suppmat_1771. 1377KB Jun 05 2011 09:30:57 PM
Teks penuh
Gambar
Garis besar
Dokumen terkait
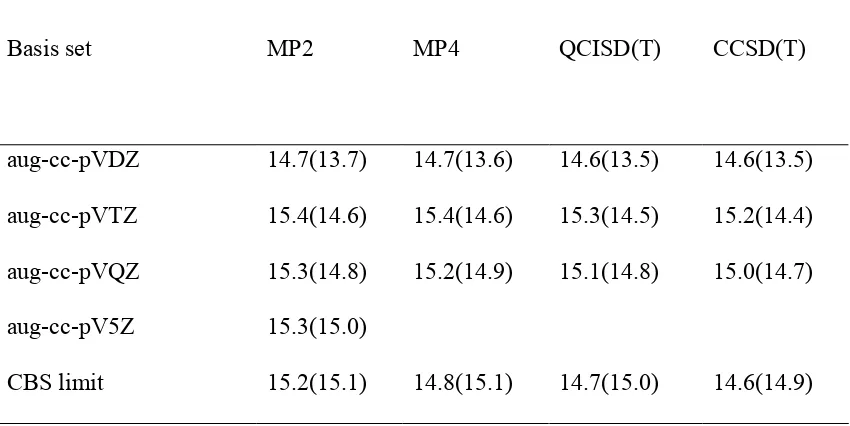
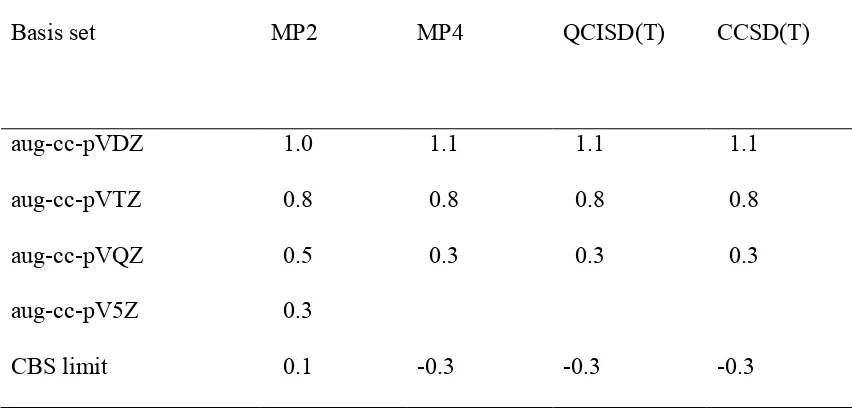
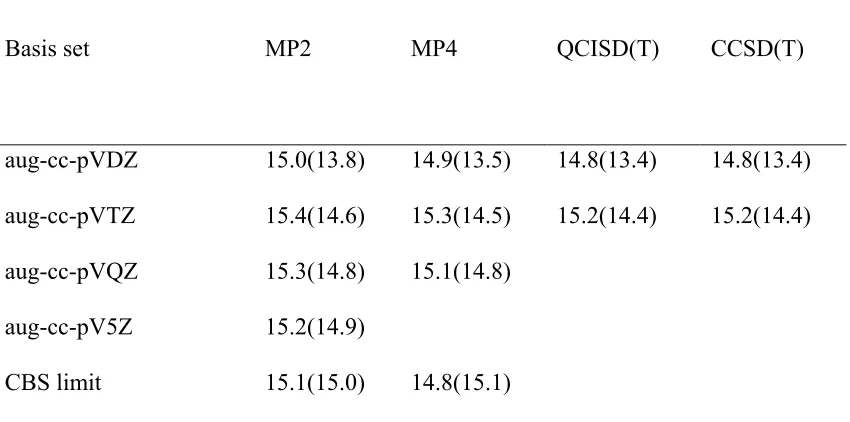
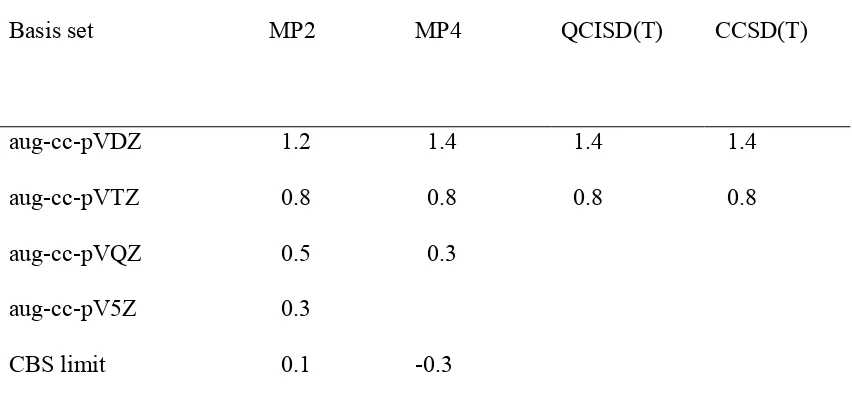
standard multiloop penalty α 2 , and unpaired bases that are inside the multiloop are given penalty α 3. The energy treatment for the exterior pseudoknot remains the same. Base
Conformational energy with soft empirical (red dashes lines) and MMFF94s van der Waals (blue dotted lines) “1-5,1-6” repulsion potential is calculated at different values of
The Chemistry of the Carbon-Carbon Triple Bond; John Wiley & Sons: New York, 1978; Vol... CODATA Key Values for
Vibrational Frequency Comparison with Ab Initio Values for Molecule 2... Normal Mode MM3 Ab Initio
poly funs. and poly funs.h, whih are also given in this supplement.. The programs are written in ANSI-ompliant C, and should
The following are documented Value Express bugs that can affect various calculations in the program. Each bug described below is followed by a reference to a fax that will correct
temperatures are to be converted to Fahrenheit temperatures according to the following formula: FAHRENHEIT= (9/5) CELSIUS +32One solution is as follows:.. MULTIPLY 9 BY CELSIUS
Identify reasons why depreciation methods are selected.Explain the accounting issues related to asset impairment.Explain the accounting procedures for the depletion of