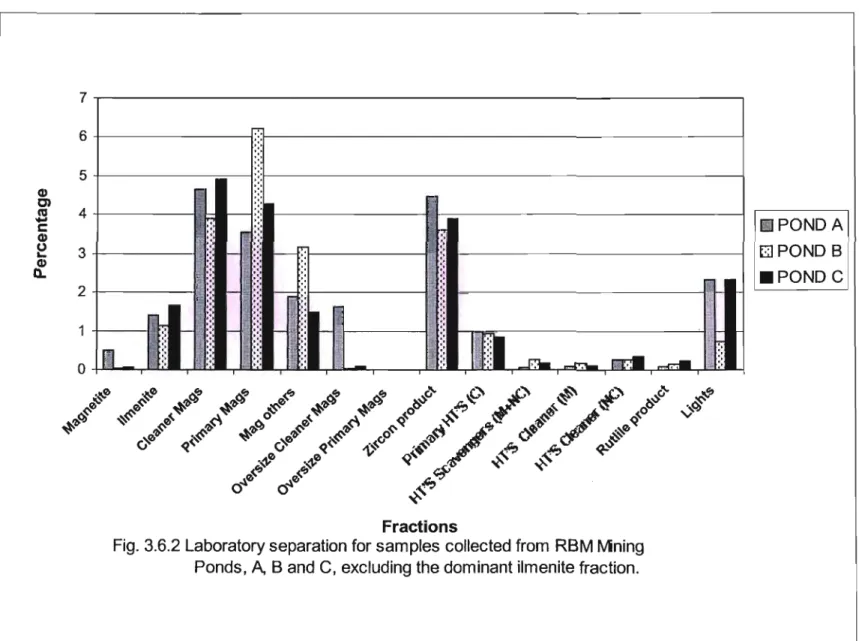
A Carpco High Tension Roll Separator was used for the electrostatic separation of the non-magnetic samples (Appendix A2). The dominant fraction from all three mine ponds was the ilmenite fraction (Fig. 3.6.1), as this made up almost 80% of the heavy mineral concentrate (HMC) in all cases. The 1menites from the 0.6 Amp subfraction were titanium enriched; however, most of the fraction consisted of silicates, Le.
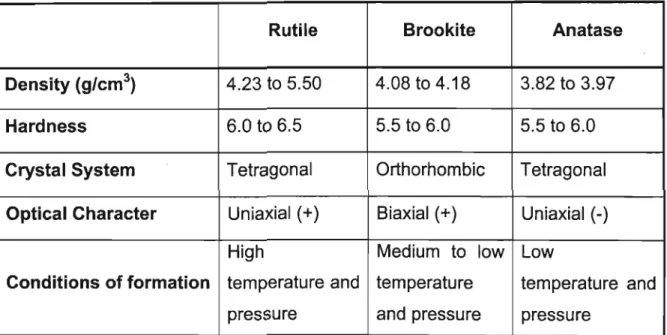
Rutile Mineral Chemistry
- Crystal Structure
- Rutile Mineralogy
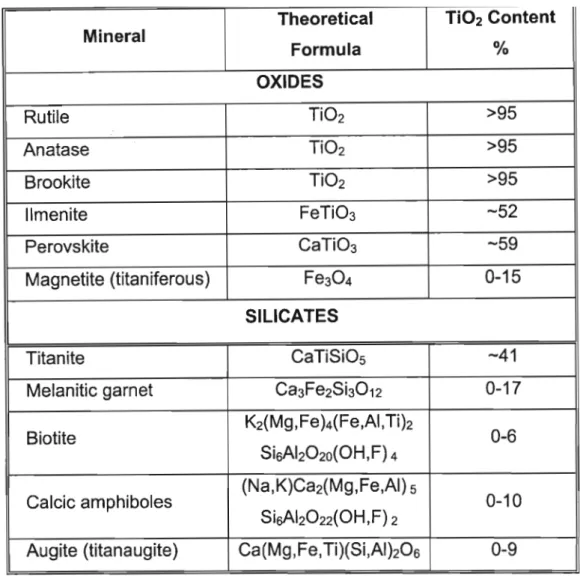
Four unit cells of rutile showing oxygen octahedra around Ti at the center of each cell. The two anatase unit cells showing a distorted oxygen octahedra around Ti (shared edges (s)) are shorter than non-shared edges. Although magnetite, ilmenite, and hematite are common minerals, their precise chemical composition is difficult to determine due to extensive chemical substitution and difficulty in determining the oxidation states of the ions (Force 1991).
Fe g 04 magnetite
Mineralogy of the Iron-Titanium Oxides
FeTizOs
Solid Solution
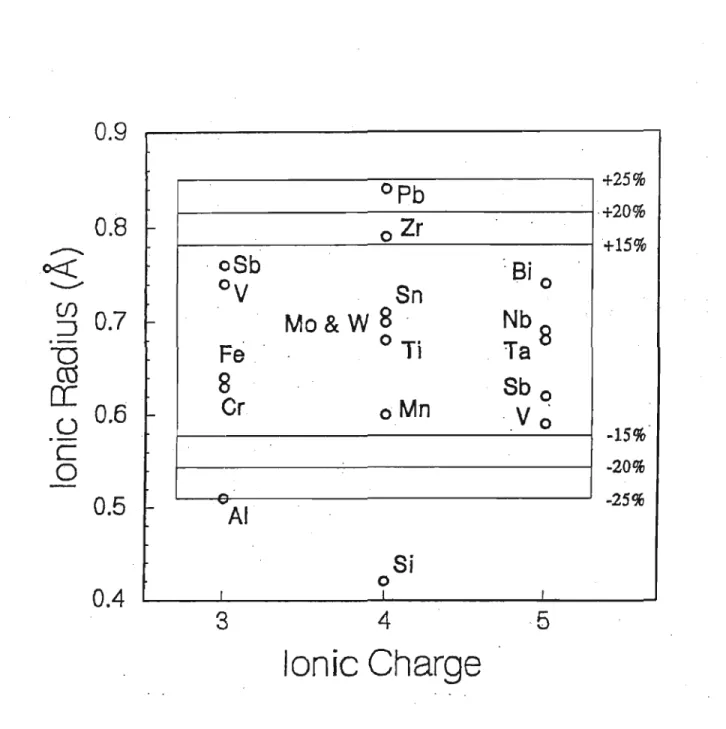
The three mechanisms of solid dissolution are substitutional solid dissolution, omitted solid dissolution, and interstitial solid dissolution. Solid solvation is the removal of atoms from the structure, leaving vacancies in the structure to maintain charge balance during a substitution mechanism. Solid dissolution is governed by Goldschmidt's rules, which state (Blossions with similar radii « 15% size difference) and with a charge difference of at most one can enter the same crystal lattice site.
Rutile Chemistry
- Hydrogen in Rutile
- Ti 3 + and Fe 3 + in rutile
- Aluminium in rutile
- Niobium and Tungsten in rutile
- Tungsten in rutile
- Niobium and chromium in rutile
- Chromium in rutile
- Zirconium in rutile
Some rutile crystals contain significant amounts of Fe2+ and Fe3+, large amounts of niobium and tantalum, and small amounts of chromium, aluminum, and silicon. Paired substitution of types:. are common in synthetic rutile to preserve electrostatically neutral compounds. Linnen and Keppler (1997), found that rutile forms an extended solid solution series with columbite due to structural similarities of niobium and tantalum ions, and also reported rutile grains with up to 66 wt% columbite or tantalum components.
Linnen and Keppler (1997) concluded that most of the Nb5+ and Ta5+ incorporated into rutile is through solid solution with columbite-tantalite end-members. As much as 16 wt% Fe2O3 can be accommodated in rutile crystals through crystallographic dislocation structures and replacement of Ti3+ by Fe3+ (Blanchin and Bursill, 1989). According to Banfield and Veblen (1991), the occurrence of regularly spaced iron-rich lamellae throughout rutile crystals is due to the dissolution of iron originally present in the rutile structure.
However, coexisting fluorine-rich aqueous fluids and rutile crystal growth dynamics were also of considerable importance. Given limitations based on charge and ionic radius, only chromium ions with Cr3+ and Cr4+ charges are able to replace the Ti4+ ion. During a study of the Khibina Alkaline Complex, northwestern Russia, Nb - Zr-bearing rutile was found to be an accessory mineral (Barkov et al. 1997).
The high content of alkali and Zr in the magmatic melt is believed to contribute to the formation of this unusual replacement.
Rutile in geological environments
Data Analysis
- Oversize and Magnetite Fractions
- IImenite
- Magnetic IImenite
- Non-Magnetic IImenite .1 Pond A
- Mag Others
- Magnetic Mag Others .1 Pond A
- Non-Magnetic Mag Others .1 Pond A
- Cleaner Mags .1 Pond A
- Pond B
- Pond C
- Primary Mags .1 Pond A
- Pond B
- Pond C
- Primary HT'S Conductors .1 Pond A
- Pond 8
- Pond C
- HT'S Scavengers
- HT'S Cleaner Mids .1 Pond A
- Pond B
- Pond C
- HT'S Cleaner Non-Conductors .1 Pond A
- Pond B
- Pond C
- HT'S Cleaner Conductors .1 Pond A
- Pond B
- Pond C
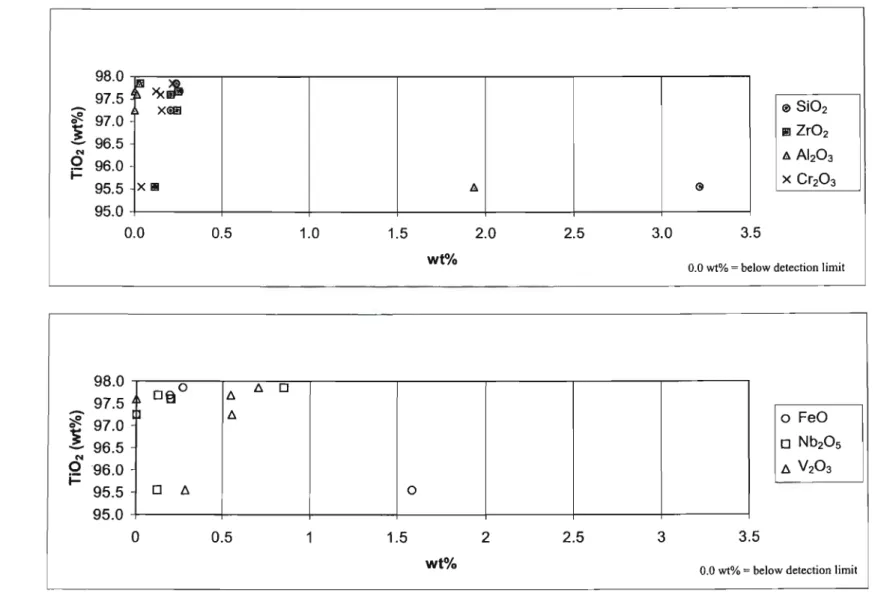
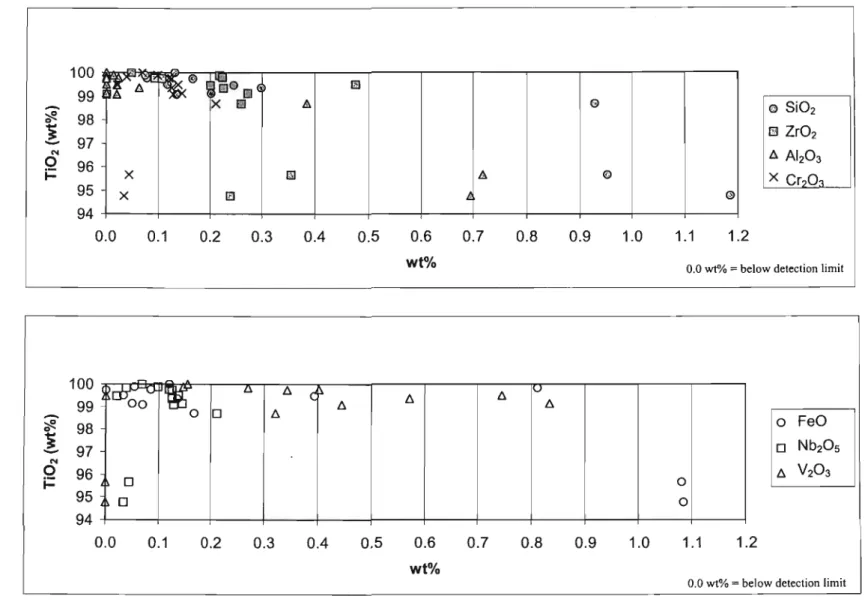
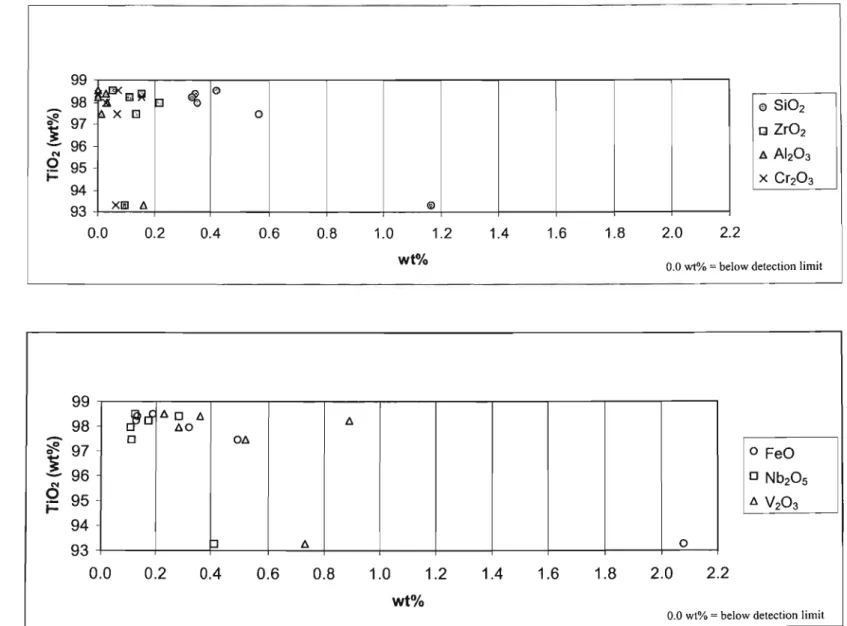
These rutile grains contained very little ZrO2 with values below the detection limit of 0.017 wt. % to 0.160 wt. %. The concentration of FeO in the rutile grains varied from below the detection limit (0.027 wt. %) to 1.974 wt. %. The rutile grains from the 'HT'S cleaner mids' fraction of pond A contained a significant proportion of SiO2 and lr02.
In the 'HT'S cleaner mids' fraction of pond B, SiOz and ZrOz were found at levels significantly above the detection limit in all rutile grains analyzed. Only four of the nine rutile grains contained AI2O3 in concentrations above the detection limit. All rutile grains from the 'HT'S purer conductors' fraction of pond B had a high proportion of SiO2.
Some of the rutile grains from the 'HT'S cleaner conductors' fraction from pond C had high concentrations of V203, Nb20and FeO.
Discussion
- Rutile Chemistry in relation to physical properties During mineral separation four magnetic fractions were produced
- Magnetic effect
- Electrostatic effect
- Rutile colour
- Overview of rutile geochemistry in the magnetic fractions
- Magnetic ilmenite fraction
- Non-magnetic ilmenite fraction
- Magnetic mag others fraction
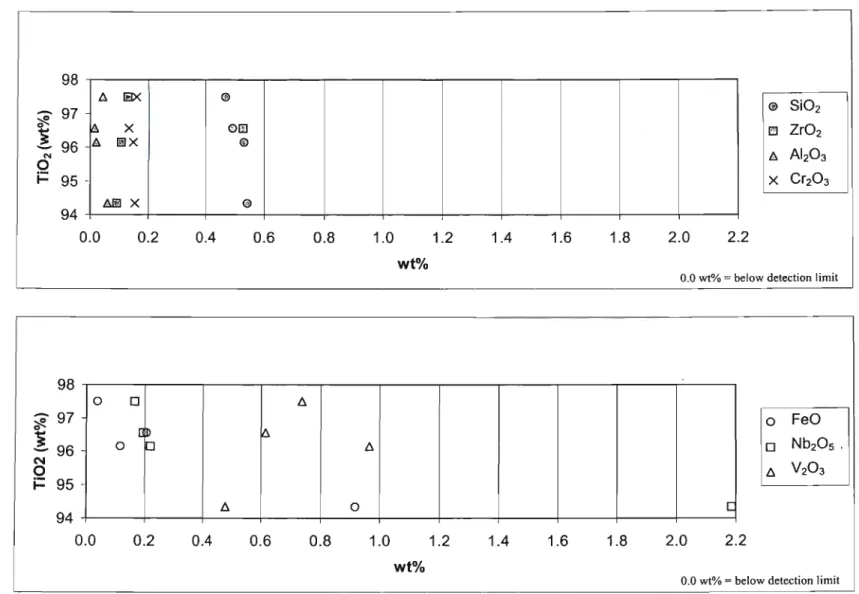
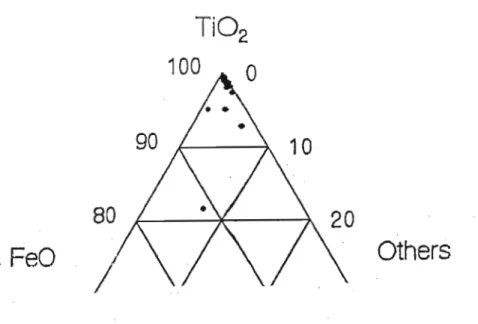
Rutile grains from this fraction had low FeO contents, ranging from below the detection limit of 0.027 wt% to approximately 1.6 wt%. All other oxides in the rutile grains accounted for approximately 5% grain weight. Rutile grains from the 'HT'S primary conductors' fraction (Fig. 6.1.2.1) showed that the rutile grains consisted almost entirely of Ti02.
Ternary plot of Ti02, FeO and other oxides for rutile grains of the 'primary HT's conductors' fraction. The most common colors for rutile grains were reddish brown and black followed by yellow and blue respectively (see Appendix A5 for color plates). However, in all the red-brown rutile grains, this high FeO content was not visible.
Geochemical data for black rutile grains (brownish black 5YR 2/1 - Committee on Rock and Color Charts, 1979) are presented in Fig. A graphic display of geochemical data for yellow rutile grains (dark yellowish orange 10YR 6/6 - rock and Color Chart Committee, 1979) is shown in fig. Yellow rutile grains had higher FeO and Nb20s contents compared to other oxides.
Therefore, the yellow color of the rutile grains may be the result of the substitution of FeO and Nb20 in the rutile lattice.
Pond C V203
Pond C
Non magnetic mag others fraction
Cleaner mags fraction
Primary mags fraction
Pond B Pond C
Pond B
Overview of rutile geochemistry in the electrostatic fractions
- Primary HT'S conductors fraction
- HT'S scavengers fraction of Pond B
- HT'S cleaner mids fraction
- HT'S cleaner non-conductors fraction
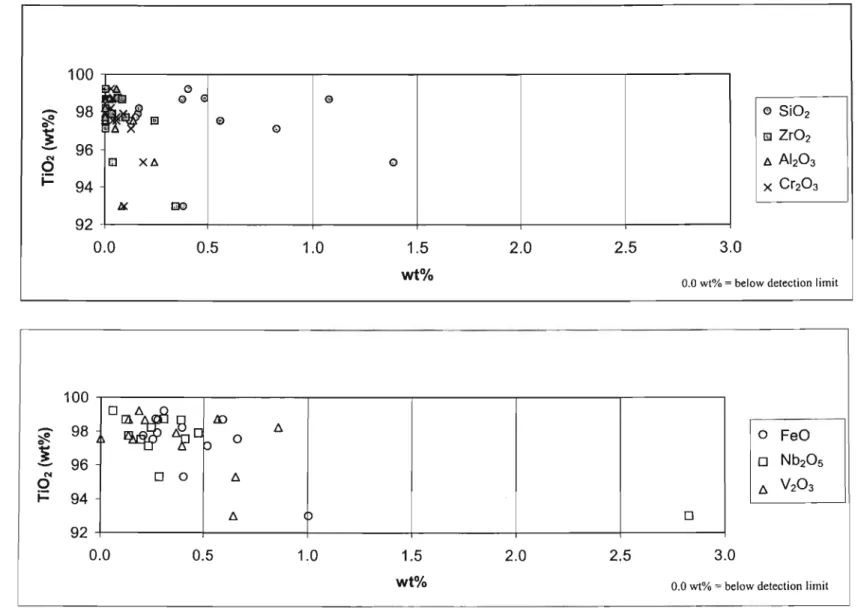
All ponds had very similar trends (Fig. 6.3.1), with SiO2 being the dominant component in the rutile grains, with approx. 0.38% by weight for all ponds. Also evident was the lower FeO and Zr02 content in rutile grains from all three ponds. The radar plot (Fig. 6.3.2) shows that SiO2 and V2O3 were the only oxides that occurred at concentrations greater than 0.25% by weight.
Rutile grains from dam A consisted of SiO2 and V20 3 , while grains from dam B were dominated by SiO2 and Nb20 s. All three dams contained virtually no A1203, as well as a much lower FeO content than the magnetic fractions.
Si02 PandA
Si02 FeO
PandA
HT'S cleaner conductors fraction
All three basins showed a similar trend regarding the oxides SiOz, VZ03 and NbzOs (Fig. 6.3.5).
A brief summary on Radar graphs
Conclusions
- Special features of Ponds A, Band C
- Magnetic ilmenite fraction
- Non-magnetic ilmenite fraction
- Magnetic mag others fraction
- Non magnetic mag others fraction
- Cleaner mags fraction
- Primary mags fraction
- Primary HT'S conductors fraction
- HT'S scavengers fraction of Pond B
- HT'S cleaner mids fraction
- HT'S cleaner non-conductors fraction
- HT'S cleaner conductors fraction
- Rutile chemistry in relation to magnetic susceptibility, electrostatic conductivity and colour
- Magnetic Susceptibility
- Electrostatic Conductivity
- Colour
No element in particular dominated the 'cleaner mags' fraction of dams A, Band C, although most grains contained varying concentrations of other ions. All the rutile grains analyzed in the 'primary mags' fraction of dam A contained some SiO2 and FeO. The rutile grains of 'primary HT'S conductors' fraction of ponds A, Band C, are almost pure TiO2, and contain very limited substitutions of any other oxides.
Grains from the HT'S scavengers fraction of pond B all contained SiO 2 , ZrO 2 , and FeO at low concentrations, with only V 2 O 3 having concentrations close to 1 wt%. Rutile grains from the 'HT'S cleaner middle fraction' of all three ponds contained significant amounts of SiO2 and ZrO2. Although the average V203 content of grains from all ponds was higher than SiO2 or Zr02, not all grains contained a higher proportion of V203. .
All grains from pond B contained enriched Nb20S and FeO, with Nb20S in the highest proportion in the grains, while grains from pond C had the highest proportion of FeO. All grains analyzed in the 'HT'S cleaner conductor' fraction of Ponds A, lane C, contained significant amounts of SiO2 and FeO, with one grain in Pond A containing 1.167 wt. % FeO. Red rutile grains have slightly higher Cr203 and Nb20s contents, while black rutile grains are generally enriched in V203 and Nb20s.
The blue color of rutile grains appears to be the product of multiple substitutions with SiO 2 , AbO 3 , and Nb 2 O s .
Coastal and Environmental Services (1993) Environmental Impact Study, Eastern Shore of Lake St Lucia (KingsafTojan Lease). Coastal and Environmental Services (1982) Environmental Impact Study, Eastern Shore of Lake St Lucia (KingsafTojan Lease). The study of titanium-bearing oxides in heavy minerals deposited along the east coast of South Africa.
1997) Solubility of columbite in granitic melts: implications for the enrichment and fractionation of Nb and Ta in the Earth's crust. 1976). 1976) Oxide Minerals Mineralogical Society of America Short Course Notes Vol 3. Southern Printing Company, Virginia L1-Hg300. 2000) Southeast and South Coastal Deposits, 19-32, (in Partridge T.C. and Maud, RR, The Cenozoic of Southern Africa.
An EPMA and SEM study of niobic-tungsten rutile from the Fanos Aplitic Granite, Central Macedonia, Northern Greece. A survey of the stratigraphy and sedimentary environments of the Karoo basins of southern Africa. Lithostratigraphy of the Republic of South Africa, South West Africa/Namibia and the Republics of Bophuthatswana, Transkei and Venda.
In: An Introduction to the Geological and Mining Heritage of South Africa. and minor elements in rutile and consequences of high OH contents in Nb- and Cr-rich rutile from the upper mantle. 1998).
APPENDIXA1