ADDITIONAL NEST STRUCTURE AND FLOWER
CONSTANCY OF STINGLESS BEES
(HYMENOPTERA: APIDAE)
NORITA WIDYA PANGESTIKA
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
STATEMENT OF THESIS AND
INFORMATION SOURCES
I hereby declare that thesis entitled Additional Nest Structure and Flower Constancy of Stingless Bees (Hymenoptera: Apidae) is original result of my own research supervised by supervisory committee and has never been submitted in any form at any institution before. All information from other authors cited here are mentioned in the text and listed in the reference at the end part of the thesis.
I hereby assign copyright of my thesis to the Bogor Agricultural University.
Bogor, September 2016
Norita Widya Pangestika
RINGKASAN
NORITA WIDYA PANGESTIKA. Struktur Sarang Tambahan dan Flower Constancy pada Lebah Tak Bersengat (Hymenoptera: Apidae). Dibimbing oleh TRI ATMOWIDI dan SIH KAHONO.
Lebah tak bersengat (Hymenoptera: Apidae) tersebar luas di daerah tropik dan subtropik. Lebah ini mulai diternakkan oleh masyarakat karena dapat menghasilkan madu dan propolis. Keberadaan musuh alami dapat mengganggu koloni yang mempengaruhi struktur sarang dan produktivitas lebah tersebut. Tujuan penelitian ini untuk mempelajari struktur sarang umum dan struktur sarang tambahan pada lebah tak bersengat yang disebabkan oleh musuh alami. Penelitian ini juga bertujuan untuk menghitung jumlah polen yang menempel pada tubuh lebah dan flower constancy pada tiga spesies lebah tak bersengat. Penelitian ini dilakukan pada bulan Agustus sampai November 2015. Sampel lebah tak bersengat diambil dari tiga lokasi, yaitu Pasuruan (Jawa Timur), Tasikmalaya (Jawa Barat), dan Rangkasbitung (Banten). Analisis deskriptif digunakan untuk membandingkan struktur dan komposisi sarang pada bambu dan struktur sarang tambahan yang disebabkan oleh musuh alami. Pollen load dan flower constancy diukur pada tiga spesies lebah tak bersengat yaitu Heterotrigona itama, Lepidotrigona terminata, dan Tetragonula laeviceps. Analisis korelasi Pearson digunakan untuk menguji hubungan antara bobot tubuh individu lebah tak bersengat dengan jumlah polen yang dibawanya.
Struktur dan komposisi sarang lebah tak bersengat secara umum adalah saluran masuk sarang, pot pakan (madu dan polen), dan sel anakan. Koloni lebah tak bersengat dari Pasuruan dan Tasikmalaya tidak ditemukan adanya musuh alami dan tidak membuat struktur sarang tambahan. Koloni lebah tak bersengat dari Rangkasbitung ditemukan struktur sarang tambahan, seperti saluran masuk bagian dalam dilapisi dengan batumen dan cerumen yang tebal serta membuat struktur seperti akar. Musuh alami ditemukan pada koloni asal Rangkasbitung, yaitu kecoa (Blattodea: Blattidae), kumbang Platysoma leconti (Coleoptera: Histeridae), kumbang Carpophilus sp. (Coleoptera: Nitidulidae), dan semut Pheidole sp. (Hymenoptera: Formicidae). Koloni lebah tak bersengat yang bersarang di bawah tanah memiliki struktur berbentuk bulat seperti bola dengan sel anakan di bagian dasar dan dilapisi oleh pot pakan dan involucrum.
Flower constancy pada tiga spesies stingless bees diukur berdasarkan persentase tipe polen yang dibawa pada tubuh setiap individu. Spesies lebah tak bersengat H. itama membawa polen pada tubuhnya paling banyak (31392 butir polen), diikuti lebah L. terminata (23017 butir polen), dan T. laeviceps (8015 butir polen). Spesies ini juga menunjukkan perbedaan flower constancy. Lebah T. laeviceps menyukai bunga dari famili Poaceae (74.49%), L. terminata pada bunga Euphorbiaceae (80.46%), dan T. itama pada bunga Solanaceae (83.33%).
SUMMARY
NORITA WIDYA PANGESTIKA. Additional Nest Structure and Flower Constancy of Stingless Bees (Hymenoptera: Apidae). Supervised by TRI ATMOWIDI and SIH KAHONO.
Stingless bees (Hymenoptera: Apidae) are widely distributed in tropics and subtropics areas. Now these bees are farmed by the human because they produce honey and propolis. Natural enemies can disturb the colony that affects to nest structure and productivity of these bees. This study aimed to study the common nest structure and additional nest structure of stingless bees which caused by natural enemies. This study also aimed to analyze pollen loads and flower constancy of three species of stingless bees. This research was conducted from August to November 2015. Samples were taken from three locations, i.e, Pasuruan (East Java), Tasikmalaya (West Java), and Rangkasbitung (Banten). Descriptive analysis was used to compare the structure and composition of the nest in bamboo and additional nest structure in relation with natural enemies. Analyses of pollen loads and flower constancy were conducted in three species of stingless bees, i.e. Heterotrigona itama, Lepidotrigona terminata, and Tetragonula laeviceps. Pearson correlation test was used to examine the relationship between the weight of each individual of stingless bees and the number of pollens loads.
Generally, the structure and composition of the nest of stingless bees in bamboo consist of nest entrance, storage pots (honey and pollen), and brood cells. We didn’t found natural enemies in Pasuruan and Tasikmalaya. These colonies did not create additional nest structures. In contrast, the colonies in Rangkasbitung created additional nest structures i.e. the inner entrance covered by batumen and cerumen and also build a root-like structure. Natural enemies found in the colony of Rangkasbitung were cockroaches (Blattodea: Blattidae), histerid beetles, Platysoma leconti (Coleoptera: Histeridae), nitidulid beetles, Carphophilus sp. (Coleoptera: Nitidulidae), and ants, Pheidole sp. (Hymenoptera: Formicidae). An underground colony of stingless bees has a round ball-like nest structure with the brood cells at the base of the nest, surrounded by storage pots and involucrum.
Flower constancy in three species of stingless bees was measured based on percentage of the pollen type attached on the body of each individual. The pollen load of H. itama was highest (31392 pollen grains), followed by L. terminata (23017 pollen grains), and T. laeviceps (8015 pollen grains). These species also demonstrated differences in flower constancy. T. laeviceps showed their preference on Poaceae flowers (74.49%), L. terminata on Euphorbiaceae flowers (80.46%), and T. itama on Solanaceae flowers (83.33%).
Copyright @ 2016, Bogor Agricultural University
Copyright are protected by law
Prohibited to cite all or a part of this thesis without referring to and mentioning the source. Citation only permits to the purposes of education, research, scientific paper, report, or criticism writing only; and it does not defame the name and honor of Bogor Agricultural University.
ADDITIONAL NEST STRUCTURE AND FLOWER
CONSTANCY OF STINGLESS BEES
(HYMENOPTERA: APIDAE)
NORITA WIDYA PANGESTIKA
Thesis
as one of the requirements for achieving Master of Science
in
Animal Bioscience Program
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
PREFACE
Praise and gratitude to Allah SWT so that the thesis was successfully completed. I would like to express my appreciation to Dr Tri Atmowidi, MSi and Dr Sih Kahono, MSc as supervisors for their advice, knowledge, and supports during my research, as well as thanks to lecturer of Animal Biosciences Program. The author thanks, Indonesia endowment fund for education (LPDP) which has given scholarships for this research and zoology office and staff of Indonesian Institut of Science (LIPI) Cibinong for permission to conduct this research. Especially, the author can only say many thanks to Reggie Surya Ph.D for correction on writing, Expressions of thanks are also extended to Drs. Utomo and Masminah (my parents), Tyas Puji Pramesti S.Pd (sister) and Fais Arma Tricaraka S.IP (brother), Nanda Perdana Putra S.Pt, Dra. Erniwati, the team of BSH 2014 and all of my friends who always give help and support. Hopefully, this thesis could be useful.
Bogor, September 2016
TABLE OF CONTENT
LIST OF TABLE ix
LIST OF FIGURE x
LIST OF ENCLOSURE xi
1 GENERAL INTRODUCTION
Background 1
Aims of this study 1
Advantages of the research 2
2 ADDITIONAL NEST STRUCTURES ARISED AS A RESULT OF NATURAL ENEMIES OF STINGLESS BEES
Introduction 3
Materials and Methods 4
Results 5
Discussion 10
Conclusion 11
3 POLLEN LOAD AND FLOWER CONSTANCY OF STINGLESS BEES
Introduction 12
Materials and Methods 13
Results 14
Discussion 17
Conclusion 18
4 GENERAL DISCUSSION 19
REFERENCES 20
LIST OF TABLE
1 The common nest-structure (C) and additional nest-structure (A) of
Stingless bees species from each sampling area 6 2 The number of colony of stingless bees were attacked by natural enemies 9
LIST OF FIGURES
1.1 Problems chart of the research 2 1.2 The morphology of three species of stingless bees found in this study 5 1.3 The relationship between the nest structure and natural enemies of
Stingless bees colonies 6 1.4 The structure and composition of the cavity-nesting of stingless bees in
bamboo 7
1
GENERAL INTRODUCTION
Background
Stingless bees (Hymenoptera: Apidae) are eusocial bees that live in the colony, with an overlapping generation in the colony and cooperative brood care (Oldyord and Wongsiri 2006). Stingless bees possess a system of caste in the colony, consisting of reproductive castes (queens and males) and non-reproductive caste (workers) (Michener 2000). One colony has a queen, hundreds of drones and thousands of workers (Michener 2007). The queen sets the work system in the colony and lays eggs. The drones mate the queen, while the workers are assigned to forage outside the nest to take pollens and nectars as nutrient sources for colony, as well as to keep the colony from predators and parasites.
Stingless bees are found in the tropics and subtropics areas (Free 1993). Approximately, 200 species of stingless bees have been identified, 50 species of them are found in Southeast Asia (Inoue et al. 1985). Stingless bees are also widely distributed in Indonesia i.e., 31 species in Kalimantan, 41 species in Sumatra, and 9 species in Java (Schwarz 1937). Sakagami et al. (1990) reported six species of stingless bees found in Java, those are Tetragonula laeviceps, Heterotrigona itama, Tetragonula drescheri, Tetrigona apicalis, Geniotrigona thoracica, and Lepidotrigona terminata. SubgeneraHomotrigona, Lepidotrigona, and Heterotrigona are endemic for the tropics and subtropics areas in Asia (Michener 2000).
These bees have local name i.e., teuweul (West Java), klanceng (Central Java), lanceng (East Java), galo-galo (Sumatera), and kelulut (Kalimantan). The nests of stingless bees are found in the trunks of living trees, dead trees, brick walls and crevices of rocks (Chinh et al. 2005). Stingless bees possess economic value since they produce honey and propolis. In addition, these bees are more adaptive to the environment and generalize on nutrient sources (Michener 2000). These bees also contribute to the ecosystem by helping the process of pollination of wild plants and agricultural crops. But, the effectiveness of stingless bees as pollinators of agricultural crops have not been investigated completely (Heard 1999). Moreover, natural enemies, like predators and parasites can reduce the productivity of bees, disturb the colony, as well as affect the structure and composition of the nest, or even kill the members of colony.
Advantages of the Research
1. Data of bee preferences on plants (flower constancy) can be used by beekeepers to select plants as resources.
2. Data of flower constancy can be used to predict the potential of the species to pollination of plants.
3. Data of natural enemies that attacks the colony of stingless bees can be used to minimize its effects to the structure and composition of the nest.
Figure 1.1 Problems chart of the research.
Stingless bees Economical aspects: - produce
honey - propolis Ecological aspects:
- as pollinators - pollen load - flower constancy
3
ADDITIONAL NEST STRUCTURES ARISED AS A RESULT OF NATURAL ENEMIES OF STINGLESS BEES
INTRODUCTION Background
Honey bees and stingless bees are classified in the family of Apidae. Their nests located in the cavities. However, the structure and the composition of their nests are different. Honey bee (genus Apis) has three types of nesting, i.e., cavity-nesting with multi-combs (Apis mellifera and A. cerana), single comb nest outside by hanging or attached on the branch (Apis dorsata) and single comb nest on tree branches on dwarf honey bees (A. florea and A. andreniformis). Multi combs of cavity-nesting honeybees are built mainly by wax (Oldroyd and Wongsiri 2006).
Stingless bees have two types of nest i.e., cavity and base nests. Cavity nest is located on a trunk characterized by the outer entrance located on trunk hollows. This type of nests is usually built in a certain height. Base nest is usually located below or at the base of the tree characterized by outer entrance attached to the outer wall of the tree and is usually hidden (Nunes et al. 2011). These bees often built their nest in the hollow trunk as well as on termite and ants nests (Michener, 1974). Some of these species also build nests underground (Jalil and Shuib 2014). Nest of stingless bees is constructed mainly by resin. The arrangement of the cells in the nest of stingless bees consists of cluster and comb form (Michener 1974).
Stingless bees generally build their nests in tree holes (Buchwald and Breed 2005). Stingless bees also build their nest in tree trunks, wood, cracks in the walls or under the roof of residence (Kumar et al. 2012). These bees can be found in the forests, some types can also be adaptable in open forest areas, grasslands, and there is also in the settlement (Inoue et al. 1985).
Nest is an artificial construction used by bees to lay eggs, spend time, and nurture young bees. The nest of stingless bees consists of entrance, cerumen, batumen, involucrum, storage pots, and brood cells. Cerumen is a mixture of propolis (resin) and wax to construct storage pots and brood cells. Batumen consists of resin or wax as a layer of cavity nest (Jalil and Shuib 2014). Batumen and cerumen are used to protect the inner part of the hive. Involucrum is a layer around brood cells (Michener 2007).
Predators are animals that prey on other animals (Estes et al. 2001). Some predators attack the colony of stingless bees, i.e. wasps, ants, spiders, and centipedes. Centipedes may damage bee colonies within 2-5 seconds because they have a morphological variation and adaptation to take, catch, and hold prey (Kumar et al. 2012). Suicide bite is usually done by the bees as a defense mechanism against predators (Shackleton et al. 2015). Parasites are organisms that live on other organisms. The parasites commonly found in honey bees are mites, fleas, moths, and small beetles (Strauss et al. 2013). These parasites can be harmful to the health of honey bees (Shen et al. 2005).
Aims of the Study
The research aimed to study the natural enemies and additional nest structure of stingless bees related to natural enemies.
MATERIALS AND METHODS
Samples Collection
Collection of stingless bees colonies were taken from August to November 2015. Ten colonies were taken from Pasuruan (East Java), eleven colonies from Tasikmalaya (West Java), and nineteen colonies from Rangkasbitung (Banten province).
Observation of Nest Structure of Stingless bees
Each colony of stingless bees collected was observed and classified based on their caste systems. The structure and composition of the nest were observed i.e., the outer entrance, inner entrance channel, storage pots (honey and pollen), and brood cells. Each colony observed then was moved to wood box, sized 30 cm x 25 cm x 15 cm.
Identification of Stingless Bees and Natural Enemies
Description of samples collected of stingless bees from each area were conducted based on morphological study. Samples identification of stingless bees was conducted based on Sakagami (1990) and Michener (2007). Natural enemies were studied and collected from the stingless bees in bamboo. Afterward, both samples, stingless bees and their natural enemies were preserved in 70% ethanol. Samples were identified in the Laboratory of Entomology, Indonesian Institute of Sciences (LIPI) at Cibinong, Bogor.
Data Analysis
5 This species has two strong mandibular teeth, propodeum glabrous and shiny, and mesoscutellum backward exceeded propodeum. Species of T. moorei has two weak mandibular teeth, propodeum glabrous, and shiny, and mesoscutellum almost the same as propodeum. The species of stingless bees nesting in underground found in Tasikmalaya has not been identified (Figure 1.2).
Figure 1.2 The morphology of three species of stingless bees found in this study: T. laeviceps (a), T. moorei (b), Unidentified sp. (c). Scale = 5mm
A total of 27 colonies of T. laeviceps observed (81.48%) have a common nest structure and 18.52% have additional nest-structure. However, 75% of T. moorei have additional nest structure and 25% have a common nest-structure. A total 40 colonies found, 65% colonies have the common nest structure and the remaining (35%) with the additional nest structure (Table 1).
Table 1 The common nest-structure (C) and additional nest-structure (A) of stingless bees species from each sampling area.
Location of colony
Nest Structure
T. laeviceps T. moorei Unidentified sp.
Stingless bees colonies of Pasuruan and Tasikmalaya did not create the additional nest structure. Contrary, more colony of Rangkasbitung created additional nest structure (73.68%) and the remaining have common nest-structure (26.32%). Observation in the fields showed that natural enemies were not found in colonies of Pasuruan and Tasikmalaya. In contrast, more colonies of
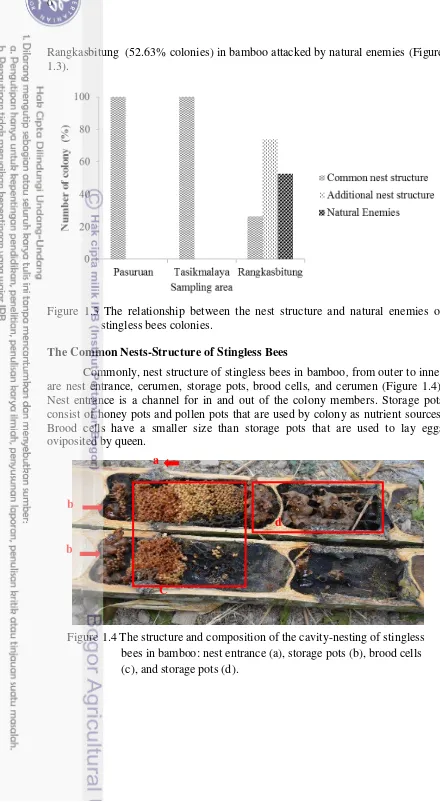
Rangkasbitung (52.63% colonies) in bamboo attacked by natural enemies (Figure 1.3).
Figure 1.3 The relationship between the nest structure and natural enemies of
…………..stingless bees colonies.
The Common Nests-Structure of Stingless Bees
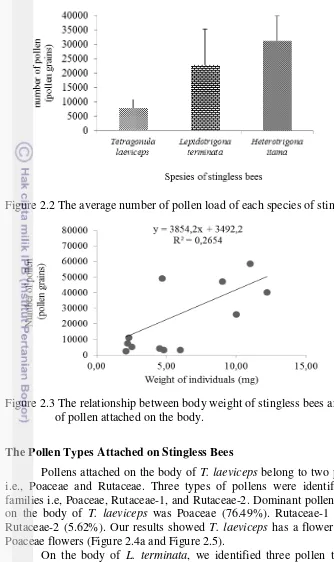
Commonly, nest structure of stingless bees in bamboo, from outer to inner are nest entrance, cerumen, storage pots, brood cells, and cerumen (Figure 1.4). Nest entrance is a channel for in and out of the colony members. Storage pots consist of honey pots and pollen pots that are used by colony as nutrient sources. Brood cells have a smaller size than storage pots that are used to lay eggs oviposited by queen.
Figure 1.4 The structure and composition of the cavity-nesting of stingless bees in bamboo: nest entrance (a), storage pots (b), brood cells (c), and storage pots (d).
a
b b
C
7
Stingless bee colony collected from the underground has blob shape with brood cells surrounded by involucrum (length=7 cm, diameter=13 cm). Outer entrance channel has about 3 cm in length. This nest located at 50-60 cm from the ground surface. Afterward, the colony was transferred to the wood box. In the wood box, this colony built a new nest with the same structure and composition like their previous nest that found underground (Figure 1.5).
Figure 1.5 The structure and composition of nest of stingless bee found in the ground: outer entrance channel (a), general view of the nest (b), brood cells with blob-shape surrounded involucrum (c), the nest in the wood box, and brood cells were covered by storage pots and involucrum (d).
a b
The Additional Nest Structures of Stingless Bees
Colonies of Rangkasbitung created the additional nest structure, when their nest is damaged or attacked by natural enemies, such as predators and parasites. Results showed that some additional nest structure were inner entrance channel with much batumen and cerumen and also create the root-like structure (Figure 1.6).
Natural Enemies of Stingless Bees
Ten colonies in bamboos at Pasuruan and 10 colonies at Tasikmalaya were found without their natural enemies. However, 10 colonies at Rangkasbitung were found with their natural enemies, such as cockroaches (Blattodea: Blattidae), Platysoma leconti (Coleoptera: Histeridae), Carpophilus sp. (Coleoptera: Nitidulidae) (Table 2, Figure 1.7). We also found several eggs of lizard on the nest and larva of termites on below layers of the bamboo colony.
Figure 1.6 The additional nest structure of stingless bees: the inner entrance channel with batumen (a), the inner entrance channel with cerumen (b), nest with batumen (c), and root-like structure (d).
a b
9
Table 2 The number of colony of stingless bees were attacked by natural enemies. Natural enemies The number of colonies attacked Total
Pasuruan Tasikmalaya Rangkasbitung Platysoma leconti
(Coleoptera: Histeridae)
- - 6 6
Carpophilus sp.
(Coleoptera: Nitidulidae)
- - 2 2
Cockroaches
(Blattodea: Blattidae)
- - 2 2
Total 0 - 10 10
Figure 1.7 Natural enemies attacked the stingless bees colonies in bamboo: Platysoma leconti (mummified) (a), P. leconti stuck in sticky cerumen (b), eggs of lizard on bamboo colony (c), eggs of lizard on plastic cover of wooden box (d), and larva of termitesnesting on bamboo layers (e), and the cockroach (f).
a b
c d
Discussion
Nests Structure of Stingless Bees
Results showed that the nest structure and composition of stingless bees in bamboo, from the outside to the inside, consist of nest entrance, cerumen, storage pots (honey and pollen), brood cells, and storage pots. Generally, the structure and composition of stingless bees nests consist of nest entrance, inner tunnel, brood cells, storage pots (honey and pollen), and batumen layers (Sakagami et al. 1983). The nests are generally built by materials, such as wax, resin, and mud. Wille (1983) stated that bees used olfactory cues to locate the resin and food sources (nectar and pollen). Lack of plant resins causes a pot of food storage and brood cells are transparent (Leonhardt et al. 2010). Cerumen is a resin material mixed with wax. The wax of the stingless bee has a higher melting point compared to the wax of honey bees, Apis mellifera (Blomquist et al. 1985). If the wax is present in a higher concentration than resin, the texture of the nest becomes harder. The waxy nest can furthermore be hardened by mud to form batumen. While involucrum is cerumen are located around the brood cells to protect the nest from predators and parasites (Michener 2007).
There are two types of brood cells, cluster and comb form. Brood cells which do not contain eggs have darker in color, whereas those containing eggs are transparent. Chinh et al. (2005) stated the new cells were yellow-brownish in color but after the wax eroded to be transparent. Cells are connected with another cell by pillars. Leonhardt et al. (2010) showed brood cells associated with one pillar with a small ball shape. Nectar contains carbohydrates that are used by adult bees as sources of energy to forage and to defend their colony. While pollen contains protein that is used by young colony members (Jalil and Shuib 2014). Honey of stingless bees has more acidic and has a bright yellow in color (Chinh et al. 2005).
11
Natural Enemies of Stingless Bees
Natural enemies were not found in colonies of Tasikmalaya (West Java) and Pasuruan (East Java). Contrary, some predators, such as cockroaches (Blattidae), histerid beetles (Platysoma leconti), and nitidulids beetles (Carphophilus sp.) were found in the colony of Rangkasbitung. Some of the lizard eggs also were found in the bamboo or box colonies. Ants (Pheidole sp.) were found in the stingless bee colonies that moved to wood box. In India, Kumar et al. (2012) reported some predators attacked the colony of stingless bees, including wasps, ants, spiders, and centipedes. Ants are predators, if the number exceeds the limit, it can interfere the colony of stingless bees.
The resin of plants is used as a defense mechanism against natural enemies (Roubik 2006; Leonhardt et al. 2010). Defense mechanism against predators also was showed by workers of the stingless bee by standing and hovering. Standing is the behavior of worker bees which keep the entrance of the hive. While, hovering is the behavior of worker bees which fly near the entrance of the nest. In the evening, some species of stingless bees close the outer entrance channel by wax with a thickness of <0.05 mm (Gruter et al. 2010). If the colonies were disturbed by humans, workers showed defense behavior, like biting and attacking the hair, eyes, nose, and ears (Wille 1983).
CONCLUSIONS
POLLEN LOAD AND FLOWER CONSTANCY ON STINGLESS BEES
INTRODUCTION Background
Stingless bees are social insects that play an important role as pollinator of plants (Wille 1983; Inoue et al. 1985). Bees actively visit to agricultural crops, such as peppers and tomatoes (Cruz et al. 2005; Santos et al. 2009). There are about 100 species of plants visited by 37 species of stingless bees belonging to the families of Acanthaceae, Begoniaceae, Caesalpiniaceae, Cucurbitaceae, Poaceae, Malvaceae, Myrtaceae, and Rutaceae (Engel and Bakels 1980).
Many flowering plants are capable to self-pollinate, but cross-pollination is needed to increase genetic diversity of plants. In cross-pollination, pollens are transferred by wind, water, birds, bats, or bees. This latter are identified as the most common agents involved in cross-pollination (Davies 1988). Pollination is transfer process of pollen to the stigma. Effective pollination occurs when pollen grains out of the anther and reach the stigma (Dafni 1992).
Pollen is attractive main sources for bees. Pollen grains have thin walls composed of exine and intine (Lersten 2004). Pollen contains protein and amino acid as nutrient sources (Szczesna 2006). Size is an important parameter for pollen identification (Erdtman 1952). Size of pollen is classified into very small (<10 μm), small (10‒12 μm), medium (25‒50 μm), large (50‒100 μm), very large (100‒200 μm), and gigantic (>200 μm). Generally, pollen diameter is 15‒60 μm (Kearns and Inouye 1993). Pollen is identified based on five principal properties there are polarity, symmetry, aperture, shape, and size (Erdtman 1952).
The number of pollens attached to the body of bees in one foraging trip is called pollen load. Generally, pollen contains proteins, fats, carbohydrates, and some chemical substances. Pollen was taken by corbicula located at the tibia of hind legs of worker bees for their colonies (Dafni 1992). During foraging, bees also showed a flower constancy. Flower constancy is the preference of bees to visit flower of a particular plant species in one trip (Waser 1986). Ramalho et al. (1994) reported that in one foraging, commonly stingless bee visits only one flower type (97%) and the remaining (3%) taken from other plants. Several factors influence flower constancy, i.e. odor or chemical compound, colors, and shapes of flowers (Gruter and Ratnieks 2011).
The effectiveness of stingless bees as pollinators can be measured by the ability of bees to carry pollens. These bees can be used as alternatives for commercial pollination (Heard 1999), because the colony are more resistant to pests and parasites than honey bees and can be used to pollinate the plants cultivated in residential areas and in the greenhouses. But the role of these bees as pollinators of agricultural crops has not been widely reported.
Aims of the study
13
Each individual of worker of stingless bee collected was anesthetized using ethyl acetate and then was put in a 1.5 ml micro-tube contain 0.5 ml 70% ethanol:glycerol (4:1). Then, the samples were rotated during 24 hours, after which, the bee was removed from the micro-tube, the remaining solution only contained pollens. Afterwards, the solution was centrifuged at 2000 rpm for 10 minutes. The supernatant was discarded and the 0.1 ml sediment contain pollens was homogenized using shaker.
Measurements of Pollens Loads
The 0.1 ml solution contain pollens is used to measure pollen loads. As much as 0.02 ml solution is dripped into the Improved Neubauer-type hemocytometer. Then the number of pollens in the solution were counted under a compound microscope. The method was replicated 5 times, so total pollen loads in 0.1 ml was counted.
Pollen Identification and Determination of Flower Constancy
Pollen loads on the body of bees were identified based on Huang (1972) and Erdtman (1972). Several morphological structures were observed to identify the pollens: aperture, surface pattern, polar and equatorial forms and pollen size by using a compound microscope equipped with Optilab camera. Image Raster software was used to measure pollen size. The flower constancy of each species of bee was determined based on the identification of pollen type.
RESULTS AND DISCUSSION
Results
Body Weight of Stingless Bees
The morphology features of three species used in the study, i.e. T. laeviceps (Figure 2.1a), L. terminata (Figure 2.1b), and H. itama (Figure 2.1c) were shown in Figure 1. There were several differences on the morphology and size of these species. The average of fresh body weight of T. laeviceps, L. terminata, and H. itama were 2.67 mg ± 1.01 (n=6), 5.00 mg ± 0.53 (n=6), and 10.55 mg ± 5.5 (n=4), respectively.
Figure 2.1 Morphological features of stingless bees: Tetragonula laeviceps (a), Lepidotrigona terminata (b), and Heterotrigona itama (c). Scale 5 mm.
Species H. itama have larger body size (3‒7.5 mm), dominantly blackish, with one weak tooth on the mandible. Species L. terminata has medium to large body size (4.0‒5.5 mm) and yellow-brownish on mesoscutellum. The feature of T. laeviceps is a small body size (about 3.5 mm), brown-blackish body with transparent wings.
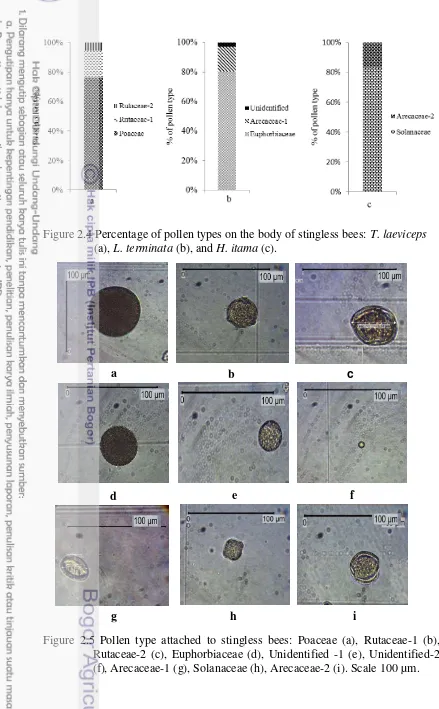
Pollen Loads on Stingless Bees
Results showed that average pollen loads on each species of stingless bees varied. Highest pollen loads were observed in H. itama (31392 pollen grains), followed by L. terminata (23017 pollen grains), and T. laeviceps (8015 pollen grains) (Figure 2.2). The number of pollens attached to the body of stingless bee was positively correlated with body weight (y=3854.2x+3492.2, R2=0.2654, p=0.017) (Figure 2.3).
15
Figure 2.2 The average number of pollen load of each species of stingless bees.
Figure 2.3 The relationship between body weight of stingless bees and the number of pollen attached on the body.
The Pollen Types Attached on Stingless Bees
Pollens attached on the body of T. laeviceps belong to two plant families, i.e., Poaceae and Rutaceae. Three types of pollens were identified from two families i.e, Poaceae, Rutaceae-1, and Rutaceae-2. Dominant pollen type attached on the body of T. laeviceps was Poaceae (76.49%). Rutaceae-1 (17.89%) and Rutaceae-2 (5.62%). Our results showed T. laeviceps has a flower constancy on Poaceae flowers (Figure 2.4a and Figure 2.5).
Figure 2.4 Percentage of pollen types on the body of stingless bees: T. laeviceps (a), L. terminata (b), and H. itama (c).
Figure 2.5 Pollen type attached to stingless bees: Poaceae (a), Rutaceae-1 (b), Rutaceae-2 (c), Euphorbiaceae (d), Unidentified -1 (e), Unidentified-2 (f), Arecaceae-1 (g), Solanaceae(h), Arecaceae-2 (i). Scale 100 µm.
a b c
d e f
17
DISCUSSION
The Number of Pollen Loads on Stingless Bees
Heterotrigona itama has the highest body weight compared to the other species used in this study. The body weight of worker bees correlated positively with their pollen loads. H. Itama, the heaviest species used in this study, had the highest pollen loads (31392 pollen grains). Whereas T. laeviceps, the lightest species used in this study had the lowest pollen loads (8015 pollen grains). This result supported the study previously performed by Reinaldo (2015) that the pollen loads on T. laeviceps were lower than in Apis cerana (4228 pollen grains). The ability of worker bees to carry the pollens also depends on the size of the pollen basket (Shuel 1992) and body size (Sadeh 2007). The pollen loads were also demonstrated correlated to positively with the number of visits to the flowers (Raw 2000).
Flower Constancy on Stingless Bees
Tetragonula laeviceps showed flower constancy on the flowers of family Poaceae (76.49%). Poaceae (grasses) are generally found near stingless bees colonies. Foraging habitat of stingless bees is generally closer to their hive compared with honey bees. In bees, foraging in a limited space showed increasing the efficiency (Kakutani et al. 1993). Other individuals of stingless bees also forage on Rutaceae plants. Stingless bees also forage on the flowers of Caesalpiniaceae, Begoniaceae, Myrtaceae, Malvaceae, and Rutaceae (Engel and Bakels 1980). Pollinator bees also tend to choose flowers that are easily accessible and those with simple morphological feature (Gruter and Ratnieks 2011).
Lepidotrigona terminata showed flower constancy on the flowers of Euphorbiaceae (80.46%). This result supported the study of Ramalho et al. (1994) that 62‒89% of stingless bees took pollens from the Euphorbiaceae plants. One individual of L. terminata carried pollens from Arecaceae-1 plant (16.67%) and the remaining individuals carried pollens from unidentified-1 and unidentified-2 plants (2.87%). Two types of pollen can not be identified because the polar and equator form of this pollen were not clearly visible. Polar and equatorial form are indispensable characteristics for pollen identification (Huang 1972).
CONCLUSION
Pollen load on three species of stingless bees varied. Pollen load on H. itama was highest (31392 pollen grains), followed by L. terminata (23017 pollen grains) and T. laeviceps (8015 pollen grains). The number of pollens attached to the body of stingless bees was positively correlated with body weight. Three species of stingless bees also showed different flower constancy. T. laeviceps showed flower constancy on the flowers of Poaceae (76.49%). L. terminata on Euphorbiaceae (80.46%), and H. itama on Solanaceae (83.33%).
19
GENERAL DISCUSSION
Additional Nest Structures of Stingless Bees Arised as a Results of Natural Enemies
The shape of the nest entrance of stingless bees varied. These colonies built nest entrance surrounded by cerumen. Chinh et al. (2005) reported the shape of the nest entrance and inner entrance channel usually are not found in T. laeviceps. Nest entrance is usually found at the tree cavity an average height of 90 cm above the ground and or in artificial constructs of human (Sakagami et al. 1983)
The nests structure of stingless bees has two main parts i.e., storage pots and brood cells. Storage pots are usually found on the top and bottom of the nest, round form, thin walls, and dark brown in color. The thickness of storage pots is less than 1 mm. Brood cells have the smaller size than storage pots (Chinh et al. 2005). Brood cells are usually open up and closed after the egg is laid. Commonly, brood cells arranged regularly in the horizontal combs and pollen pots are commonly found near the brood cells. Brood cells contain nutrient for the development of eggs and larvae, such as pollen, nectar, and honey (Michener 2000).
Additional nest structures is built to defend against predators. Several predators found in this study were cockroaches (Blattodea: Blattidae), histerid beetles Platysoma leconti (Coleoptera: Histeridae), nitidulid beetles Carphophilus sp. (Coleoptera: Nitidulidae) and ants Pheidole sp. (Hymenoptera: Formicidae). The beetle larvae live and growth in colonies of bees in order to obtain nutrient sources. Whereas, antsin the bee colonies aim to taking a food.Chinh et al. (2005) reported that worker bees defend their colony against ants by biting and using a resin to block the outer and inner entrance of the nest.
Pollen Load and Flowers Constancy on Stingless Bees
Three species of stingless bees have different preference to visit the flowers. It is showed that the effectiveness of the pollination in some species of bees highly depends on the plant species and each species showing differences in flower constancy (Kearns and Inouye 1993). Flower constancy of social pollinator insects is also a strategy to avoid competition in the colony. Even though, the competition in the colony was weaker than competition between colonies (Gruter and Ratnieks 2011). Foraging of bees were performed in about 10.00 am- 14:00 pm (Chinh et al. 2005). Bees carried pollen and move from one plant to another plant will help the process of pollination. But, each species tends to limit their visits to the same species in one trip (Dafni 1992). During foraging, stingless bees showed more efficiency than honeybees on some plant species (Free 1993, Heard 1999).
REFERENCES
Barbosa FM, Alves RMO, Souza BA, Carvalho CAL. Nest Architecture of the Stingless bee Geotrigona subterranea (Friese, 1901) (Hymenoptera: Apidae: Meliponini). Biota Neotrop. 13(1):147-152.
Blomquist GJ, Roubik DW, Buchmann SL. 1985. Wax Chemistry of Two Stingless bees of The Trigonisca Group (Apidae: Meliponinae). Comp. Biochem. Physiol. 82(1):137-142.
Buchwald R, Breed MD. 2005. Nestmate Recognition Cues in a Stingless Bee, Trigona fulviventris. Anim. Behav. 70:1331-1337.
Chinh TX, Sommeijer MJ, Boot WJ, Michener CD. 2005. Nest Architecture and Colony Characteristic of Three Stingless bees in North Vietnam with the First Description of the Nest of Lisotrigona carpenter Engel (Hymenoptera: Apidae, Meliponini). J.Kans Entomol. Soc. 3:26-39. Cruz DO, Freitas BM, Silva LA, Silva EMS, Bomfim IGA. 2005. Pollination
Efficiency of the Stingless Bee Melipona Subnitida on Green House Sweet Pepper. Pesq Agropec bras. 40(12):1197-1201.
Dafni A. 1992. Pollination Ecology a Practical Approach. New York (US): Oxford Univ Pr.
Davies RG. 1988. Outlines of Entomology. Cambrigde (GB): University of London.
Engel MS, Bakels FD. 1980. Nectar and Pollen Resources for Stingless Bees (Meliponinae, Hymenoptera) in Surinam (South America). Apidologie 11(4):341-350.
Fatoni A. 2008. Pengaruh propolis Trigona spp. asal Bukittinggi terhadap beberapa bakteri usus halus sapi dan penelusuran komponen aktifnya [Tesis]. Bogor (ID): Program Pascasarjana, Institut Pertanian Bogor, Bogor. Free JB. 1993. Insect Pollination of Crops. Cambridge (GB): Academic Press. Garedew A, Schmolz E, Ingolf L. 2003. The Antimicrobial Activity of Honey of
the Stingless Bee Trigona spp. Journal of Apicultural Science. 47(1):37-49. Gruter C, Karcher MH, Ratnieks FLW. 2010. The Natural History of Nest
21
Inoue T, Salmah S, Abbas I, Yusuf E. 1985. Foraging Behavior of Individual Workers and Foraging Dynamics of Colonies of Three Sumatran Stingless bees. Res. Popul. Ecol. 27:373-392.
Jalil AB, Shuib I. 2014. Beescape for Meliponines. Singapore [SG]: Partridge Publishing.
Kakutani T, Inoue T, Tezuka T, Maeta Y. 1993. Pollination of Strawberry by The Stingless Bee, Trigona minangkabau and The Honey Bee, Apis mellifera: an Experimental Study of Fertilization Efficiency. Res Popul Ecol. 35:95-111. Kearns AN & Inouye WD. 1993. Techniques for Pollination Biologist. Colorado
(US): University Press of Colorado
Kek SP, Chin NL, Yusof YA, Tan SW, Chua LS. 2014. Total Phenolic Contents and Colour Intensity of Malaysian Honeys from the Apis spp. and Trigona spp. Bees. Agriculture and Agricutural Science Procedia. 2:150-155. Kumar MS, Singh AJAR, Alagumuthu G. 2012. Traditional Beekeeping of
Stingless Bee (Trigona asp) by Kani Tribes of Western Ghats, Tamil Nadu, India. Indian Journal of Tradional Knowledge. 11(2):342-345.
Leonhardt SD, Zeilhofer S, Blu¨ thgen N, Schmitt T. 2010. Stingless Bees Use Terpenes as Olfactory Cues to Find Resin Sources. Chem. Senses 35: 603-611.
Lersten NR. 2004. Flowering Plant Embryology. Australia (AU): Blackwell Publishing
Michener CD. 1974. The Social Behavior of the Bees. Cambrigde (GB): Harvard University Press
Michener CD. 2000. The Bees of the World. Edition 1. [Digital]. JHU Press. Michener CD. 2007. The Bees of the World. Edition 2. Maryland (MD): JHU
Press.
Nunes TM, Mateus S, Turatti IC, Morgan ED, Zucchi R. 2011. Nestmate Recognition in the Stingless Bee Frieseomelitta Varia (Hymenoptera, Apidae, Meliponini): Sources of Chemical Signals. Animal Behaviour. 81:463-467 Oldroyd BP, Wongsiri S. 2006. Asian Honey Bees. Cambridge (GB): Harvard
University Press.
Ramalho M, Giannini TC, Malagodi-Braga KS, Imperatriz-Fonseca VL. 1994. Pollen Harvest by Stingless Bee Foragers (Hymenoptera, Apidae, Meliponinae). Grana. 33:239-244.
Raw A. 2000. Foraging Behaviour of Wild Bees at Hot Pepper Flower (Capsicum annum) and Its Possible Influence on Cross Pollination. Ann Bot. 85:487-492.
Reinaldo D. 2015. Diversitas Serangga Penyerbuk pada Tanaman Salak Kalimantan (Salacca affinis) di Taman Buah Mekarsari, Bogor [skripsi]. Bogor (ID): Institut Pertanian Bogor.
Roubik DW. Stingless bee Nesting Biology. 2006. Apidologie. 37:124-143.
Sadeh A, Shmida A dan Keasar T. 2007. The Carpenter Bee Xylocopa Pubescens as an Agricultural Pollinator in Greenhouse. Apidologie. 38:508-517. Sakagami SF. 1983. Nest Architecture and Colony Composition of the Sumatran
Sakagami SF, Inoue T, Salmah S. 1990. Stingless bees of Central Sumatra. In: Ohgushi R., Sakagami SF, and Roubik, D. W. (Eds). Natural History of Social bees in Equatorial Sumatra. Japan (JP): Hokkaido University Press. Sakagami SF, Ohgushi R, Roubik DW. 1990. Natural History of Social Wasps
and Bees in Equatorial Sumatra. Sapporo (JP): Hokkaido University Press. Santos SAB, Roselino AC, Hrncir M, Bego LR. 2009. Pollination of Tomatoes by
the Stingless Bee Melipona quadrifasciata and The Honey Bee Apis mellifera (Hymenoptera, Apidae). Gen and Mol Res. 8(2):751-757.
Schwarz HF. 1937. The Indo-Malayan Species of Trigona. Bull Am Mus Nat Hist. 76:83-141.
Sforcin JM, Bankova V. 2010. Propolis: Is There a Potential For the Development of New Drugs. Journal of Ethnopharmacology. 133:253-260.
Shackleton K, Toufailia HA, Balfour NJ, Nascimento FS, Alves DA, Francis L, Ratnieks W. 2015. Appetite for Self-Destruction: Suicidal Biting as a Nest Defense Strategy in Trigona Stingless bees. Behav Ecol Sociobiol. 69:273-281.
Shen M, Cui L, Ostiguy N, Cox-Foster D. 2005. Intricate Transmission Routes and Interactions Between Picorna-like Viruses (Kashmir Bee Virus and Sacbrood Virus) With The Honeybee Host and The Parasitic Varroa Mite. Journal of General Virology. 86:2281-2289.
Shuel RW. 1992. The Hive and The Honey Bee. Illinois (US): Dadant & Hamilton, Ltd.
Slaa EJ, Sanchez LA, Sampaio MB, Hofstede FE. 2006. Stingless Bees in Applied Pollination: Practice and Perspectives. Apidologie 37:293-315.
23
BIOGRAPHY
The author was born in Kudus, November 16th 1989 as the second child of Drs. Utomo and Masminah. The author was graduated from SMAN 1 Bae (Kudus) in 2007 and accepted in Department Biology, Faculty of Mathematics and Natural Sciences, Bogor Agricultural University (IPB) through Undangan Seleksi Masuk IPB (USMI) and the author graduated in 2011. Afterward, the author continued to Master of Science in Animal Bioscience Program, Graduate School IPB in 2014.