ALTITUDE AND SHADING CONDITIONS
AFFECT VEGETATIVE GROWTH OF
Kaempferia parviflora
EVI
A24070015
ABSTRACT
EVI. ALTITUDE AND SHADING CONDITIONS AFFECT VEGETATIVE GROWTH OF Kaempferia parviflora. (Supervised by NURUL KHUMAIDA and SINTHO W. ARDIE)
Kaempferia parviflora is a native plant of Thailand which potentially developed in Indonesia because of its pharmacological values. Thus, in order to develop appropriate cultivation system of K. parviflora in Indonesia, this research was conducted to study the effect of different altitudes and shading conditions on the vegetative growth of K. parviflora.
The experiment was arranged in Split-plot Nested design, where the main plot was altitude with two factors (1 200 m asl at Pasir Sarongge Experimental Farm and 240 m asl at Cikabayan Experimental Farm), the subplot was three levels of shading condition (0% shading, 55% shading, and natural shading), and replication was nested at subplot.
The results showed that there was no significant different in plant height, number of leaves, and leaf area of K. parviflora grown at 1 200 m asl and 240 m asl respectively. However, higher altitude affects the color of K. parviflora leaves. Plants grown at higher altitude (1 200 m asl) had greener adaxial leaf color and more reddish abaxial leaf color than plants grown at lower altitude. The vegetative growth of K. parviflora was more affected by shading conditions. Kaempferia parviflora had taller plant, higher leaf area, and greener adaxial leaf color under natural shading than under full sun condition. Plants grown under natural shading and 55% artificial shading also had higher number of leaves at the early vegetative growth than those grown under full sun condition. Plants grown under 55% artificial shading showed similar growth with plants grown under natural shading, except that plants under natural shading had higher leaf area than plants grown under 55% artificial shading. The best combination between altitude and shading was of 240 m asl with natural shading. Based on the early vegetative growth,
ABSTRACT
EVI. ALTITUDE AND SHADING CONDITIONS AFFECT VEGETATIVE GROWTH OF Kaempferia parviflora. (Supervised by NURUL KHUMAIDA and SINTHO W. ARDIE)
Kaempferia parviflora is a native plant of Thailand which potentially developed in Indonesia because of its pharmacological values. Thus, in order to develop appropriate cultivation system of K. parviflora in Indonesia, this research was conducted to study the effect of different altitudes and shading conditions on the vegetative growth of K. parviflora.
The experiment was arranged in Split-plot Nested design, where the main plot was altitude with two factors (1 200 m asl at Pasir Sarongge Experimental Farm and 240 m asl at Cikabayan Experimental Farm), the subplot was three levels of shading condition (0% shading, 55% shading, and natural shading), and replication was nested at subplot.
ALTITUDE AND SHADING CONDITIONS
AFFECT VEGETATIVE GROWTH OF
Kaempferia parviflora
The undergraduate thesis is submitted to the Faculty of Agriculture
as partial fulfillment of the requirements
for the degree of Bachelor of Agricultural Science
Evi
A24070015
DEPARTMENT OF AGRONOMY AND HORTICULTURE
FACULTY OF AGRICULTURE
Title :
ALTITUDE AND SHADING CONDITIONS AFFECT
VEGETATIVE GROWTH OF
Kaempferia parviflora
Name : EVI
NIM : A2070015
First Supervisor Second Supervisor
Dr. Ir. Nurul Khumaida, M.Si Dr. Sintho Wahyuning Ardie, S.P., M.Si NIP.19650719 199512 2 001 NIP. 19820706 200501 2 001
Head of Department of Agronomy and Horticulture Faculty of Agriculture
Dr.Ir. Agus Purwito, MSc. Agr. NIP. 19611101 198703 1 003
BIOGRAPHY
Author was born at 08 September 1990 in Batam, Kepulauan Riau. Author was first child with two siblings from Irianti and Song Key Dong. Author spends her childhood in Jakarta.
Author started her formal education at 1995 in SD Nasional I Bekasi, 2001 in SLTP Nasional I Bekasi, and continued to SMA Nasional I Bekasi at 2004. At 2007, author continued her education in Department of Agronomy and Horticulture, Bogor Agricultural University.
PREFACE
Author was so grateful to Allah SWT for His blessing so author can finished this thesis “Altitude and Shading Conditions Affect Vegetative Growth of
Kaempferia parviflora”. This research had presented at PERHORTI National Seminar of Horticulture at November 2011. Author was lucky enough to get so much help, so author would like to express her gratitude to these people:
1. Dr. Ir. Nurul Khumaida, M.Si and Dr. Sintho W. Ardie as supervisor who gave idea, guidance, and advice in the making process of this undergraduate thesis
2. Dr.Ir. Sudrajat MS. as examiner who gave advise and support so this undergraduate thesis can be better
3. Prof. Dr. Ir. Bambang S. Purwoko, M.Sc as academic counselor who has helped author when she was a student of Department of Agronomy and Horticulture
4. Dad, Mom, Bora, and Irfan, beloved family who gave a lot of supports, love, and prayers
5. Mr. Juhana, Pasir Sarongge experimental field manager and Mr. Milin, Cikabayan experimental field manager, with all staff who helped author did her research
6. Haveel, Afdhol, Andina, Indin, Ardoyo, Nasuha bestfriends considered as authors own family who gave so much help and support. Tri Handayani, SP. and Sumiyati, SP. who gave a lot of advice to author. And all AGH 44 friends for kindness and accpetance to author.
Bogor, January 2011
LIST OF CONTENT
Altitude Effect on Plant Growth ... 5
Shading Effect on Plant Growth ... 6
MATERIAL AND METHOD ... 8
Morphology of Kaempferia parviflora ... 15
Growth of Kaempferia parviflora ... 18
Altitude Affect the Early Vegetative Growth of K. parviflora ... 18
Shading Condition Affect Vegetative Growth of K.parviflora differently 20 Combination of Altitude of Altitude and Shading Treatment ... 23
Leaf Anatomy of Kaempferia parviflora at Different Treatment ... 26
Leaf Size... 26
CONCLUSION AND SUGGESTION ... 31
Conclusion ... 31
Suggestion ... 31
REFERENCES ... 32
LIST OF TABLES
No Page
1. Recapitulation of Treatments Analysis of Variance ... 15
2. Effect of Altitude on Plant Height, Number of Leaves, And Leaf Length of K. parviflora ... ...19
3. Effect of Altitude at Abaxial and Adaxial Leaf Color ... 19
4. Effect of Shading Conditions on Plant Height ... 20
5. Effect of Shading at Number of Leaves ... 21
6. Effect of Shading at Number of Plant per Rhizome Planted……… .... 22
7. Effect of Shading to Adaxial Leaf Color at 16WAP ... 22
8. Combination of Treatments at Number of Shoot Emerged, Leaf Area, and Adaxial Leaf Color.. ... 23
9. Combination of Treatments at Plant Height.. ... 24
10. Effect of Shading Conditions on Leaf Size… ... 27
11. Leaf Thickness of K. parviflora at Different Shading Condition.. ... 28
12. Effect of Different Altitude at Stomatal Density (per cm2).. ... 29
LIST OF FIGURES
No. Page
1. Kaempferia parviflora plant ... 5
2. Experimental condition ... 9
3. Leaf Measurement Equipment ... 10
4. Score of Abaxial Leaf Color Composition ... 11
5. Pest Control Method Used during the Experiment ... 14
6. Phenotye of K. parviflora ... 16
7. Kaempferia parviflora Flower ... 17
8. Kaempferia galanga Plant ... 17
9. Growth of K. parviflora ... 18
10. Different growth of K. parviflora on 12 WAP at 240 m asl ... 25
11. Physiological Changes Due to Dehydration ... ...26
12. Thickness of K. parviflora at Different Treatment ... 28
LIST OF APPENDIX
No. Page
1. Climate Data at Darmaga Area during March-July 2011... 37
2. Climate Data at Pasir Sarongge Area during March-July 2011 ... 39
3. Soil Sample Analysis Result ... 41
INTRODUCTION
Background
The use of medicinal plants as herbal drugs is increasing rapidly. Ginger is the common name given to members of the Zingiberaceae family, a group of tropical, rhizomatous, herbaceous perennials which have been gained much notoriety in the list of medicinal plants. The rhizomes of species from this family are known to have many pharmacological values.
Kaempferia parviflora is one of the plants in the Zingiberaceae family originated from Thailand. In its origin, K. parviflora is known as kra-chai-dam, Thailand ginseng, or black galingale (Putiyanan et al., 2008). Recently, K. parviflora has been reported to possess anti-mycobacterial, anti-plasmodial (Yenjai et al., 2004), anti-peptic ulcer (Rujjanawate et al., 2005), and anti-viral protease effects (Sookkongwaree et al., 2006) as well as modulators of multi-drug resistance in cancer cells (Patanasethanont et al., 2007). Because of its pharmacological benefits and the increasing trend of herbal consumption in Indonesia, K. parviflora is potentially developed in Indonesia.
Studies on the plant adaptation on the local agro-climate are necessary in order to domesticate K. parviflora in Indonesia. As the member of Zingiberaceae family, K. parviflora might share similar cultivation system as other member grown in Indonesia, i.e. ginger (Zingiber officinale), galingale (Kaempferia galanga), galangal (Alpinia galanga), and turmeric (Curcuma longa). However, as a medicinal plant, cultivation system should be designed to produce high yield in biomass as well as in the level of bioactive compound. Previous studies showed that the level of fenolic compound in the rhizome of K. parviflora was affected by light intensity (Chansakaow et al.,2005) and by altitude (Pojanagaroon, 2008).
Chansakaow et al. (2005), showed that K. parviflora grown at 60% shading produce highest fenolic compound, and at 80% shading produce highest antioxidant. Pojanagaroon (2008) showed that low temperature increased fenolic compound and coloring material of K. parviflora.
Therefore, as a preliminary step to determine the best cultivation system of
conditions) on the vegetative growth of K. parviflora was studied. Two altitude was used are 240 m asl and 1 200 m asl. Three shading conditions was used as treatment was no shading, artificial shading (55% shading), and natural shading.
Natural shading treatment was used to compare K. parviflora growth under natural shading compare and artificial shading. One of best cultivation criteria was economically profitable. Natural shading could provide shade with less cost than artificial shading. It can be provided using intercroping system.
Objectives
The objective of this research was to study the effect of altitude and different shading conditions on the vegetative growth of K. parviflora to determine the best cultivation practice of this plant in Indonesia.
Hypothesis
LITERATURE REVIEW
Member of Kaempferia Genus
Kaempferia galanga
Kaempferia galanga is one of the members of Kaempferia genus that commonly cultivated in Indonesia. Galingale or kencur is a rhizomatous perennial plant. The rhizomes of this plant produce an essential oil which can be utilized in the manufacture of jamu, cosmetic industry, food additive, and bioinsecticide. Galingale is used as expectorant, tonic, cough medicine, bacterial infection, flatulent, and disentry medicine (Rostiana and Effendi, 2007).
Galingalerequires a warm humid climate. It grows well up to an elevation of 1500 m. A well distributed annual rainfall of 1 500 – 2 500 mm during the growing period and less rain fall during land preparation and harvesting are ideal. Rich loamy soil with good drainage is suitable for the cultivation of the crop. Indonesia. Kaempferia rotunda is an aromatic herb with tuberous root-stalk and very short stem. The leaves are simple, few, erect, oblong, or ovate-lanceolate, acuminate, 30 cm long, 10 cm wide, variegated green above and tinged with purple below. Flowers are fragrant, white, tip purple or lilac arranged in crowded spikes opening successively. The plant produces a subglobose tuberous rhizome from which many roots bearing small oblong or rounded tubers arise (Warrier et al, 1995).
complaints, improves complexion, and cures burning sensation, and insomnia (Sivarajan and Balachandran, 1994).
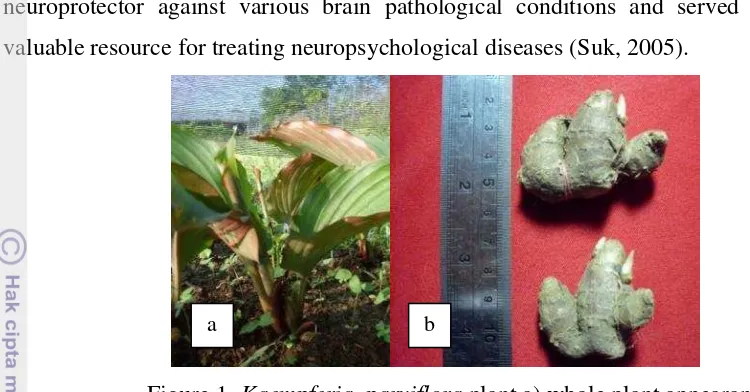
Kaempferia parviflora Wall.
Kaempferia parviflora Wall. is indigenous to north-eastern Thailand.
Kaempferia parviflora belong to Kingdom plantae, phylum Anthophyta, class Monocotyledones, ordo Zingiberales, family Zingiberaceae, genus Kaempferia, and species K. parviflora.Kaempferia parviflora is rhizomatous herb. Rhizome is a horizontal underground stems that often serve as a storage organ and a means to asexual reproduction (Graham et al., 2006).
This plant is best grown at highland about 500-700 m above sea level.
Kaempferia parviflora grow very well in a good aerated soil under mild sunlight. Old rhizomes aged 11-12 months; germ free should be kept in dry and cool place for 1-3 months before growing. Fertilizer formula 15-15-15 (N-P2O-K2O) about 125-150 kg/ha is recommended. Harvesting time for the best crop is at 8-9 months after planting (ICS UNINDO, 2009).
Kaempferia parviflora or Krachai Dam in Thailand, is an indigenous plant of Thailand which very popular for health promotion in the country. Rhizomes of
K. parviflora have been used as traditional medicine for various medicinal purposes including ease body pains and gastrointestinal disorders among local people in the Northeast of Thailand. Many bioactive compounds have been discovered from this plant rhizome extract. For example, methoxyflavones isolated from this plant had anti-gastric ulcer activity by experimental models in rat (Rujjanawate et al., 2005). In addition, the ethanol extract and their flavones constituents from K. parviflora tincture (called Ya-dong in Thailand) have been shown to inhibit the P-glycoprotein function in a transfected epithelial cell line which may be mainly attributed to 3,5,7,3′4′-pentamethoxyflavone (Patanasethanont et al., 2007). Recently, Krachai Dam rhizome extracts also have excellent antioxidant potential, as evidenced by their ability to scavenge free radicals. Yenjai et al. (2004) have reported that this plant contained high amount
neuroprotector against various brain pathological conditions and served as a valuable resource for treating neuropsychological diseases (Suk, 2005).
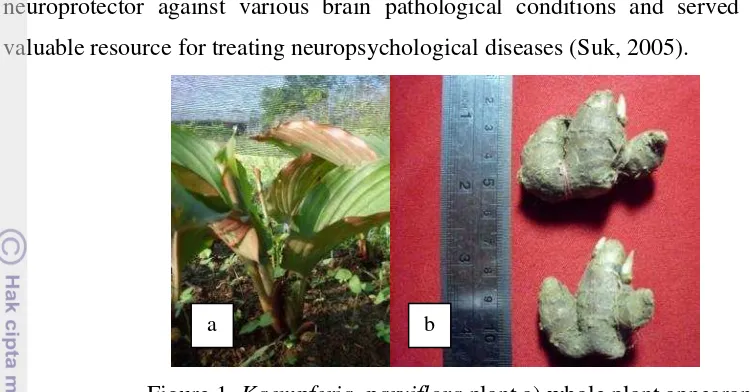
Figure 1. Kaempferia parviflora plant a) whole plant appearance above the ground and b) Rhizome
Altitude Effect on Plant Growth
There are environmental changes with altitude, such as atmospheric pressure, temperature, and clear-sky turbidity. These changes cause plant species
diversity. At increasing altitudes, plants are exposed to decreasing average temperatures and increasing light intensities, so they must have developed mechanisms, by which to prevent damage caused by chilling, by freezing or by photodestruction (Ren et al., 1999). Every plant species has it own minimum, optimum, and maximum temperature. Plant will not grow well below its minimum temperature or above its maximum temperature (Salisbury and Ross, 1995).
Plant can adapt to low temperature through morphological and physiological mechanism. Physiological adaptation can occur as alteration in photosynthesis, respiration, solute transport, or reproduction. At low temperature plant shows declination of photosynthesis rate as the effect of decreasing metabolic reaction. Low temperature can also reducing respiration rate because of stomata closure (Oquist and Martin, 1986). In the other hand, Scott (2008) stated that plant can adapt at high temperature by lowering its leaves temperature by transpiration. If water is not available, then cooling process is reduced so plants are more vulnerable to high temperature damage.
Plant stress induces defense system. This defense system can be alteration of physical structure or chemical defense. Three major plant chemical defense
systems are terpene, fenolic compound, and nitrogen organic compound (Scott, 2008). Pojanagaroon (2008) showed that low temperature increased fenolic compound and coloring material of K. parviflora.
Shading Effect on Plant Growth
Light plays important roles on physiological process on plants, such as photosynthesis, respiration, growth, stomata closure, phototropism, and germination (Salisbury and Ross, 1995; Taiz and Zeiger, 2002). Light component that affect plant are light quality, quantity, and photoperiod (Runkle, 2008).
The duration of light in a 24-hour period is known as the daylength or photoperiod. Light quantity refers to the intensity of light that can be measured instantaneously or as a daily light sum. Plant growth is primarily influenced by the average amount of light received each day. In other words, plant growth is influenced by the number of hours of light and the intensity of light during the day. Light quality refers to the spectral distribution of light, or the relative number of photons of blue, green, red, far-red and other portions of the light spectrum that is emitted from a light source. Some of these portions are visible, whereas others are not. The energy of each photon is dependent on its wavelength. Photons with a short wavelength, such as that of ultra-violet (UV), have more energy than photons with a longer wavelength, such as red light (Runkle, 2008).
Plant ability to adapt at changing environment is determined by genetic factor. Shade plants have lower photosynthesis rate, lower light compensation point, and lower point of saturated photosynthesis than sun plants. Lower light saturation point on shade plant happen because of low respiration rate, so with only a little nett photosynthesis produced allowed nett CO2 exchange rate become zero. Lower respiration rate is a basic adaptation, allowing shade plant survive at low light environment (Salisbury and Ross, 1995).
Shade plant can adjust its leaves to light intensity so at low light condition chloroplast gather near to epidermis thus leaf color is greener (Taiz and Zeiger, 2002). Sukaesih (2002) suggest that plant height is increase as shading increase, on the contrary nodes, branches, and trunk diameter is decrease at soybean (Glycine max). Stem elongation occurs to maximize sun radiation accepted to maintain photosynthesis rate.
Excess of light intensity can decrease yield. This happen because of three things, which are; first, chlorophyll is decreased causing yellowish green leaf thus light absorption rate is low. Second, leaf temperature is increased cause of excess light intensity so transpiration rate is increased and not balanced with water absorption, stomata are closed and photosynthesis decreased. Third, light intensity affect leaf temperature and affect particular enzyme which is deactivated enzyme that alter sugar to starch, so sugar concentration is high and lowering photosynthesis rate (Harjadi,1989).
MATERIAL AND METHOD
Time and Place
This research was conducted from January to August 2011 at Pasir Sarongge Experimental Field, Cipanas (altitude 1 200 m above sea levels) and Cikabayan Experimental Field, Darmaga (altitude 240 m above sea levels). Examination of stomata and leaf thickness was held at Microtechnique Laboratory of Department Agronomy and Horticulture, Bogor Agricultural University.
Material
Material used in this research were K. parviflora rhizomes which was provided by PT Ogawa Indonesia, fertilizer containing nitrogen (N), phosphate (P), and potash (K), manure, Carbofuran, Dithane, Agrept, artificial shading, and pesticide. Equipment used were bamboo, ruler, weigh scale, camera, SPAD (Soil Plant Analysis Development), portable leaf area meter LI-3000C, and common farming tools.
Method
The experiment was arranged in Split-plot Nested design, with altitudes (1 200 m asl and 240 m asl) as the main plot and shading conditions (0% shading, 55% artificial shading, and natural shading) as the sub-plot and three replications were nested in the subplot. Every replication is a raised bed 60 x 200 cm sized containing 39 plants. From each replication, ten examples plant are examined. Natural shading used for this research was orange tree (Citrus sp.) at Cikabayan and avocado tree (Perse americana) at Pasir Sarongge.
Mathematical model from design used was:
Data was test for normality then tested with F-test. Bartlett test was conducted to ensure the homogeneity between each condition. All data, except for abaxial leaf color variable, was tested with Duncan Multiple Range Test using SAS 9.1.3 software. Adaxial leaf color tested with Kruskall-Wallis test and homogenity t-test used PAWStatistic 18 Software.
Experimental Treatment
Plant material used in this research was K. parviflora rhizomes which were received from PT Ogawa Indonesia. Rhizomes with one shoot (+ 2 mm length) were cut to approximately 15 g, dipped in 2% (w/v) Dithane and 2% (w/v) Agrept for ten minutes, and air dried for 24 hours. Rhizomes were then planted at 5 cm depth and 20 cm x 15 cm plant spacing in a raised bed. The raised bed size was 60 cm x 200 cm x 30 cm (width x length x height), thus there were 39 rhizomes planted in one raised bed.
Manure (30 ton/ha) and compost (30 ton/ha) was applicated on each bed. Planting was held two weeks after manure application. To homogenize watering both in volume and frequency, plastic roof was built on each site using bamboo as its main structure. For 55% shading treatment, paranet was put below the plastic and surrounded the structure. For natural shading, the plastic roof put below a tree. Each structure was 2.5 m x 3 m sized upon three raised bed. The experimental condition are shown in figure 2.
Figure 2. Experimental condition. a) no shading, b) 55% shading, and c) natural shading
Fertilizer was applied following standard procedure of common galingale, that are 30 ton/ha manure, 30 ton/ha compost, 300 kg/ha Urea, 250 kg/ha SP-36, and 250 kg/ha KCl (Rostiana and Effendi, 2007). Manure and compost were
applied on each bed two weeks before planting. Half dosage of urea and full dosage of SP-36 and KCl were applied at 5 week after planting (WAP) when root was established. Half dosage of urea was applied at 16 WAP. Weeding was done manually every two weeks. Pest control was done manually and physically.
Observation
In order to study the growth of K. parviflora, some parameters have been observed. Variable measured was:
1. Plant growth.
Plant growth was measured every two week by measuring “plant height”, number of leaves per rhizome planted, number of plants per rhizome planted, leaf width, and leaf length. First time flower appeared and rhizome appeared was also noted. All leaf measurement used first leaf opened of each plant.
a) “Plant height” was measured from land surface to the tip of the longest leaf because the true stem of this plant is difficult to determine.
b) Number of leaves per rhizome planted was measured by counting all leaves produce by one rhizome.
c) Number of plants per rhizome planted was measured by counting all plant produced by one rhizome.
d) Leaf width and leaf length was measured on first leaf that fully expanded
e) Specific leaf area measured using portable leaf area meter LI-3000C (LI-COR, USA) (Figure 3a)
Figure 3. Leaf Measurement Equipment. a) Spesific Leaf Area Meter and b) SPAD
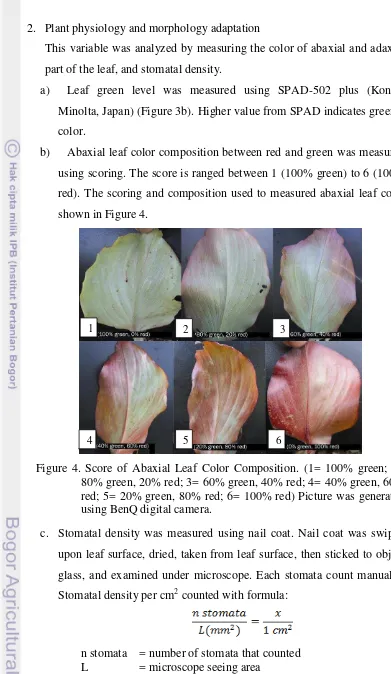
2. Plant physiology and morphology adaptation
This variable was analyzed by measuring the color of abaxial and adaxial part of the leaf, and stomatal density.
a) Leaf green level was measured using SPAD-502 plus (Konica Minolta, Japan) (Figure 3b). Higher value from SPAD indicates greener color.
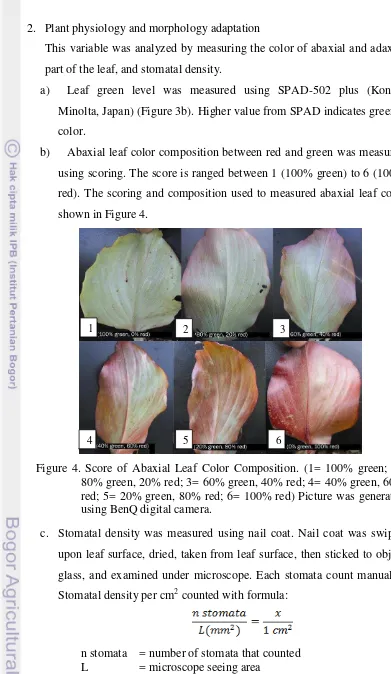
b) Abaxial leaf color composition between red and green was measured using scoring. The score is ranged between 1 (100% green) to 6 (100% red). The scoring and composition used to measured abaxial leaf color shown in Figure 4.
Figure 4. Score of Abaxial Leaf Color Composition. (1= 100% green; 2= 80% green, 20% red; 3= 60% green, 40% red; 4= 40% green, 60% red; 5= 20% green, 80% red; 6= 100% red) Picture was generated using BenQ digital camera.
c. Stomatal density was measured using nail coat. Nail coat was swiped upon leaf surface, dried, taken from leaf surface, then sticked to object glass, and examined under microscope. Each stomata count manually. Stomatal density per cm2 counted with formula:
n stomata = number of stomata that counted L = microscope seeing area
x = stomatal density per cm2
1 2 3
Microscope seeing area was counted with circle area formula: πd2
π = 3.14
d = 1.1 mm (Olympus Microscope with 400X magnification)
3. Environmental factors
RESULT AND DISCUSSION
General Condition
This research was conducted in two locations, Pasir Sarongge Experimental Field (1 200 m asl) and Cikabayan Experimental Field (240 m asl). The different altitude between two locations resulted in different daily temperature, relative humidity, and different light exposure. Based on data collected from Meteorology and Geophysics Institution from March to July 2011, the average day temperature in Pasir Sarongge was 20oC, with maximum temperature was 27oC, and minimum temperature was 15oC. Cikabayan Experimental Field had higher daily temperature than Pasir Sarongge. Average day temperature in Cikabayan Experimental Field was 26oC, with maximum temperature was 33oC, and minimum temperature was 19oC. The average relative humidity in Pasir Sarongge Experimental Field (78%) was lower than in Cikabayan Experimental Field (83%). Pasir Sarongge Experimental Field was cloudy, thus the average light exposure per day was relatively low (48%). In contrast, the average light exposure per day was relatively high in Cikabayan Experimental Field (80%). Data is shown in Appendix 1 and 2.
altered soil structure and made soil ability to retain water increased. Acidity of soil samples showed for all sample pH was about 5. Soils have a cation exchange capacity (CEC), the total amount of exchangeable cations that can be held by a given mass of soil. The higher CEC means higher amount of cation can be exchanged thus can be absorb by plants (Singer and Munns, 2006). The highest CEC was SR B (24.33 cmolc/kg), followed SR A (13.19 cmolc/kg), then CB A (10.76 cmolc/kg), and the smallest was CB B (10. 61 cmolc/kg). Full soil analysis result was shown at Appendix 2.
There was no major effect because of disease examined. Destruction mostly caused by insect. At Pasir Sarongge, insect that became problem was many kind of caterpillar. The destruction was varied from light to severe. For controlling this pest, insecticide was given. At Cikabayan, pest that caused most destruction was grasshopper. Trap was put to decreasing amount of this pest. Trap was made from yellow plastic and covered with rat glue (Figure 3).
Figure 5. Pest Control Method Used during the Experiment. Arrowhead shows a) Yellow Plastic Trap and b) Trapped Grasshopper
Rhizomes were planted following planting space for K. galanga (20 cm x 15 cm) since there was no detailed information for K parviflora cultivation. However, as the plant grew it seems that K. parviflora need bigger spaces. In separate experiment, we planted K. parviflora in 30 cm x 30 cm planting space which seems to be more sufficient.
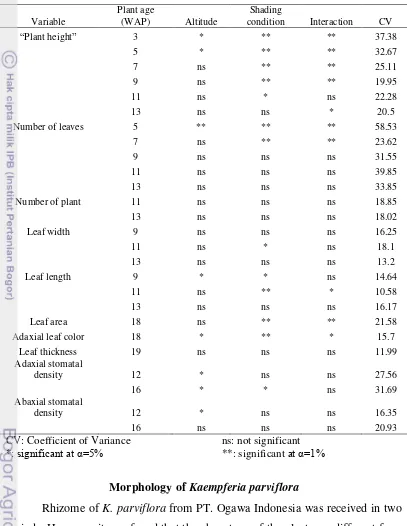
Bartlett test showed that condition between treatments was homogen, thus F-test can be done. Recapitulation of treatments analysis of variance was shown at Table1. Variables including “plant height”, number of leaves, number of plants
per rhizome planted, leaf width, leaf length, leaf area, adaxial leaf color, leaf thickness, adaxial stomata density, and abaxial stomata density.
Table 1. Recapitulation of Treatments Analysis of Variance
Variable
CV: Coefficient of Variance ns: not significant
*: significant at α=5% **: significant at α=1%
Morphology of Kaempferia parviflora
Only one week after germination, plant from the first phenotype formed several leaves. In contrast, plants germinated from later phenotype grew slowly by forming only one leaf after four weeks in the field. Both phenotypes showed similarities, in the color of rhizome and in the color and shape of flower. The most significant different between them is their shoot phenotype. The first phenotype had narrower leaf than the later phenotype which had broader leaf (figure 6a and 6b). The other differences observed were the petiole, stem, and leaf color.
The first phenotype had longer petiole, less green leaf color, and no purplish color at the abaxial part of the leaves. Furthermore, the first phenotype had slightly smaller and flat-shaped stem. The first phenotype of K. parviflora
was planted at individual pot at Bogor Agricultural University, Darmaga.
Figure 6. Phenotype of K. parviflora, a) First Phenotype Plant, b) Narrower Leaf of First Phenotype, c) Second Phenotype Plant and d) Broader Leaf of Second Phenotype
The second phenotype of K. parviflora is shown in Figure 6 (c and d). The plane of disitchy of leaves is parallel to rhizomes. This plant had single blade leaf. The leaf was broadly elliptic with rounded base, 8-21 cm long, and 5-11 cm wide. The border of the leaf was wavy and often red. Adaxial part of K. parviflora type leaf was dark green to green, and abaxial part was gradation of red and green. The Adaxial part color and the composition between red and green on abaxial part of leaf was vary depend on environment acclimation.
Inflorescence appears on the terminal of the stem between the leaves. Graham et al. (2006) stated that inflorescence are cluster of flowers with one main
a b
flower stalk, the peduncle, from which emerge many secondary flowers stalks, termed pedicels, each with flower as it tip. Kaempferia parviflora have one to four flowers from one inflorescence. Flower of K. parviflora are white with purple spot. The flower is irregular or bilaterally symmetrical; the flower can be cut only along one plane to produce equal halves. Plant, inflorescence, and flower are shown in Figure 7.
Figure 7. Kaempferia parviflora Flower, a) Arrowhead shows Inflorescence and b) Flower
Cross section of K. parviflora rhizome is orbicular or ellipse and have a circle line in the center. The flesh color is violet to blackish purple. Rhizome contains bud that will grow to individual plant. As dormancy broken, a shoot appear from this bud. The energy needed to form this shoot taken from the rhizome.
Kaempferia parviflora can be distinguished with the K. galanga or galingale, the common herbaceous plant from Indonesia, from the absence of purplish color that K. parviflora has in its leaf and stem, the color of the rhizome, and also from the flower. Galingale has a bigger flower (about 2.5-3 cm) than K. parviflora and have ribbon-like petal flower (Figure 8).
Figure 8. Kaempferia galanga Plant. a) Rhizome and b) Appearance above the Ground (Rostiana and Effendi (2007))
a b
Growth of Kaempferia parviflora
Kaempferia parviflora was propagated using rhizome. Bud appeared from this rhizome and elongated. Inside this bud, leaf primordial and inflorescence was formed. The root appeared near the bottom side of the shoot. The rhizome became smaller as the shoot grew longer then leaf expanded. After the leaves expanded, flower bloomed. Some plant has only one leaf while other can have two to three leaves. Then, new plant grew from another bud on the rhizome or from the base of the plant. The plant then formed a new rhizome which harvested. The speed of growth was different depend on the treatment given. The sequence of K. parviflora growth is shown in Figure 9.
Figure 9. Growth of K. parviflora from Rhizome to Expanded Leaf, a) Rhizome Planted, b) Shoot Emerged, c) Folded Leaf, d) One Leaf Opened Other Leaf Folded, and e) Leaves Opened.
Altitude Affect the Early Vegetative Growth of K. parviflora
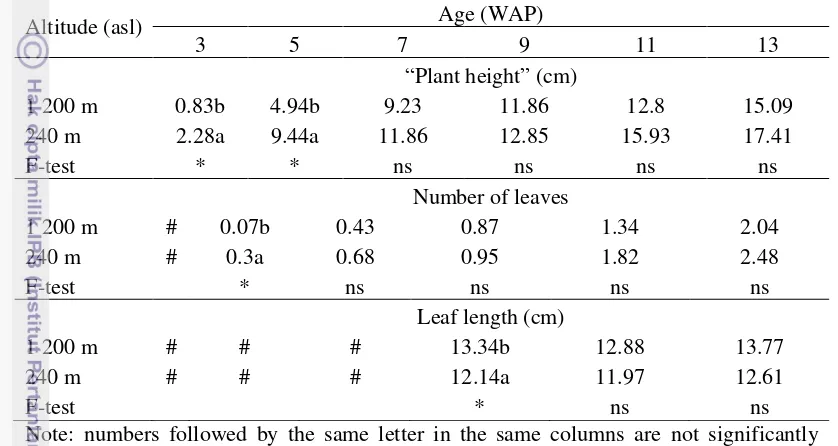
The results showed that different altitude affect the early vegetative growth of K. parviflora. As shown in Table 2, plants grown at Cikabayan Experimental Field (240 m asl) had higher “plant height” and higher number of leaves until 5 WAP compared to plants grown at Pasir Sarongge Experimental Field (1 200 m asl). At 9 WAP, plants grown at Cikabayan Experimental field also had longer leaf than those grown at Pasir Sarongge Experimental Field.
Altitude only affects the early vegetative growth of K. parviflora. At 7 WAP, “plant height” and number of leaves showed no significant different. At early stage of K. parviflora growth, this plant used nutrition stored in the rhizome through respiration process. Respiration process is temperature dependent. For many species of plants, Q10 reaction usually 2.0 to 2.5 at temperature between 5 and 25oC. If temperature goes higher until 30 or 35oC, respiration rate still increased, yet slower (Salisbury and Ross, 1995). Higher temperature at low altitude made respiration rate of K. parviflora higher. Thus, K. parviflora grew
faster at low altitude. At high altitude the growth was slower and after the growth reached a critical point, altitude showed no different effect on “plant height”, number of leaves, and leaf length.
Table β. Effect of Altitude on “Plant Height”, Number of Leaves, and Leaf Length of K. parviflora
* significant at P < 0.05; # not observed; ns not significant WAP: week after planting
Leaf color was affected by altitude. As shown in Table 3, both adaxial and abaxial leaf color showed different response at different altitude. Higher value of abaxial leaf color means higher composition of red color to green. Higher value of adaxial color means greener color. At 1 200 m asl, abaxial leaf color had more red composition than 240 m asl. Adaxial leaf color also greener at 1 200 m asl.
Table 3. Effect of Altitude at Abaxial and Adaxial Leaf Color
Altitude (asl) Abaxial leaf color
Analyzed using Kruskal-Wallis test; *significant at 0.01 < P < 0.05; **significant at P < 0.01.
Abaxial leaf color of K. parviflora has reddish color that appeared mostly at high altitude. Red coloration occurs commonly in the vegetative organs of vascular plants. This coloration is due to the accumulation of anthocyanin. Anthocyanin has function of photoprotection or free radical scavenging (Lee and Collins, 2001). Leaves commonly synthesize anthocyanins during the nascent and/or senescing stages of their ontogeny, or upon exposure to environmental stressor such as drought, strong light, and low temperature. In those situations, plants probably at their most vulnerable to the effect of oxidative stress. Anthocyanin have the potential to mitigate photoinhibitory and photooxidative damage in the leaves by reducing the incidence of high-energy quanta striking the chloroplasts, and by scavenging free radicals before they cause structural injury to membranes (Neill and Gould, 2003).
Anthocyanin is one of flavonoid that becomes the reason why K. parviflora planted. Anthocyanin is well known as antioxidant. The high content of leaf anthocyanin on K. parviflora at high altitude maybe has a correlation to anthocyanin in its rhizome, but it needs further analysis after the rhizome harvested.
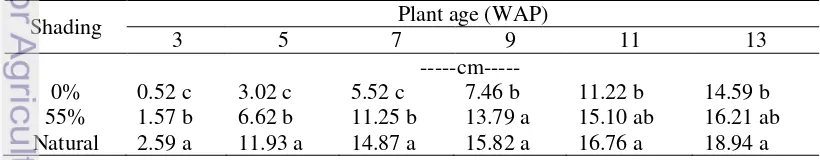
Shading Conditions Affect Vegetative Growth of K. parviflora Differently Shading affects vegetative growth of K. parviflora at “plant height”, number of leaves per rhizome planted, number of plant per rhizome planted, leaf size, and leaf color. Plants grown under natural shading condition had taller plants than those grown under 55% artificial shading and full sun conditions at their early growth until 7 WAP (Table 4).
The increased stem elongation is thought to allow the plants to place their leaves above their neighbors, increasing light interception. Light that has passed through a canopy of leaves has a reduced red to farred ratio (R : FR). Via the elongation. Simulated canopy shading (high levels of far-red light) induced these plants to allocate more of their resources to growing taller. This correlation did not hold for “shade plants,” which normally grow in a shaded environment. Shade plants showed little or no reduction in their stem extension rate as they were exposed to higher R/FR values (Taiz and Zeiger, 2002).
Shading conditions showed different effects on number of leaves only at the early stage of growth until 7 WAP (Table 5). After 8 WAP, plants grown under natural shading, 55% artificial shading, and full sun conditions had similar number of leaves. As shown in Table 5, until 7 WAP plants grown under natural shading condition had the highest number of leaves, followed by those grown under 55% artificial shading, and under full sun condition.
Table 5. Effect of Shading at Number of Kaempferia parviflora Leaves
Shading Plant age (WAP)
Table 6. Effect of Shading at Number of Plant per Rhizome Planted
Based on our results, the best vegetative early growth was shown by plants grown under natural shading. Response of growth at artificial shading at some variable was as good as response at natural shading. Plants grown under full sun condition showed the slowest growth rate. It seems that K. parviflora was a shade plant. Serrano et al (1995) showed that leaf area, length of petioles, and chlorophyll content were higher for shade-plants than sun-plants. There are typical acclimation events, allowing effective absorption of light energy.
Shading condition also affected adaxial leaf color but not the abaxial leaf color. Plants grown under natural shading and 55% artificial shading had greener adaxial leaf color than those grown under full sun condition (Table 7). Salisbury and Ross (1995) suggested that low light intensity was affecting the orientation of chloroplast. At low light intensity, chloroplast will gather at two part at the nearest and farest area from the light. This caused shaded leaf appears greener than full sun leaf.
Table 7. Effect of Shading to Adaxial Leaf Colorof Kaempferia parviflora
on light harvesting complex II (Sasmita, 2006). Kisman et al. (2007) showed that soybean (Glycine max) chlorophyll a and b content under low light intensity stress was higher. The ratio of chlorophyll a/b was lower at tolerant genotype than sensitive genotype. Increased of chlorophyll b was faster than increased of chrolophyll a, resulted in low ratio of chlorophyll a/b on tolerant genotype.
When grown without shading, leaves of K. parviflora were yellowish green. The yellowish green color of adaxial leaf was probably because the presence of carotenoid. Sarijave et al. (2007) reported that sun leaves of ginkgo (Ginkgo biloba L.) and beech (Fagus sylvatica L.) possessed higher levels in chlorophylls (Chl) and carotenoids on a leaf area basis, higher values for the ratio Chl a/b and lower values for the ratio Chl/carotenoids which resulted in lighter leaf color in comparison to shade leaves.
Carotenoid is an accessory pigment in plant. It absorbs light wavelength from 450 to 550 nm, indicating that energy transfer from carotenoids to chlorophylls is not as effective as energy transfer between chlorophylls. In addition to their role as accessory pigments, carotenoids play an essential role in photoprotection. Carotenoids exert their photoprotective action by rapidly quenching the excited state of chlorophyll. The excited state of carotenoids does not have sufficient energy to form singlet oxygen, so it decays back to its ground state while losing its energy as heat (Taiz and Zeiger, 2002).
Combination of Altitude and Shading Treatment
To know the best combination treatment resulted in best vegetative growth some variables were analyzed further, which was “plant height” (Table 8), number shoot emerged, leaf area, and adaxial leaf color (Table 9).
Number of shoot emerged was measured by counting rhizome that showed
Altitude (asl) Shading Grew shoot (%) Leaf area (cm
2
Plants grown at 240 m asl under natural shading had the highest value of leaf area. Plants grown at 240 m asl without shading treatment had the lowest leaf area. There were no significant difference in leaf area of plants grown at 240 asl under 55% shading and those grown at 1 200 m asl. Plants grown at 240 m asl under natural shading condition also had the highest value of adaxial leaf color. In contrast, plants grown at 240 m asl without shading treatment showed the lowest value of adaxial leaf color.
expand more to catch light more efficiently. On the contrary, at same altitude without shading, the leaves of the plant were more erect to reduce light acceptance. The difference acclimation between treatments showed at Figure 10.
Figure 10. Different Growth of K. parviflora on 12 WAP at 240 m asl with a) Natural Shading, 55% Shading and c) Without Shading
Kaempferia parviflora is shade plant. Most of Zingiberaceae grow at the floor of tropical forest, where light intensity was low. Lower light compensation point and CO2 compensation point is one of characteristic of shade plant photosynthesis (Yanyou et al., 2007). It means, shade plants have a lower light saturation point. Excess of light can caused photo-destruction and limiting growth. Plants grown at 240 m asl without shading might experience photoinhibition because of high temperature and light intensity. Chronic photoinhibition results from exposure to high levels of excess light that damage the photosynthetic system and decrease both quantum efficiency and maximum photosynthetic rate. In contrast to dynamic photoinhibition, these effects are relatively long-lasting, persisting for weeks or months (Taiz and Zeiger, 2002). Disturbed photosynthesis means depletion on solute produced. It leads to inhibition of growth.
Mostly, reaction which catalyzed by enzyme will increase as temperature increased from 0o to 35o or 40oC. Reaction was doubled or tripled by 10o increased temperature. Higher than 40oC, the reaction is depleted because enzyme starts to denaturized (Salisbury and Ross, 1995).
Low altitude and no shading created a condition that high in light intensity and temperature. The maximum air temperature reached 33oC and with absence of shading, the energy received by plant was high. So, low rate of growth at 240 m asl with no shading perhaps because enzyme denaturation.
b
Both artificial shading and natural shading, which have lower temperature than 0% shading, resulted in highest growth rate. Although the air temperature was not significantly different among treatment without shading and with shading at same altitude, the energy received by plant at shaded condition was lower because shading reduced light intensity. The energy from light at high temperature with shading was high enough to increase enzyme activity but not to denaturize it. At high altitude, there was no significant different between shading treatment.
The temperature at 240 m asl under full sun condition was higher, while the RH was lower than other treatment at same altitude. The environment at 240 m asl without shading condition resulted in higher temperature and lower relative humidity. At noon, temperature at 240 m asl without shading can reach approximately 33oC. High temperature resulted in higher evapotranspiration. Thus, at the same amount of water given, under full sun condition there was higher water loss than other treatment trough evaporation and transpiration. Water deficits can lead to inhibition of plant growth and photosynthesis. Figure 11 showed physiological changes that plant experience due to dehydration. The intensity of the bar color corresponds to the magnitude of the process, darker color indicate higher magnitude.
Figure 11. Physiological changes due to dehydration (Taiz and Zeiger, 2002)
shading. Plants grown under artificial shading 55% at 240 m asl had similar vegetative growth with those grown at 1 200 m asl.
Leaf Anatomy of Kaempferia parviflora at Different Treatment Leaf Size
Leaf size was affected mostly by shading conditions, while altitude did not affect leaf width, leaf length, leaf area, and leaf thickness. There was no different in leaf width between plants grown under different shading conditions from 9 until 13 WAP (data not shown). The narrowest leaf ever counted was 4 cm, and the broader leaf was 12 cm. The average leaf width was 7-8 cm.
Plants grown under different shading conditions had different leaf length, which resulted in different leaf area (Table 10). Plants grown under natural shading had the longest leaf at 13 WAP, and the biggest leaf area at 16 WAP. Leaf of the plant grown under shade was bigger in order to enhance the light absorption (Taiz and Zeiger, 2002).
Table 10. Effect of Shading Conditions on Leaf Size of K. parviflora
Shading Leaf length (cm) Leaf area (cm
2
properties that allow light to pass through, and the spongy mesophyll cell properties that are conducive to light scattering, result in more uniform light absorption throughout the leaf. This result showed that under shading the leaf is thinner (Table 11, Figure 12) however statistically not different.
Table 11. Leaf Thickness of K. parviflora at Different Shading Condition at 18 WAP (measure on first fully opened leaf)
Shading Leaf Thickness (μm)
0% 319.15 a
55% 300.88 a
Natural 286.92 a
Note: numbers followed by the same letter in the same columns are not significantly different based on DMRT at level α = 5%.
Different characters at leaf area and leaf thickness is shade avoidance response from plant that grown under low light intensity is aimed to maximize light absorption thus more efficient photosynthesis (Taiz and Zeiger, 2002).
Kaempferia. parviflora showed no signifficant difference among treatment perhaps because this plant did not have a great ability to adapt at high light intensity.
Figure12. Leaf Thickness of K. parviflora at Different Treatment, a) 1 200 m asl 0% shading, b) 1 200 m asl 55%, c) 1 200 m asl natural shading, d) 240 m asl 0% shading, e) 240 m asl 55% shading, and f) 240 m asl natural shading
a b c
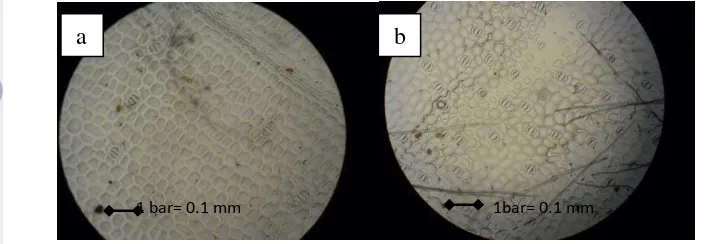
Stomatal density
Stomatal density observed using microscope with 400X magnification. Sample observed is shown at Figure 13. Stomatal density was higher at abaxial part of leaf than adaxial part of leaf.
Figure 13. Stomatal density of K. parviflora at a) adaxial leaf part and b) abaxial leaf part oak) leaves, and Nothofagus solandri var. cliffortioides (mountain beech). While Belhadj et al. (2011) showed that abaxial leaf stomatal density decreases significantly compared to that of lowland habitats for pistachio (Pistachia atlantica).
* significant at P < 0.05; # not observed; ns not significant WAP: week after planting
Stomatal density was affected by shading condition. Under 0% shading, stomatal density was higher. Both under artificial and natural shading stomatal density was fewer than under full sun condition (Table 13).
1 bar= 0.1 mm 1bar= 0.1 mm
Table 13. Effect of Different Shading Condition at Stomatal Density
Shading Adaxial Abaxial
12 WAP 16 WAP 12 WAP 16 WAP 0% 486.92 a 644.84 a 3303.2 a 3330.6 a 55% 434.28 a 460.60 b 2803.1 ab 2816.2 a Natural 394.80 a 421.12 b 2671.5 b 3040.0 a Note: numbers followed by the same letter in the same columns are not significantly
different based on DMRT at level α = 5%.
In open places, plants are exposed to higher fluence rates of photosynthetically active radiation and to higher red to far-red ratios than under the shade of neighbor plants. Boccalandro (2009) showed that higher red to far-red ratios increase stomatal density in Arabidopsis (Arabidopsis thaliana). This was goes along with our result which showed that without shading (high red-far red ratio) had higher stomatal density.
According Büssis et al. (2006) stomatal density was not the only factor affected CO2 assimilation. Using transgenic Arabidopsis thaliana over-expressing the SDD1 (stomatal density and distribution) and sdd1-1 mutant plants, CO2 assimilation rate and stomatal conductance of expressers plants and the sdd1-1
CONCLUSION AND SUGGESTION
Conclusion
The conclusion of this research is that K. parviflora is shade plant and best grown under shade condition. The adaptation range of K. parviflora is wide enough, including both altitudes 240 m asl and 1200 m asl respectively.
The best combination treatment resulted in best early vegetative growth of
K. parviflora is at 240 m asl under natural shading. Growing K. parviflora at 240 m asl without shading is not appropriate. This does not mean that K. parviflora
should be planted at low altitude with natural shading because the observations done in this research were only at the early vegetative growth and not including yield of rhizome harvested or bioactive content.
Suggestion
The suggestion can be given from this research is to cultivate K. parviflora
under shading conditions, both artificial and natural shading. With economic consideration, using artificial shading is less economic than used natural shading at intercropping system. Because of K. parviflora took a relatively long time before harvested (about 12 months) maybe it best to intercrop this plant with woody perennial.
REFERENCES
Ammer, C. 2003. Growth and biomass partitioning of Fagus sylvatica L. and
Quercus robur L. seedlings in response to shading and small changes in the R/FR-ratio of radiation. Ann. For.Sci. 60:163-171.
Ardie, S. W. 2006. Pengaruh Intensitas Cahaya dan Pemupukan terhadap Pertumbuhan dan Pembungaan Hoya diversifolia Blume. Tesis. Institut Pertanian Bogor. Bogor. 55 hal.
Bartlett, G. A. and W. R. Remphrey. 1998. The effect of reduced quantities of photosynthetically active radiation on Fraxinus pennsylvanica growth and architecture. Can. J. Bot. 76:1259-1365
Belhadj, S., A. Derridj, A. Moriana, M. D. C. Gijon, J. Mevy, and T. Gauquelin. 2011. Comparative analysis of stomatal characters in eightwild atlas pistachio populations Pistacia atlantica Desf. (Anacardiaceae). Intern. Res. J. Plant Sci. 2(3): 060-069
Büssis, D., U. V. Groll, J. Fisahn, and T. Altmann. 2006. Stomatal aperture can compensate altered stomatal density in Arabidopsis thaliana at growth light conditions. Func. Plant Biol. 33:1037-1043.
Boccalandro, H. E., M. L. Rugnone, J. E. Moreno, E. L. Ploschuk, L. Serna, M.J. Yanovsky, and J. J. Casal. 2009. Phytochrome B enhances photosynthesis at the expense of water-use efficiency in Arabidopsis. Plant Physiol. 150:1083-1092
Bron, V. H., C. Robin, C. Varlrt-Grancher, D. Afif, and A. Guckert. 1999. Light quality (red:far-red ratio): does it affect photosynthetic activity, net CO2 assimilation, and morphology of young white clover leaves?. Can. J. Bot. 77:1425-1431.
Chansakaow, S., Y. Srigiofun, L. Chitarree, and K. Panyakard. 2005. Effect of Light Intensity and Soil Amendments on Total Phenolics and Antioxidant Activity of Kaempferia parviflora Wall. Ex. Bak. 31st Congress on Science and Technology of Thailand at Suranaree University of Technology
Graham, L. E., J. M. Graham, and L. W. Wilcox. 2006. Plant Biology, Second Edition. Pearson Education, Inc. USA. 670 p.
Harjadi, S. S. 1989. Dasar-dasar Hortikultura. Jurusan Budidaya Pertanian Faperta. IPB. Bogor. 500 hal.
Joy, P. P., J. Thomas, S. Mathew, and B. P. Skaria. 1998. Zingiberaceous Medicinal and Aromatic Plants. Aromatic and Medicinal Plants Research Station, Odakkali, Asamannoor P.O., Kerala, India.
Kisman, N. Khumaida, Trikoesoemaningtyas, Sobir, dan D. Sopandie. 2007. Karakter morfo-fisiologi daun, penciri adaptasi kedelai terhadap intensitas cahaya rendah. Bul. Agron. 35 (2): 96-102
Kouwenberg, L. L. R., W. M. Kurschner, and J. C. McElwain. 2007. Stomatal frequency change over altitudinal gradients: prospects for paleoaltimetry. Reviews in Mineralogy & Geochemistry.66: 215-241
Lee, D. W. and T. M. Collins. 2001. Phylogenetic and ontogenetic influences on the distribution of anthocyanins and betacyanins in leaves of tropical plants. Int. J. Plant Sci. 162(5):1141-1153
Lee, D. W., S. F. Oberbauer, P. Johnson, B. Krishnapilay, M. Mansor, H. Mohamad, and S. K. Yap. 2000. Effect of irradiance and spectral quality on leaf structure and function in seedlings of two Southeast Asean Hopea
(Diterocarpaceae) species. Am. J. Bot. 87:447-455
Maliakal, S. K., K. McDonnell, S. A. Dudley, and J.Schmitt. 1999. Effects of red to far-red ratio and plant density on biomass allocation and gas exchange in Impatiens capenensis. Int. J. Plant Sci. 160(4):723-733
Neill, S. O. and K. S. Gould. 2003. Athocyanins in leaves: light attenuators or antioxidants?. Funct. Plant Biol. 30. 865-873
Nybe, E. V. and N. M. Raj. 2004. Ginger Production in India and Other South Asian Countries. In P. N. Ravindran and K. N. Babu (Eds). Ginger: The Genus Zingiber. CRC Press. Florida. 550 p.
Onwueme, I. C. and M. Johnston. 2000. Influence of shade on stomatal density, leaf size, and other characteristics in the major tropical root ceops, tannia, sweet potato, yam, cassava, and taro. Exprimental Agric. 36:509-516 Oquist, G. and B. Martin. 1986. Cold Climate. In N. R. Baker and S.P. Long
(Eds). Photosynthesis in Contrasting Environtment. Elsevier Science Publisher. New York. 423 p.
Patanasethanont D., J. Nagai, R. Yumoto, T. Murakami, K. Sutthanut, B. Sripanidkulchai, C. Yenjai, and M. Takano. 2007. Effects of Kaempferia
parviflora extracts and their flavone constituents on P-glycoproteinfunction. J. Pharmaceutical Sci. 96:223-233.
Wall. ex Baker). Proceeding of the 46th Kasetsart University Annual Conference. p 118-124.
Putiyanan, S., S. Chansakaow, A. Phrutivorapongkul, and W. Charoensup. 2008. Standard pharmacognostic characteristic of some Thai herbal medicine.CMU.J. Nat. Sci. 7(2):239-255.
Rajagopalan, A. and P. K. Gopalakrishnan. 1985. Growth, yield and quality of
Kaempferia galanga as influenced by planting time and types of seed material. Agric. Res. J. Kerala. 23 (1):83
Ren, H. X., Z. L. Wang, X. Chen, and Y. L. Zhu. 1999. Antioxidative responses to different altitudes in Plantago major. Environ. Exp. Bot. 42: 51–59 Rostiana, O. dan D. S. Effendi. 2007. Teknologi Unggulan Kencur. Pusat
Penelitian dan Pengembangan Perkebunan. Bogor. 14 hlm.
Rujjanawate, C., D. Kanjanapothi, D. Amornlerdpison, and S. Pojanagaroon. 2005. Antigastric ulcer effect of Kaempferia parviflora. J. Ethnopharmacol 102:120–122.
Runkle, E. 2008. Principles of lights. Orchid. May:350-353
Salisbury, F. B. dan C. W. Ross. 1995. Fisiologi Tumbuhan. Terjemahan D. R. Lukman dan Sumaryono. Jilid I, II, III. ITB. Bandung. 757 hal.
Sarijave, G., M. Knapp, and H. K. Lichtenthaler. 2007. Differences in photosynthetic activity, chlorophyll and carotenoid levels, and in chlorophyll fluorescence parameters in green sun and shade leaves of
Ginko and Fagus. J. Plant Physiol. 164: 950-955.
Sasmita, P., B. S. Purwoko, S. Sujiprihati, I. Hanarida, I. S. Dewi, dan M. A. Chozin. 2006. Evaluasi pertumbuhan dan produksi padi gogo haploid ganda toleran naungan dalam sistem tumpang sari. Bul. Agron. 34(2): 79-86
Scott, P. 2008. Physiology and Behavior of Plants. John Wiley & Sons Ltd. England. 305p
Serrano, L., F. I. Pugnaire, F. Domingo, and J. A. Pardos. 1995. Absorption of radiation,photosynthesis, and biomass production in plants, p. 246-256. In: Handbook of Plant and Crop Physiology. Ed. M. Pessarakli. Marcel Dekker, Inc., New York.
Sivarajan, V. V. and I. Balachandran. 1994. Ayurvedic Drugs and Their Plant Sources. Oxford and IBH Publishing Co. Pvt. Ltd., New Delhi. 570p. Smith, H. 2000. Phytochromes and light signal perception by plants - an emerging
synthesis. Nature 407:585-591.
Sookkongwaree, K., M. Geitmann, S. Roengsumran, A. Petsom, and U. H. Danielson. 2006. Inhibition of viral proteases by Zingiberaceae extracts and flavonoids isolated from Kaempferia parviflora. Pharmazie 61:717– 721.
Suk, K., 2005. Regulation of neuroinflammation by herbal medicine and its implications for neurodegenerative diseases: A focus on traditional medicines and flavonoids. Neurosignals 14:23-33.
Sukaesih, E. 2002. Studi Karakter iklim Mikro pada Berbagai Tingkat Naungan Pohon Karet dan Pengaruhnya Terhadap Pertumbuhan 20 Genotipe Kedelai (Glycine Max (L) Merr.) Skripsi. Depertemen Budidaya, Fakultas Pertanian, Institut Pertanian Bogor. Bogor.
Taiz, L. and E. Zeiger. 2002. Plant Physiology, Third Edition. Sinauer. Sunderland. 690p.
Warrier, P. K., V. P. K. Nambiar, and C. Ramankutty. 1993-1995. Indian Medicinal Plants. Vol.1-5. Orient Longman Ltd. Madras.
Yanyou, W., P. Li, Y. Zhao, J. Wang, and X. Wu. 2007. Study on photosynthetic characteristics of Orchophragamus violaceus related to shade tolerance. Scientia Horticulturae 113:173–176
Appendix 1. Climate Data at Darmaga Area during March-July 2011, Darmaga Climatology Station (continued)
Date
MARCH APRIL MAY JUNE JULY
Average
Temperature RH
Average
Temperature RH
Average
Temperature RH
Average
Temperature RH
Average
Temperature RH (ºC) (%) (ºC) (%) (ºC) (%) (ºC) (%) (ºC) (%)
25 25.4 86 25.5 83 26.1 86 26.3 75 25.3 78
26 25.4 86 25.9 87 26.5 85 25.5 74 25.6 75
27 26.2 82 25.6 91 26.4 86 26.7 76 25.7 77
28 25.4 81 26.5 81 26.7 84 25.8 88 25.6 78
29 26.2 79 25.2 92 25.1 81 25.4 86 25.7 75
30 23.8 91 25.2 92 25.7 87 25.0 87 26.4 73
31 24.7 88 26.7 82 25.9 70
Appendix 2. Climate Data at Pasir Sarongge Area during March-July 2011, Pasir Sarongge Climatology Station (continued)
Date
MARCH APRIL MAY JUNE JULY
Average
Temperature RH
Average
Temperature RH
Average
Temperature RH
Average
Temperature RH
Average
Temperature RH (ºC) (%) (ºC) (%) (ºC) (%) (ºC) (%) (ºC) (%)
25 19 90 22 77 20 86 20 78 21 71
26 20 87 21 78 20 82 21 72 20 71
27 19 84 20 87 22 87 21 74
28 10 85 21 86 21 78 19 87
29 20 82 20 85 22 75 21 79
30 20 85 20 86 21 78 21 81
31 19 87 21 75
Appendix 3. Soil Sample Analysis Result
Sample
Texture Extract 1:5 pH Organic Material Olsen Morgan Cation exchange value (NH4- Acetat 1N, pH7)
Sand Dust Clay H2O KCl
Walkley &
Black C Kjeldahl N C/N P2O5 K2O Ca Mg K Na Amount CEC AS
---%--- ---%---- ---ppm--- ---cmolc/kg--- %
CB A 16 15 69 5.5 4.9 1.77 0.19 9 36 123 9.87 1.15 0.24 0.37 11.63 10.76 >100
CB B 40 48 12 5.5 4.9 2.34 0.23 10 76 281 10.47 1.73 0.56 0.36 13.12 10.61 >100
SR A 39 35 26 5.1 4.6 3.63 0.32 11 43 247 8.86 1.54 0.47 0.24 11.11 13.19 84
SR B 36 56 8 5.7 5.2 4.86 0.34 14 127 1098 18.91 3.81 2.12 0.54 25.38 24.33 >100
CB A: Cikabayan (240 m asl) 55% shading and 0% shading CB B: Cikabayan (240 m asl) natural shading
df MS
Adaxial stomatal density 12 WAP
df MS 16 WAP
Shading 2 532024.03
Rep (altitude) 6 414502.76
Altitude 1 2399559.59
Altitude* Shading 2 253433.50
Error 12 410885.47
ALTITUDE AND SHADING CONDITIONS
AFFECT VEGETATIVE GROWTH OF
Kaempferia parviflora
EVI
A24070015
ABSTRACT
EVI. ALTITUDE AND SHADING CONDITIONS AFFECT VEGETATIVE GROWTH OF Kaempferia parviflora. (Supervised by NURUL KHUMAIDA and SINTHO W. ARDIE)
Kaempferia parviflora is a native plant of Thailand which potentially developed in Indonesia because of its pharmacological values. Thus, in order to develop appropriate cultivation system of K. parviflora in Indonesia, this research was conducted to study the effect of different altitudes and shading conditions on the vegetative growth of K. parviflora.
The experiment was arranged in Split-plot Nested design, where the main plot was altitude with two factors (1 200 m asl at Pasir Sarongge Experimental Farm and 240 m asl at Cikabayan Experimental Farm), the subplot was three levels of shading condition (0% shading, 55% shading, and natural shading), and replication was nested at subplot.
The results showed that there was no significant different in plant height, number of leaves, and leaf area of K. parviflora grown at 1 200 m asl and 240 m asl respectively. However, higher altitude affects the color of K. parviflora leaves. Plants grown at higher altitude (1 200 m asl) had greener adaxial leaf color and more reddish abaxial leaf color than plants grown at lower altitude. The vegetative growth of K. parviflora was more affected by shading conditions. Kaempferia parviflora had taller plant, higher leaf area, and greener adaxial leaf color under natural shading than under full sun condition. Plants grown under natural shading and 55% artificial shading also had higher number of leaves at the early vegetative growth than those grown under full sun condition. Plants grown under 55% artificial shading showed similar growth with plants grown under natural shading, except that plants under natural shading had higher leaf area than plants grown under 55% artificial shading. The best combination between altitude and shading was of 240 m asl with natural shading. Based on the early vegetative growth,
INTRODUCTION
Background
The use of medicinal plants as herbal drugs is increasing rapidly. Ginger is the common name given to members of the Zingiberaceae family, a group of tropical, rhizomatous, herbaceous perennials which have been gained much notoriety in the list of medicinal plants. The rhizomes of species from this family are known to have many pharmacological values.
Kaempferia parviflora is one of the plants in the Zingiberaceae family originated from Thailand. In its origin, K. parviflora is known as kra-chai-dam, Thailand ginseng, or black galingale (Putiyanan et al., 2008). Recently, K. parviflora has been reported to possess anti-mycobacterial, anti-plasmodial (Yenjai et al., 2004), anti-peptic ulcer (Rujjanawate et al., 2005), and anti-viral protease effects (Sookkongwaree et al., 2006) as well as modulators of multi-drug resistance in cancer cells (Patanasethanont et al., 2007). Because of its pharmacological benefits and the increasing trend of herbal consumption in Indonesia, K. parviflora is potentially developed in Indonesia.
Studies on the plant adaptation on the local agro-climate are necessary in order to domesticate K. parviflora in Indonesia. As the member of Zingiberaceae family, K. parviflora might share similar cultivation system as other member grown in Indonesia, i.e. ginger (Zingiber officinale), galingale (Kaempferia galanga), galangal (Alpinia galanga), and turmeric (Curcuma longa). However, as a medicinal plant, cultivation system should be designed to produce high yield in biomass as well as in the level of bioactive compound. Previous studies showed that the level of fenolic compound in the rhizome of K. parviflora was affected by light intensity (Chansakaow et al.,2005) and by altitude (Pojanagaroon, 2008).
Chansakaow et al. (2005), showed that K. parviflora grown at 60% shading produce highest fenolic compound, and at 80% shading produce highest antioxidant. Pojanagaroon (2008) showed that low temperature increased fenolic compound and coloring material of K. parviflora.
Therefore, as a preliminary step to determine the best cultivation system of