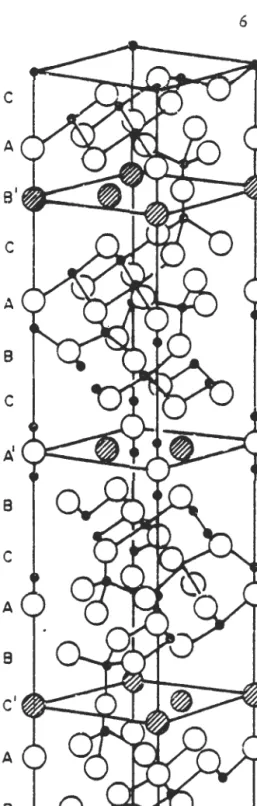
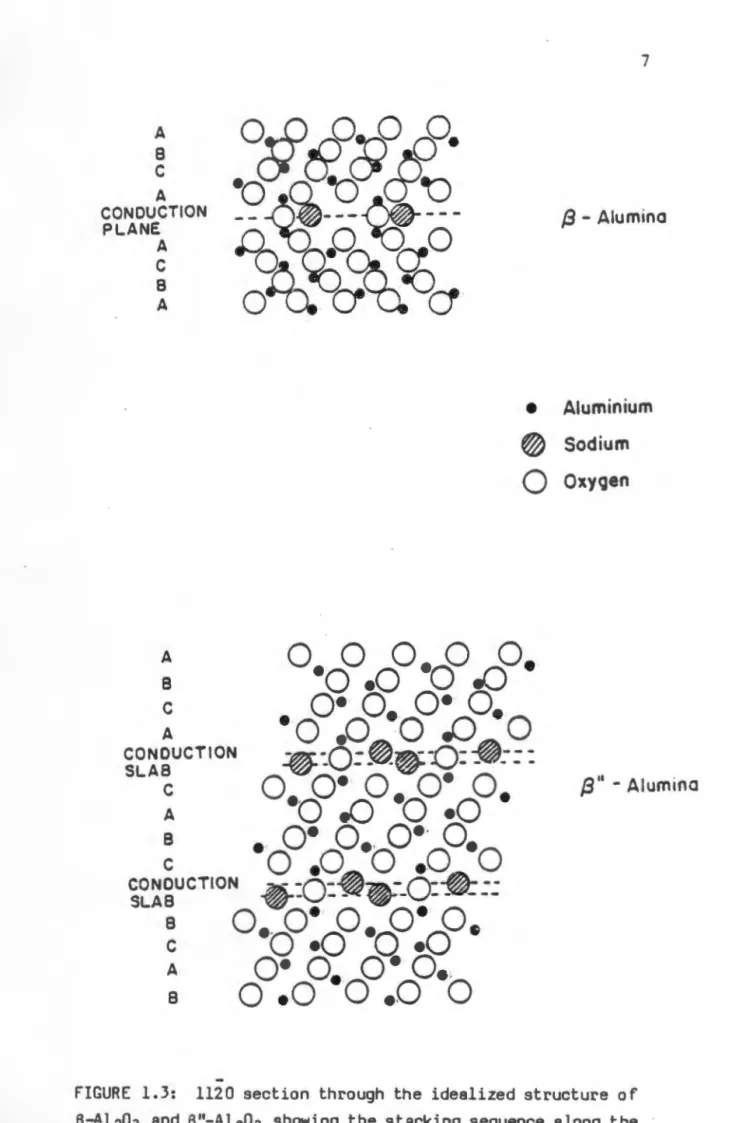
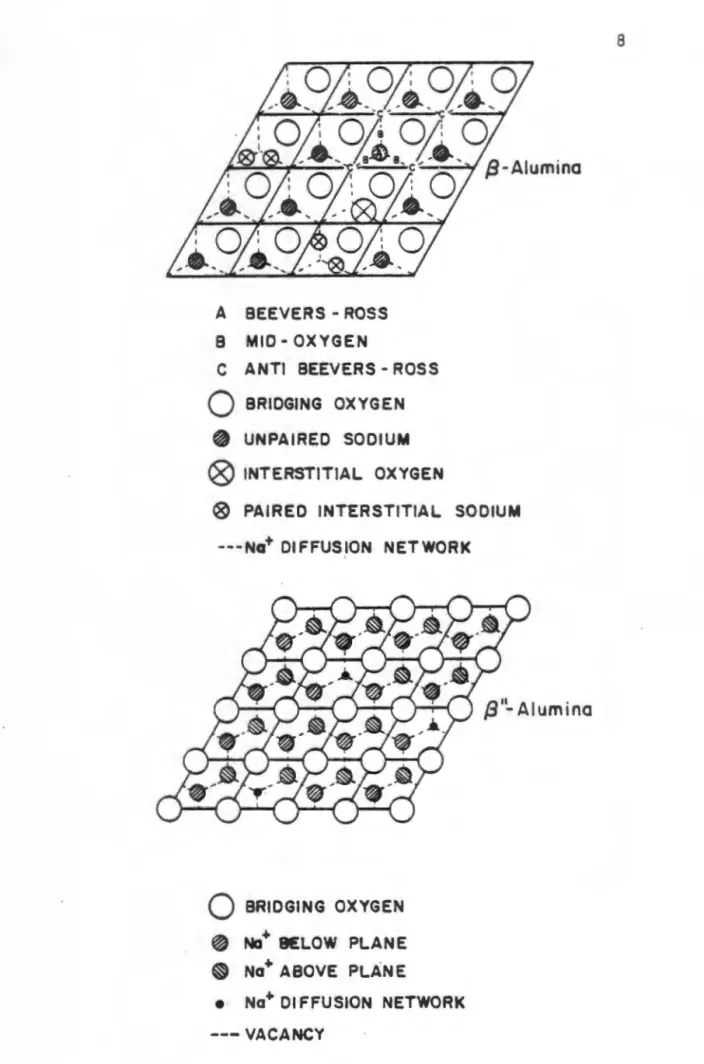
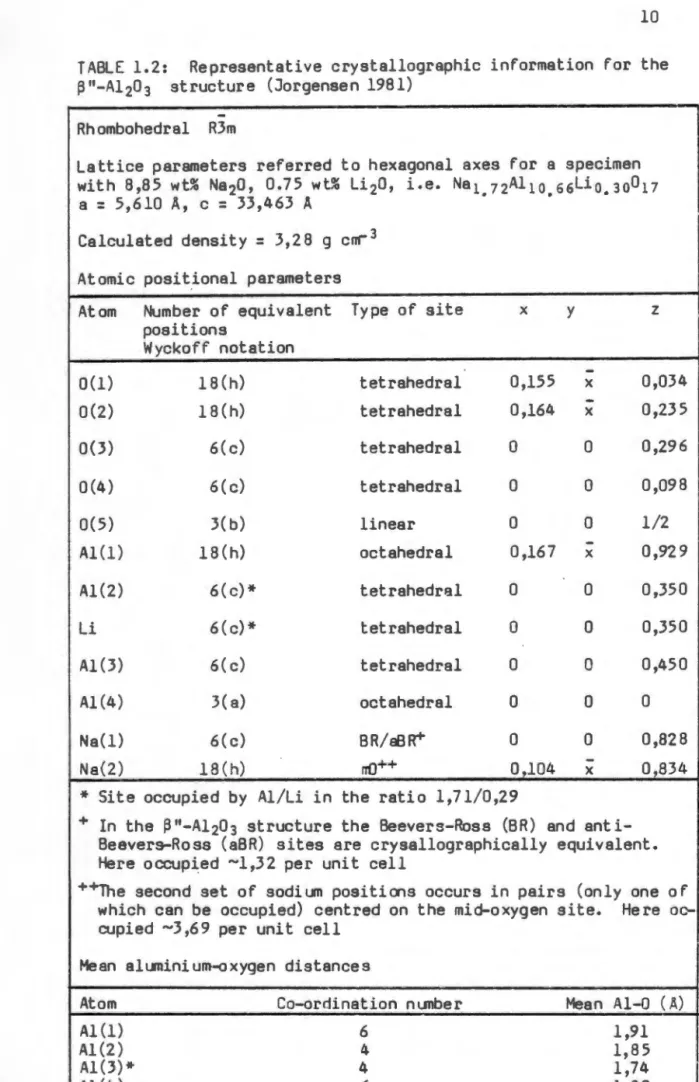
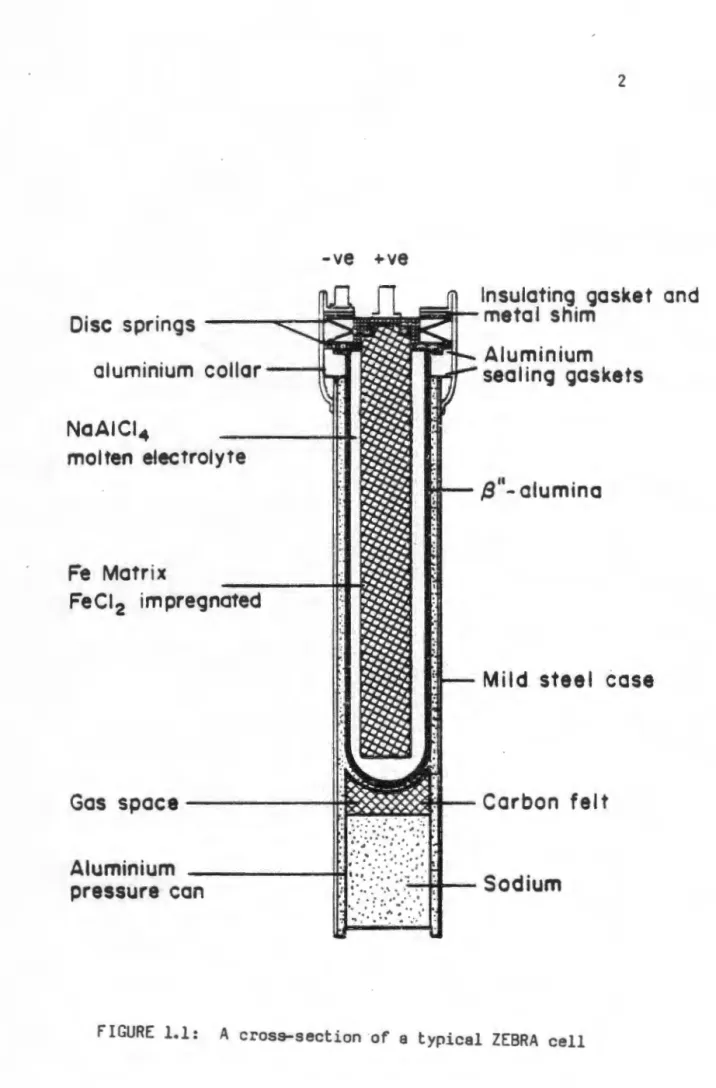
This led to the inclusion of a foreign cation in the name of the material, such as sodium beta alumina or silver beta alumina. The idealized unit cell structure of p"-Al 203 is shown in perspective in the figure. This property of the material makes it an ideal solid electrolyte for high-temperature batteries.
Q OxyQen
PHASE RELATIONSHIPS
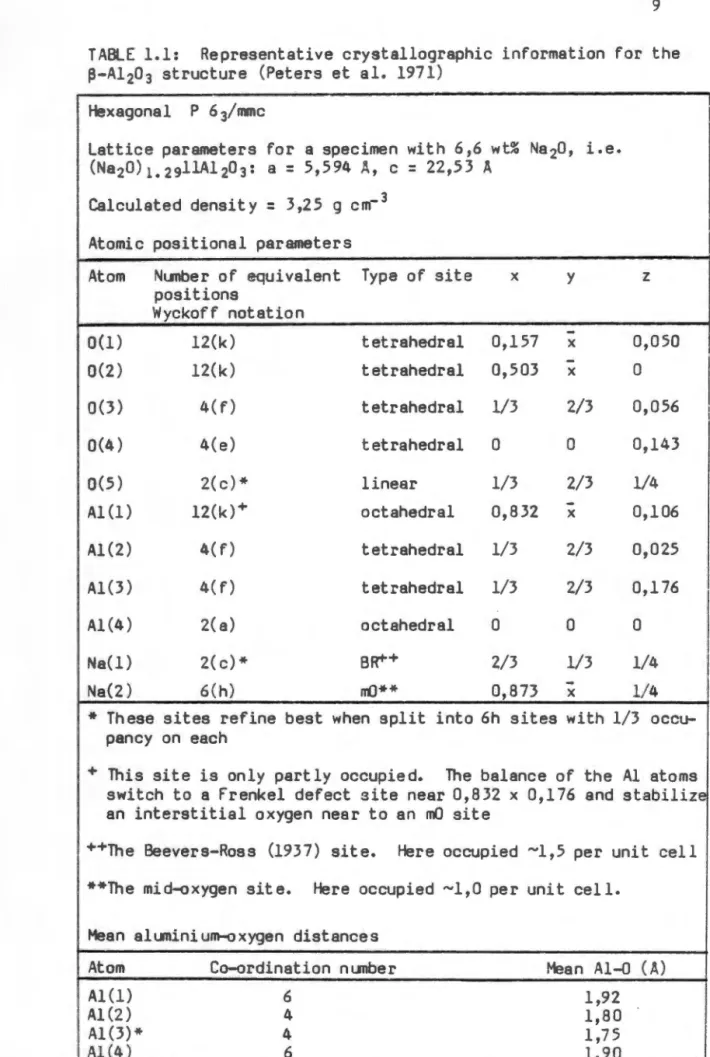
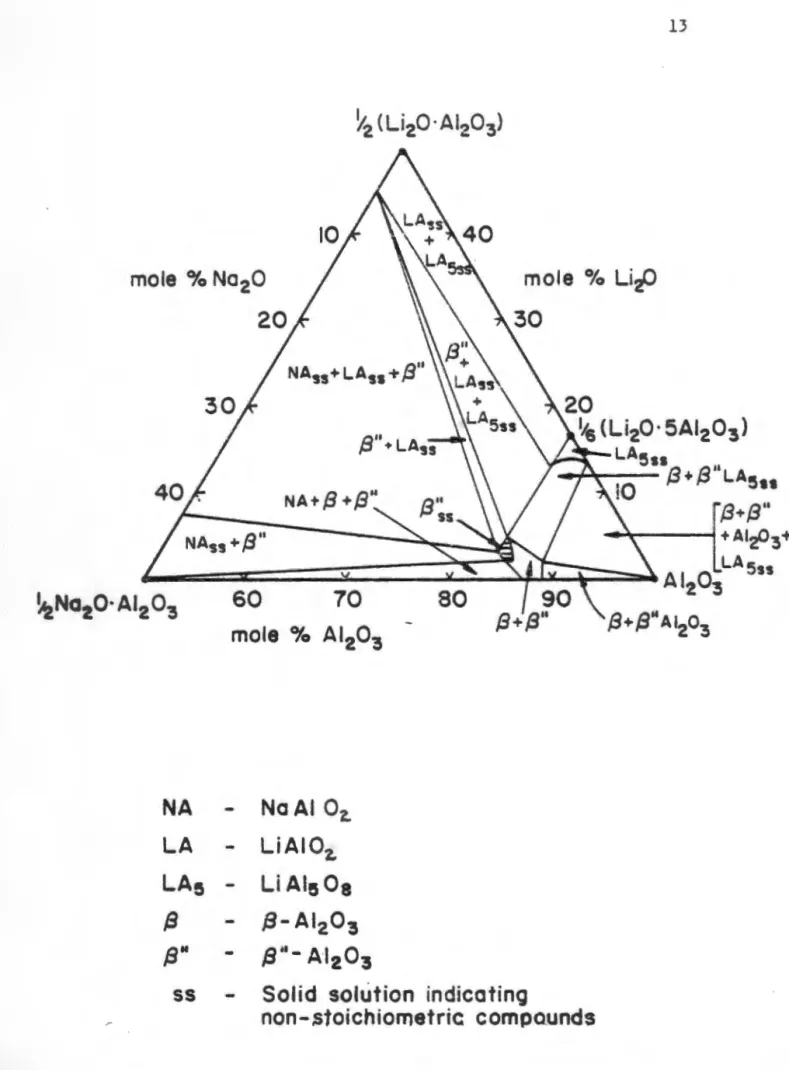
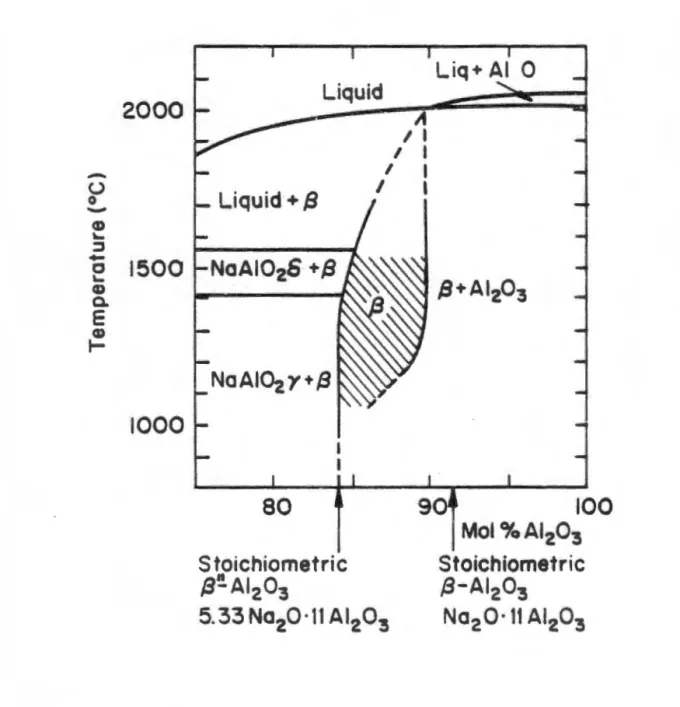
A comprehensive phase diagram for the Na2 O-Al2 O3 system, based on previous literature, was proposed by De Vries and Roth (1969). Subsequent phase diagrams for the binary system have been published by Weber and Venera (1970) and by Le Cars et al. These dopants replace the tetrahedrally coordinated aluminum urn at location Al(Z) (see Table 1.2) and occupy this location with less stress than the smaller aluminum ion.
- THE FABRICATION OF BETA ALUMINA TUBES
Beta alumina powder was prepared by mechanically mixing cx-Al2O3 with sodium salts and stabilizing dopants such as magnesium or lithium, which were then reacted into a powder of the desired composition. This process begins with the preparation of zeta LiA1 508 spinel by the solid-state reaction of cx-Al2O3 and the corresponding Li salt. the spinel is then ball milled with sodium salt and a-Al 20 to produce beta alumina powder of the desired composition. Beta alumina tubes have been formed by dry bag isostatic compression [Deplanches (1980)], extrusion [Pettet al.
CHAPTER 2
- INTRODUCTION
- CLASSIFICATION OF ALUMINIUM HYDROXIDES
- PHASE RELATIONSHIPS IN THE ALUMINA - WATER SYSTEM
- THERMAL DEHYDROXYLATION AND SUBSEQUENT REORDERING OF ALUMI- NIUM HYDROXIDES
- BETA ALUMINA CRYSTALLIZATION PATHWAYS
- SYNTHESIS OF HIGH PURITY ALUMINIUM HYDROXIDE MATERIALS
- RAW MATERIAL CHARACTERIZATION
The reaction product formed on the surface of the aluminum metal consists of "X-ray indifferent" material and pseudoboehmite i.e. the poorly crystallized hydrated form. The mutual stability relationships of the aluminum trihydroxides can be explained from a structural point of view. When the aluminum hydroxides are heated, more than 98 percent of the lattice water is removed below 600 °C.
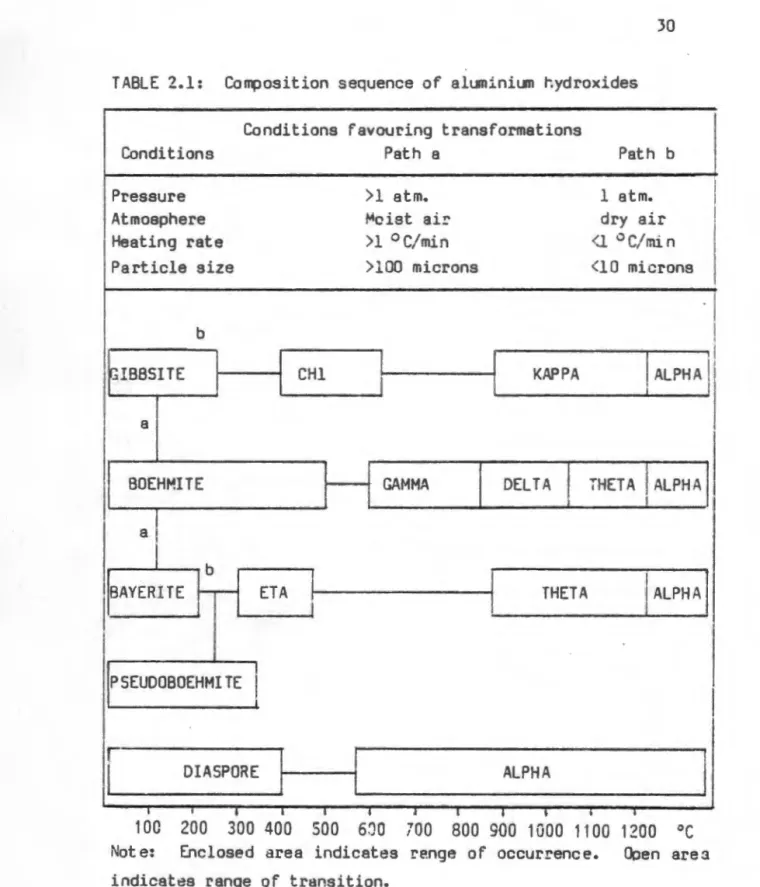
Several variables have been reported to affect the nature of the structural transformations of the aluminum hydroxides when heated. The transformations are affected by the pressure, atmosphere, heating rate and particle size of the starting material. The dehydroxylation and structural transformations of the aluminum hydroxides were reported in detail by Goton (1955) and Lippins (1961) (well crystallized boehmite), Abrams and Louw (1969) (pseudoboehmite), Torkar and Egghardt (1961) (bayerite) and Eyrand (1965) ) (gibsite).
Krischner (1971) categorized the dehydroxylation products (transitional aluminas) of the aluminum hydroxides according to the oxygen stacking order of the materials. In none of the above cases were XRD peaks corresponding to the lithium-rich phase observed. The intergrown ~/~"-Al20 3 structure is characterized by the fact that a powder XRD pattern of the.
Moreover, no systematic study of the synthesis of beta-aluminum from a wide range of aluminum hydroxides has been reported. This study aimed to characterize the dehydroxylation behavior of the materials and determine their rearrangement sequences. Pseudoboehmite shows broad bands coinciding with strong reflections of well-crystallized hydrothermal boehmite.
120 HYDROTHERMAL BOEHMITE
PSEUOOBOEHMITE
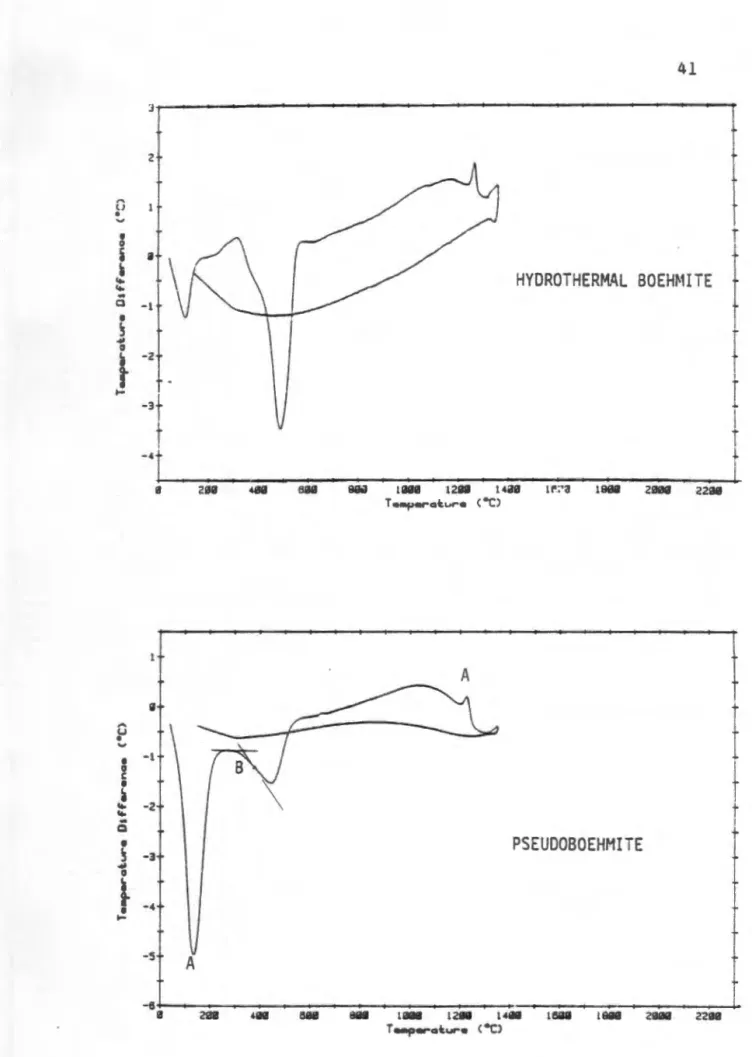
Hydrothermal boehmite showed a large dehydroxylation endotherm with an initial temperature of 4 30 ° C and an action peak of 500 ° C. A sharp exotherm with an initial temperature of 1240 ° C and a peak position of 1270 ° C indicates the shear transformation of cubic aluminum transition to hexagonally packed a-Al 203.• After cooling, we have no thermal event! observed. Around 700 oc traces of 0-Al 20 3 appeared while at 800 °C most of the material had converted to o-Al 203 with traces of y aluminum remaining.
At C, the material contained mostly 6-Al 203 and 0-Al 20 3. The identification of the phases by XRD at these temperatures is complicated by the diffuse nature cf. the XRD spectra of the various transition alumina phases. all the material had been converted to a-Al 20 3•. The differences in dehydroxylation temperature and temperature of transformation to α-Al 203 of boehmite and pseudoboehmite can be explained in terms of the relative order of these materials and their dehydroxylation products. The disturbance in the accommodation of protons and OH groups implies lower bond strengths for the protons and OH groups and thus a lower dehydroxylation temperature, as was observed. The increased d-spacing for pseudoboehmite also implies less resistance to diffusion for protons and water, resulting in a lowering of the temperature of the dehydroxylation endotherm.
The lower initial temperature and smaller intensity of the pseudoboehmite exotherm show that less energy is required to convert the material to a-Al 2D3. • The lower energy requirement for this shear transformation can be explained by suggesting that the pseudoboehmite dehydroxylation product has smaller domains of order than the boehmite dehydroxylation product. The domains of order in the dehydroxylation products of boehr.li te and pseudoboehmite are illustrated by TEM micrographs shown in figure. These photomicrographs show that hydrothermal boehmite contains substantial domains of long-range order, while pseudoboehmite consists of a fine fibrous network. of particles.
The gradual mass loss over a wide temperature range in the case of pseudoboehmite material indicates the greater mobility of the lattice water in this material and the lower diffusion resistance caused by the smaller crystallite domain size and greater disorder.
TRANSITION ALUMINA DERIVED FROM BOEHMITE
TRANSITION ALUMINA DERIVED DERIVED FROM PSEUDOBOEHMITE
RESULTS
The results of the DTA and X-ray diffraction studies of each material are discussed separately. a-Al 203 containing a reaction mixture was used to contrast the known crystallization pathway [Sugden and Duncan (1979)]. The last thermal event in the heating of the reaction mixture is an endotherm with a peak position of 780 °C.
Since there is no significant mass loss associated with this event, it is assumed to be due to the partial transformation of a-Al 203 to . The results of the equilibrium reaction in sol condition at 600 °C showed that some of the α-alumina was converted to socialuminate. The high temperature y-NaAlO 2 phase was represented at room temperature as p-NaAlO 2 .6H 2 O instead.
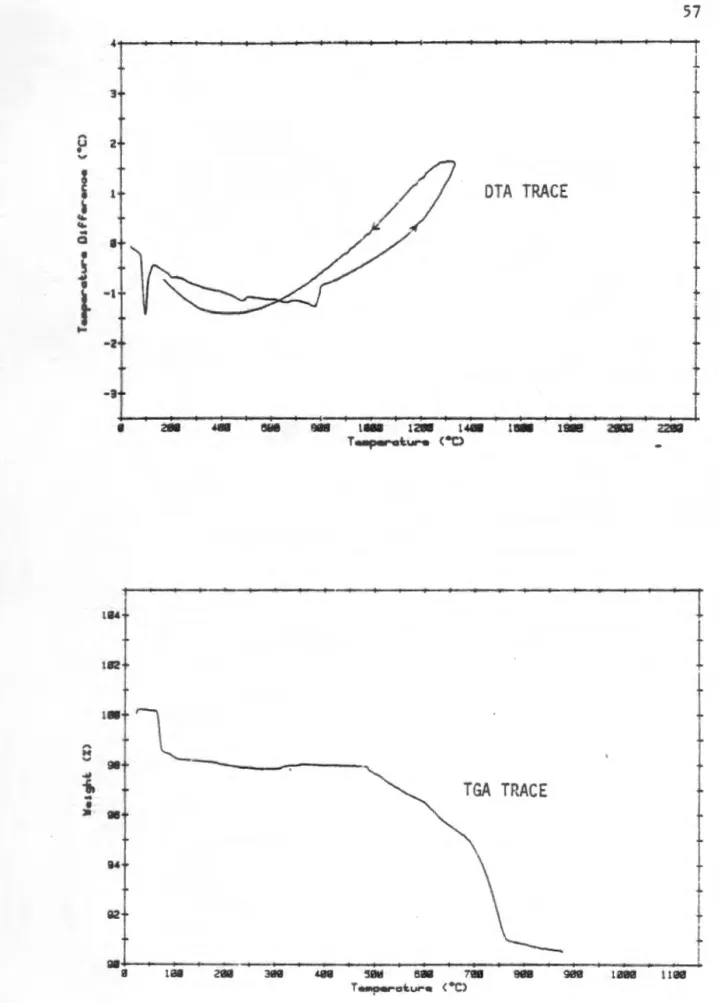
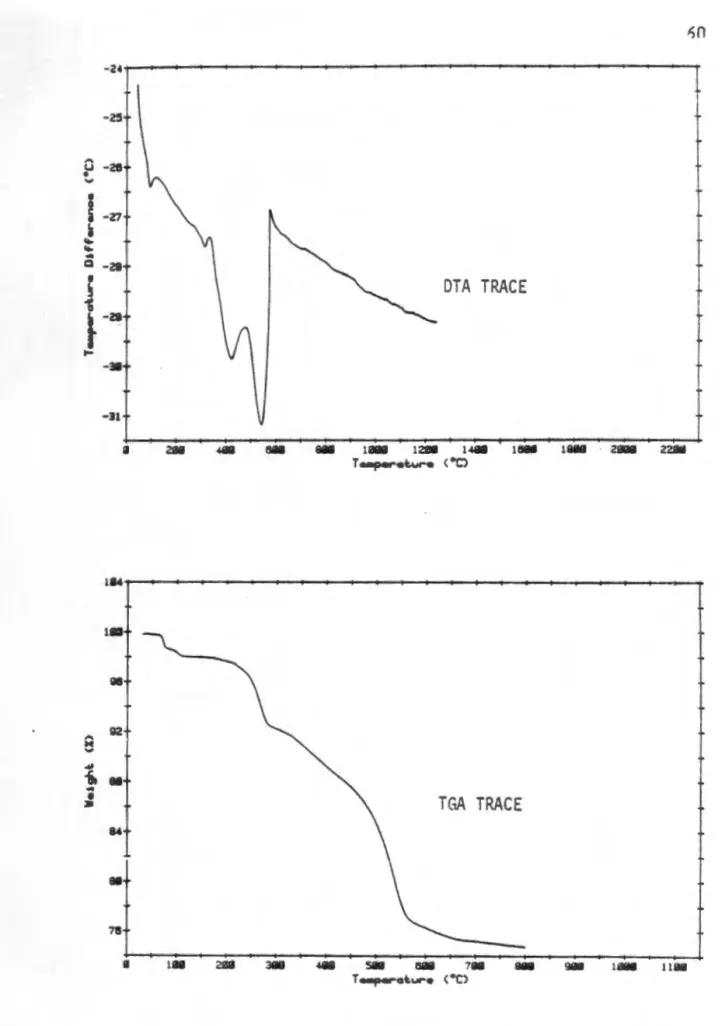
The presence of p-NaAlO 2.6H 2 O is explained by the large surface area of the reaction products, which are readily hydrated by moisture upon cooling. The appearance of ~-NaAlO2 at higher reaction temperatures is explained by the reduction of the surface area of the reaction product due to grain growth. The DTA and TGA traces of the hydrothermal boehmite, Na2CO3 and Li2Co3 reaction mixture are shown in Fig.
In addition to the characteristic strong endothermic decomposition of boehmite with a peak position at about 450 °C, two weaker endotherms are observed at 300 and LI.OO °C, respectively.
TWO 8
The absence of an endotherm of the decomposition of lithi ur.1 carbonate cannot be confirmed due to the main dehydroxylation event at 500 °C. The TGA trace supports the assumption of steam solvation of the carbonate salts, showing that most of the mass loss occurs below 650 °C. In other reaction mixtures of aluminum hydroxide, it is assumed that the same solvation reaction of carbonate salts with steam will occur.
The pattern of the sodium and lithium containing material had an additional peak in the vicinity of 3:ZO 20 while the 400 and 440 spinel peaks were shifted to lower angular values, indicating an increased d-spacing for these reflections. This broad peak can be attributed to the reaction of the sodium and lithium carbonate salts with steam as described earlier. The change in the position and shape of these carbonate reaction peaks corrected. to the hydrothermal boehmite system, can be attributed to lower temperature crystallization water loss from the pseudoboehmite material.
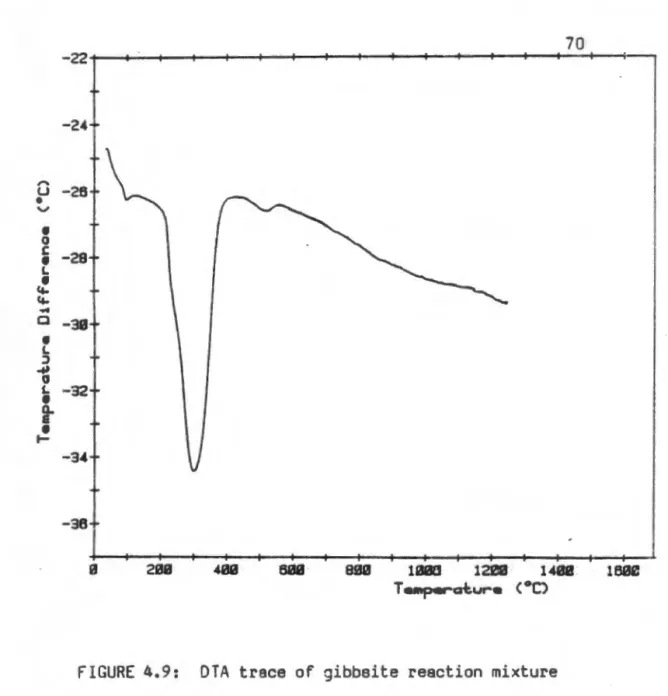
Two unidentified broad reflections appeared in 28 regions 32 and 370, but the quality of the X-ray data was too poor. The phase evolution of the intercalated β-alumina product obtained from pseudoboehmite is presented in Fig. The DTA trace of the bayerite reaction mixture (Figure 4.7) showed a loss of surface water just above 100 °C.
This indicates that, in the meantime, the reconstructive transformation of the oxygen subnet of the dehydroxylation product of bayerite does not occur.
4SS eea eea 1eaa 1200 uae 1639
TWO 9
- DISCUSSION
- CONCLUSIONS
This indicates that a larger proportion of the ~"-Al 20 3 phase is present than in the case of pseudoboehmite growths. The formation of ~ and ~'~Al203 from this precursor requires a reconstructive transformation of the oxygen sublattice. The cubic n- Al203 formed by dehydroxylation of bayerite does not retain octahedral occupancy of aluminum, resulting in a cubic spinel lattice.
The underlined layer of oxygen ions represents the conduction plane, which contains one-third of the oxygen ions of the close-packed layers. Similarity in stacking order of the y edge oxygen sublattice n-Al203 with. In the proposed mechanism, the function of lithium is to 'fix' the cubic oxygen sublattice, which is formed after hydroxylation.
Some of the transition aluminum is converted to a-Al20 3 since lithium is not present to stabilize the closely packed cubic oxygen sub-lattice. As in the case of their- and n- Al 203, sodium is deposited on the grain surface. Due to the higher density of faults and irregularities in transition alumina derived from pseudoboehmite and gibbsite, sodium dif-.
It is suggested that lithium ions diffuse into the oxygen sublattice of the transition aluminum and replace hydrogen ions by ion exchange.
CHAPTER 5
An ingrown ~/~"-Al2 O3 polytype results from the reaction of sodium ions with either a disordered alumina matrix or a ~-oxygen sublattice [ ••• ABAC ABAC ••• ] in which the hydrogen ions have been exchanged for lithium ions. Clear biphasic ~- and ~"-Al 2 O3 are the result of the reconstructive transformation of either an α-oxygen sublattice [AB AB] or by the reaction of sodium with unstabilized aluminum. The conclusions have an important impact on the raw material choice for the production of p"-Al 203 ceramic tubes.
Hydrothermal boehmite of sufficient purity is a cheaper raw material than high purity a-Al 203 and will significantly reduce production costs. Pure ~ "-Al 203 is formed when an ordered oxygen sublattice reacts with lithium and sodium salts. Further studies of aluminum urn-containing precursors that produce an ordered oxygen sublattice need to be performed.
The arguments in this thesis were based on the nature of oxygen sublation and the role of lithium. Techniques must be developed which would enable the establishment of crystallographic relationships between transition aluminum and the end products of beta aluminum. For example, .!.ow kV TEH techniques may hold some promise even though initial work has shown problems in thinning dust residues.
Duncan, J H, Solid electrolyte production, in The Sodium Sulphur Battery, (eds J Sudworth & R Tilley) Chapman Hall, Londen, 198 5.