PCST Public Communication of Science and Technology (South Africa) PIASA Pharmaceutical Industry Association of South Africa. This report is the joint work of a 13-member study panel appointed by the Council of the Academy of Science of South Africa (ASSAf).
More than half of the total expenditure on clinical research is made by the private (business) sector. In Chapter 11, the final chapter, we provide an overview of what we believe are the barriers to reinvigorating clinical research in South Africa:
An example of such an initiative is the partnership of the Health Research Capacity Building Initiative in Kenya and Malawi.
National Strategic planning, Regulation and coordination of clinical Research
Promoting an academic career path with clinical plus research (lectorates and... lectureships) in all clinical disciplines in South African institutions;
National Funding Scheme for clinical and health Research
Monitoring and Evaluation of the clinical and health Research Enterprise
INTRODUCTION
PERSPECTIVES ON CLINICAL RESEARCH
From an epidemiological perspective, clinical research is seen as the application of the methods and techniques of epidemiology (a population science) to clinical decision making. The classification of clinical research provided by Grimes and Schultz (2002) provides further insight into the epidemiological paradigm (see Figure 1.1).
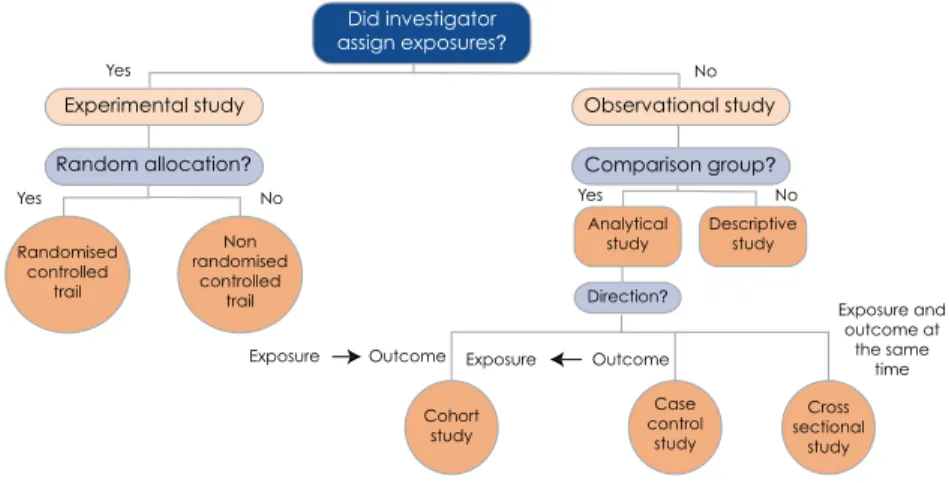
CLINICAL RESEARCH DEFINED
The UK Medical Research Council has also provided a conceptual model for understanding the links between clinical research and other forms of medical research (Figure 1.2) (Sutton, 2004). When treatment effects are inferred from observational clinical research findings rather than systematic clinical experience, participant selection biases and confounding factors may still result in false conclusions.
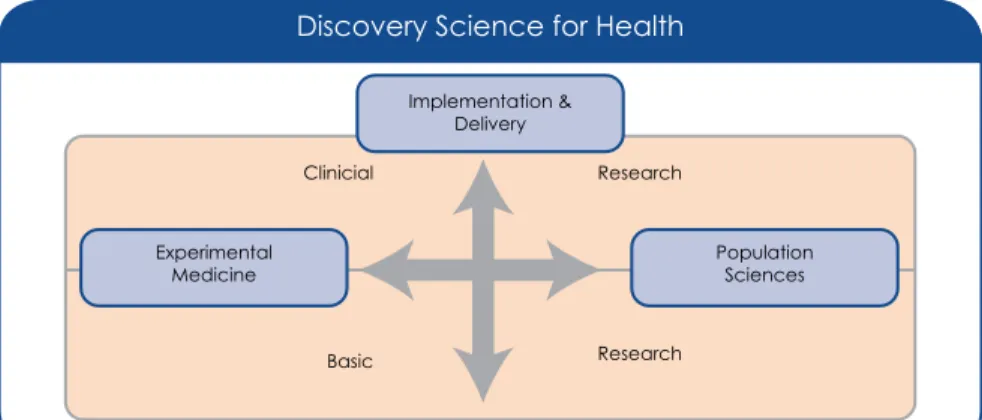
BEYOND CLINICAL TRIALS
An important consideration in evaluating the effects of treatment is that participants in comparison groups are sufficiently matched at baseline so that any differences in outcomes can be confidently attributed to treatment rather than to other factors. They are widely promoted by the international Cochrane Collaboration, a non-profit, independent organization (http://www.cochrane.org), dedicated to making up-to-date and reliable information on the effects of health care readily available worldwide.
FINDINGS
In the 19th and early 20th centuries, South African medical research was also often explicitly racist (Dubow, 1995). The demographics of the medical profession (and consequently medical researchers) in South Africa ie.
TWENTIETH-CENTURY SOUTH AFRICAN MEDICAL RESEARCH
Building on trends in the WHO to public health, a strong critique of hospital-based medicine has developed in South Africa (Niewijk, 1999). The distribution of trials conducted does not match the disease burden in the country.
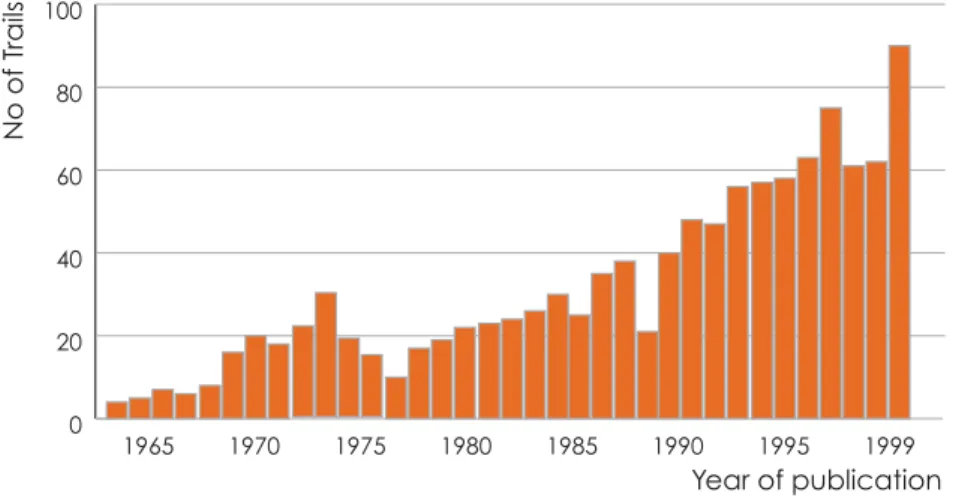
CONCLUSIONS
From the beginning of the 20th century, medical researchers in South Africa began to develop a strong scientific base that could be used to build clinical research in the future. Clinical research should actively contribute to improving the health of the nation by actively addressing the greatest disease burdens.
Public Health Then and Now: South Africa's Early Experiment in Social Medicine: Its Pioneers and Politics. White Plague, Black Labour: Tuberculosis and the Political Economy of Health and Disease in South Africa.
DEFINITIONS
An example of the latter is expert panels in the UK, which engage randomly selected members of the public on television programs and in invited correspondence with all participants, including representatives of vocal and/or self-conscious minorities.
DEVELOPING A PRODUCTIVE CULTURE OF CLINICAL RESEARCH
A STUDY OF CLINICAL RESEARCH AND RELATED EDUCATION IN SOUTH AFRICA. through round table discussions or task teams, while special methods could be followed at the general public level. A more accurate alignment of risks with benefits can create a prevailing 'culture' that promotes both clinical research and its positive impact on population health.
THE PRESENT SITUATION
The revitalization and repositioning of clinical research in South Africa can help develop new or more effective treatments for the health problems facing our population. Creating a solid and efficient clinical research infrastructure in South Africa can help attract foreign direct investment to the local economy.
AN AGENDA FOR REVITALISING CLINICAL RESEARCH
Much of the rest of this report expands on the high-level positions the Panel has taken on the narrowly focused, sectoral aspects of a national culture, “in which clinical research is seen as essential and clinical trials are widely accepted and promoted as the most reliable basis for determining the efficacy and safety of new therapies and approaches.”
NATIONAL CULTURE
Developing a national culture that supports clinical research includes gaining greater public support for and participation in research. Not enough attention has been paid to understanding and improving public understanding of clinical research in South Africa.
REGULATION
Public concerns about clinical research can hinder research recruitment, affect adherence to interventions, and even threaten the continuation of research projects (Geissler, 2005; Molyneux, et al. Geissler and Pool (2006) suggest that rumors about clinical investigators who engage in blood and organ trafficking, the deliberate spread of disease and clandestine birth control are widespread across Africa and pose a potential threat to public support and participation in clinical research.
PUBLISHING
EDUCATION
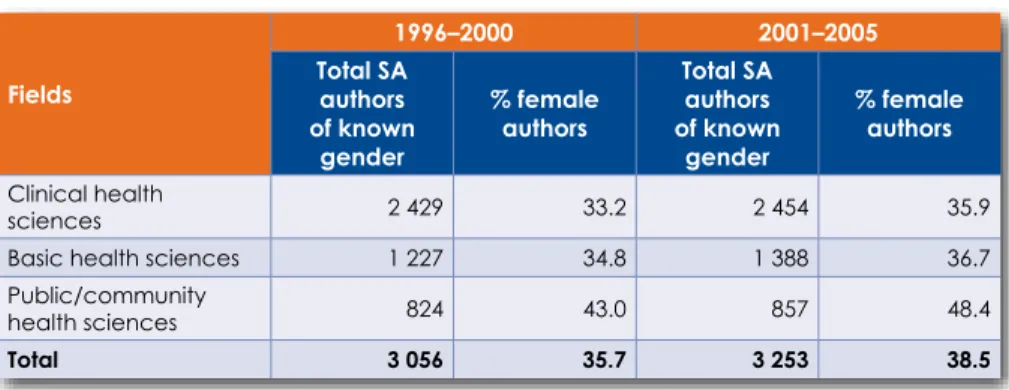
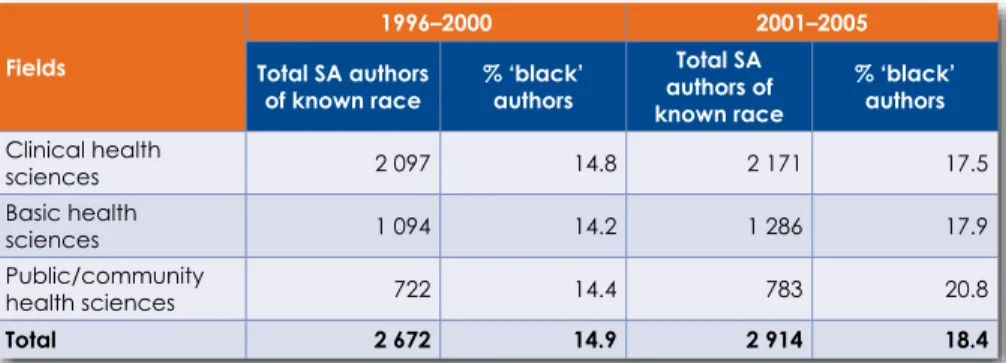
South Africa has not fully reversed the historical legacy of race and gender discrimination in clinical medicine, nor the institutional and regional imbalances in research capacity inherited from the past. Although more women and Black authors are publishing in the field of clinical medicine than before, we have not yet achieved racial or gender parity in terms of publication outcomes and demographics of physicians and researchers.
FUNDING
COORDINATION
Raising the status of clinical research both within the wider field of scientific research and within government programs that fund science;. Creating a strong public service and moral benefit, based on better programs that promote public engagement with clinical science and better risk-benefit analyzes that inform prioritization for clinical research in the country;.
How can fostering better public engagement with science in general promote a national culture that supports clinical research? Public perceptions of medical research and science in general will influence their participation in and support of clinical trials and clinical research.
PUBLIC UNDERSTANDING OF SCIENCE
Studies of school performance in science indicate that the percentage of final-year school passes in mathematics and physical science higher grade decreased slightly during the period, while pass rates in the standard grade in these subjects rose somewhat. Information about water quality or sexual behavior provided by government agencies in the rural area sampled was not always acceptable to or trusted by community members.
PUBLIC PARTICIPATION IN CLINICAL TRIALS
They are members of the International Network on Public Communication of Science and Technology (PCST). The plan is committed to promoting science and technology in achieving the Millennium Development Goals (MDGs), and three of the eight goals are health-related (child health, maternal mortality, HIV/AIDS, malaria and other diseases) ( African Union and NEPAD, 2006).
WHAT STILL NEEDS TO BE DONE
The Strategic Plan for Patient and Public Involvement aims to increase patient and public involvement in clinical research at a strategic level, improve public understanding and trust in clinical research and develop the sustainability of patient and public involvement in the UK (http://www .ukcrc.org). . To promote health and science journalism and effective interfaces between the media and the clinical research community.
UK Clinical Research Collaboration, Patient and Public Involvement Strategic Plan available at: http://www.ukcrc.org/pdf/UKCRC%20PPI%20Strategic%20Plan%2&%2Report%2April08. Addressing the challenges presented at various levels in research in South Africa would help foster the flourishing of clinical research.
THE SOUTH AFRICAN LAW GOVERNING CLINICAL RESEARCH
Chapter 9 of the National Health Care Act contains section 69 which provides for the establishment of a National Health Research Council. In accordance with the law, this committee is authorized to define the country's research priorities (Article 70).
ETHICS GOVERNANCE: REC COMPOSITION, FUNCTIONS, WORKLOADS AND FUNDING
None of the RECs in Africa require gender balance, although all consciously include women (Kass et al., 2007), with the exception of South Africa, which has regulations stating that no more than 70% must be gay (Article 4.1, Ethical guidelines). , 2004). In South Africa, the lack of diversity of expertise in RECs can be attributed to the nature of the faculty community in which most of these committees are (Moodley and Myer, 2007).
FUNCTIONS OF RECS, APPLICATION AND APPEAL PROCESSES
Multicenter research projects face additional problems, which include multiple review processes, and additional costs and delays associated with administrative errors (Green et al., 2006). Additionally, the ability of RECs to effectively review protocols and monitor approved research is at risk (Milford et al., 2006).
ETHICAL PUBLICATION
It is obviously essential that the ethics of research are monitored to protect both the participants and the clinical research enterprise itself. Addressing the challenges at different levels in research in South Africa will help clinical research flourish.
What key problems in South African clinical research can be identified from an analysis of published results. What specific interventions will best promote the overall productivity of clinical research in terms of quality and quantity.
SOUTH AFRICA’S PUBLICATION RECORD IN CLINICAL mEDICINE
In terms of the proportion of articles, by broad subject area that appeared in overseas ISI journals (ISI & Non-SA), local ISI journals (ISI & SA) and local journals not in ISI (Non-ISI & SA), respectively, only 38.5% articles were published in South African journals (Table 6.3). The proportion of articles in overseas ISI journals in the field of clinical health sciences has grown.
CONCLUSION: CREATING A VIBRANT LOCAL CULTURE OF CLINICAL RESEARCH
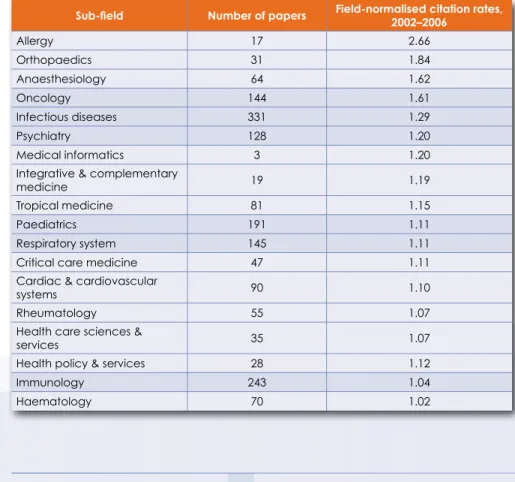
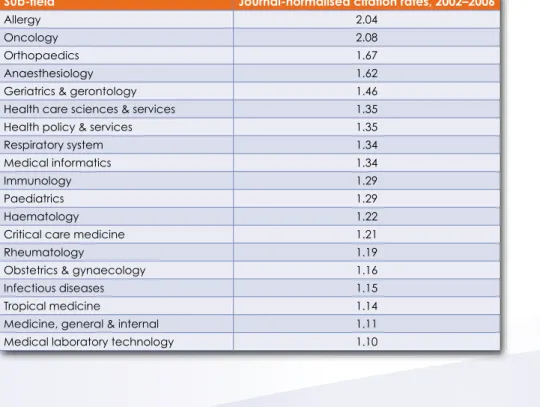
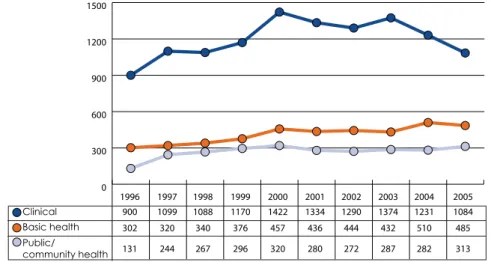
Clinical research has formed an important part of South Africa's scientific output in terms of quality and quantity. Although the number of clinical medical journal articles has declined since 2003, nearly half of the fields of clinical research recorded above average field-normalized and journal-normalized citation rates for the period 2002 to 2006.
How do we deal with the decreasing size and increasing age of the workforce actively involved in clinical research? How do we deal with the lack of effective training programs and the unattractive career path in the clinical research sector?
SHRINKING SIZE AND AGEING OF THE SCIENCE RESEARCH WORKFORCE
There are several factors that may be responsible for the declining number of outputs in clinical publications from South Africa. The two discussed below are (1) the declining size of the health research workforce and (2), the absence of effective training programs and appropriate career paths for clinical researchers in South Africa.
ABSENCE OF A NATIONAL PLAN FOR THE EDUCATION, TRAINING AND EmPLOYmENT OF CLINICAL RESEARCHERS
The mechanism's distribution of clinical training funds (R500m in 2008/09 and 2009/10) proposed by the official working group was based on a simple formula. These weightings were applied to the number of students in 2006 for the programs that qualify for clinical training funding.
STATUS OF CLINICAL TEACHING AT UNDERGRADUATE LEVEL
Clinical trials are treated in all clinical blocks as 'Evidence-based medicine' (another 'golden thread' in the curriculum). At the University of the Witwatersrand, third and fourth year medical students are exposed to various aspects of research through lectures and thematic sessions.
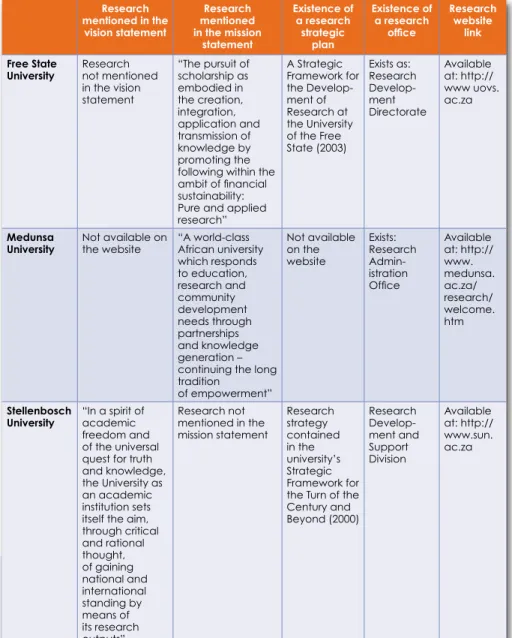
SUPPORT STRUCTURES FOR RESEARCH WITHIN INSTITUTIONS
Research strategy contained in the university's Strategic Framework for the Century and Beyond (2000). Research that is not mentioned in the university's vision, but mentioned in the health sciences faculty's vision (Strategic plan 2008).
RESEARCH ETHICS TRAINING IN SOUTH AFRICA
While research is mentioned in only three of the eight universities' vision statements, it was identified as an important mission in all but one university. Six of the eight universities had a strategic plan specifically for research or research was included in the strategic plan available on their websites.
INTERNATIONAL POLICY AND PLANNING INITIATIVES THAT SEEK TO ADDRESS THE PROBLEm
Correlated degree effects were larger in medical schools where a relatively small proportion of students received the degree; differences between medical schools are more easily explained by resource dilution (McManus et al., 1999). The clinical component of the curriculum is designed to equip students for a lifelong medical practice in a changing world with an emphasis on the acquisition of clinical skills with direct patient contact.