THE FRUIT BATS (MEGACHIROPTERA, PTEROPODIDAE)
FROM BAWAKARAENG MOUNTAIN, SOUTH SULAWESI
ELLENA YUSTI
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
STATEMENT ABOUT THESIS, INFORMATION SOURCES,
AND ACT OF SPILLING OVER COPYRIGHTS*
By this writing I clarify that the graduate thesis The Fruit Bats (Megachiroptera, Pteropodidae) From Bawakaraeng Mountain, South Sulawesi is my own work under the supervisions of the advising committee and has not been proposed for any institution. Copied information source of published and unpublished writing of other author has been mentioned in the text and incorporated in the references at the last part of this thesis.
By this writing I hand over the copyright of my thesis to Bogor Agricultural University.
Bogor, February 2015
RINGKASAN
ELLENA YUSTI. Kelelawar Pemakan Buah (Megachiroptera, Pteropodidae) Dari Gunung Bawakaraeng Sulawesi Selatan. Dibimbing oleh BAMBANG SURYOBORTO dan IBNU MARYANTO.
Kelelawar pemakan buah (Megachiroptera, Pteropodidae) berperan penting dalam ekosistem sebagai penyebar biji dan polinator, umumnya ditemui di wilayah perkebunan, hutan primer dan hutan sekunder. Keanekaragaman kelelawar pemakan buah dipengaruhi oleh sumber pakan, tipe habitat, ketinggian dan faktor-faktor lingkungan, yaitu curah hujan, angin, fase bulan yang mempengaruhi aktivitas kelelawar dan jumlah individu yang tertangkap. Penelitian kelelawar pemakan buah (Megachiroptera, Pteropodidae) di kawasan Gunung Bawakaraeng, Sulawesi Selatan bertujuan untuk mengetahui komposisi spesies, termasuk kategori umur dan status reproduksi kelelawar betina, keanekaragaman jenis kelelawar pemakan buah dan preferensi habitat di berbagai tipe habitat, serta untuk mengetahui pengaruh dari fase bulan terhadap jumlah individu yang tertangkap.
Sebanyak 265 individu kelelawar pemakan buah didapatkan dengan menggunakan perangkap jaring kabut (mist net) pada lima tipe habitat yang berbeda, yaitu hutan sekunder (1200 m dpl), perkebunan campuran (1453 m dpl), hutan pinus (1545 m dpl), hutan primer (2000 m dpl) dan gua (2200 m dpl) di kawasan Gunung Bawakaraeng, Sulawesi Selatan. Kelelawar pemakan buah yang didapatkan terbagi atas tujuh genus yang meliputi 10 spesies dengan kurva estimasi menunjukkan 12 spesies yang berarti dua spesies belum tertangkap. Komposisi kelelawar yang tertangkap meliputi Boneia bidens (159 individu; 60%), Thoopterus suhaeniahae (50 individu, 18.86 %), Rousettus celebensis (21 individu; 7.95%), Thoopterus nigrescens (11 individu; 4.16%), Eonycteris spelaea (9 individu; 3.40%), Rousettus amplexicaudatus (6 individu; 2.27%), Dobsonia viridis (4 individu; 2.27%), Styloctenium wallacei (2 individu; 0.75%), Dobsonia exoleta (2 individu; 0.75%) and Cynopterus luzonensis (1 individu; 0.37%).
Analisis keanekaragaman Shannon-Wiener menunjukkan nilai tertinggi di perkebunan campuran (1453 m asl) (H=1.80), sedangkan nilai terendah di hutan pinus (1545 m dpl) (H=0.32). Nilai indeks dominansi tertinggi di perkebunan campuran (1453 m dpl) (D=0.77) dan nilai dominansi terendah di hutan pinus (1545 m dpl) (D=0.18). Komponen analisis utama menunjukkan hubungan antara spesies dan tipe habitat. T. suhaniahae berkorelasi dengan tipe habitat terganggu, yaitu perkebunan campuran (1453 m dpl) dan hutan pinus (1545 m dpl), sementara itu T. nigrescens berkorelasi dengan hutan pinus (1545 m dpl). B. bidens berkorelasi dengan tipe habitat hutan primer dengan aliran sungai (2200 m dpl) dan R. celebensis berkorelasi dengan hutan sekunder (1200 m dpl). Berdasarkan fase bulan, keseluruhan spesies ditemukan pada fase bulan baru, bulan sabit, bulan setengah, bulan cembung dan bulan purnama. B. bidens merupakan spesies yang ditemukan pada fase bulan purnama, namun dengan jumlah individu yang sedikit.
SUMMARY
ELLENA YUSTI. The Fruit Bats (Megachiroptera, Pteropodidae) From Bawakaraeng Mountain, South Sulawesi. Advisored by BAMBANG SURYOBORTO and IBNU MARYANTO.
Fruit bats have an important role in ecosystem as seed dispersers and pollinators. They commonly found in agricultural areas with cultivated plant, secondary and primary forest that associated with food resources. The distribution and diversity of fruit bats are influenced by food resources, habitat types, elevation and environmental factors such as rainfall, wind, moon phases can all affect number captured and bat activity. Fruit bats study in Bawakaraeng Mountain aims to determine the fruit bats composition, including age catagorized and reproduction status of female fruit bats, fruit bats diversity in each habitat types, habitat preferences and relation between captured individual fruit bats with the moon phases in Bawakaraeng mountain.
A total 265 individuals of fruit bats were captured using mist net in five habitat types, which are secondary forest (1200 m asl), mixed garden (1453 m asl), pine forest (1545 m asl), primary forest (2000 m asl) and caves (2200 m asl). The sample include ten species and seven genera, with the estimation curve showed 12 species, that means 2 species were not caught yet. The fruit bats composition were caught are Boneia bidens ( (159 individuals; 60%), Thoopterus suhaeniahae (50 individuals; 18.86 %), Rousettus celebensis (21 individuals; 7.95%), Thoopterus nigrescens (11 individuals; 4.16%), Eonycteris spelaea (9 individuals; 3.40%), Rousettus amplexicaudatus (6 individuals; 2.27%), Dobsonia viridis (4 individuals; 2.27%), Styloctenium wallacei (2 individuals; 0.75%), Dobsonia exoleta (2 individuals; 0.75%) and Cynopterus luzonensis (1 individuals; 0.37%).
Shannon-Wiener diversity showed a highest value (H=1.80) in mixed garden (1453 m asl) while the lowest value (H=0.32) in pine forest (1545 m asl). The highest evenness indices value (D=0.77) in mixed garden and the lowest value (D=0.18) was in pine forest. The decreasing in Shannon-Wiener indices mainly depended on species richness in each habitat types.While the evenness shows the species dominance in each habitat types. All species were found in mixed garden (1453 m asl). Principal component analysis showed the correlation among species and habitat type. T. suhaniahae were correlated with degraded habitat, that are mixed garden (1453 m asl) and pine forest (1545 m asl), while T. nigrescens were correlated with pine forest. Both of species were correlated with mixed garden and pine forest. B. bidens were correlated with primary forest with river stream at 2000 m asl, while R. celebensis were correlated with secondary forest at 1200 m asl. Based on moon phases, all species mostly found in new moon, first quarter, third quarter and waxing gibbous, except for B. bidens that the only species was found in full moon phases with less individuals number. Bats that were found in full moon phases were more adapted in light moon intensity than other bats.
© Copy Right owned by IPB, 2015
All rights reserved
A Graduate Thesis
In partial fullfilment of Master Science degree in Animal Bioscience Faculty of Mathematics and Natural Science
THE FRUIT BATS (MEGACHIROPTERA, PTEROPODIDAE)
FROM BAWAKARAENG MOUNTAIN, SOUTH SULAWESI
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
2015
FOREWORDS
All praise to Allah SWT for all the hope and give that I have got this far. My study and thesis research would not have been accomplished without the help of many people. Special thanks to Dr Bambang Suryobroto and Prof (Ris) Ibnu Maryanto as supervisory committee, for all guidance and encouragement as well as invaluable academic advices for the whole period of my study and research. Thanks to Direktoral Jendral Pendidikan Tinggi (DIKTI) for Beasiswa Unggulan, Goverment of Gowa, South Sulawesi, staff of Biology Laboratory Makassar University, Tata and Nurdin Family and All staff of Zoology LIPI. I am highly indebted to my beloved family Juswardi Yacob Zein (father), Tuti Irda (mother), Lazuardi Akbar (brother) and Thursina (sister) for their love and support who always inspire and encourage me for higher education. My friends Husni Mubarok, Agmal Qodri and Rizaldi Triaz thanks for help in the field. All my friends in BSH 2012 thanks for support and friendship, especially for Silvia Puspitasari, Wahyudin Karim and Saudia Fitria.
Bogor, February 2015
TABLE OF CONTENTS
Sex, Age Catagorized and Reproduction Status 6
Data Analysis 6
Fruit Bats Individuals Number Captured In Relation To Moon Phases 11
DISCUSSIONS 13
Fruit Bats Captured and Distribution 13
Fruit Bats Estimation Number 14
Age Catagorized and Reproduction Status of Female Bats 14
Diversity of Fruit Bats 14
Habitat Preferences 15
Fruit Bats Individuals Number Captured in Relation To Moon Phases 16
4 CONCLUSIONS 17
REFERENCES 17
APPENDIXES 21
LIST OF TABLES
1 Habitat types description in Bawakaraeng mountain 2 2 Individual number of bats effort mist net/nights captured in different habitat
types 8
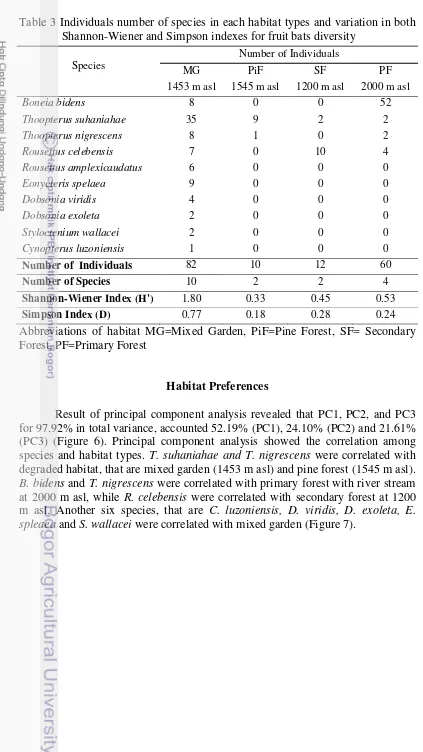
3 Individuals number of species in each habitat types and variations in both Shannon-Wiener and Simpson indexes for fruit bats diversity 10 4 Effort of individuals captured mist net/nights based on moon phases 12
LIST OF FIGURES
1 Collecting specimens sites 3
2 Ilustration of mist net replacement 4
3 External morphology measurement 5
4 Skull measurement 5
5 Estimation curve number of bats were calculated based on the individuals
number each night with Jack 1 Mean value 8
6 Female reproduction condition each month, R=reproductive, NR=non
reproductive, P=pregnant, N=nursing 9
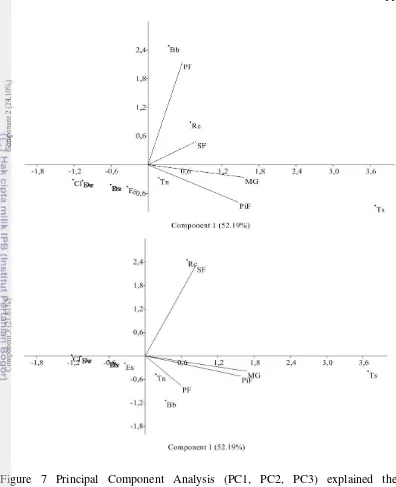
7 Principal Component Analysis (PC1, PC2, PC3) 11 8 Principal Component Analysis of Bat species and Moon Phases 12
LIST OF APPENDIXES
1
1
INTRODUCTION
Background
Bats belong to the order Chiroptera and can be distinguished from all other mammals by their ability to fly (Mickleburgh et al. 1992; Feldhamer et al. 1999; Suyanto 2001). Bats are a remarkably successful group, the second largest mammalian order in biodiversity after the Rodentia (Corbet and Hill 1992; Feldhamer et al. 1999). There are about 950 species of bats are widespreadly distibuted in the world (Mickleburgh et al. 1992). Bats divided into two suborder, they are Megachiroptera (fruit-eating bats) and Microchiroptera (insect-eating bats) (Corbet and Hill 1992; Feldhamer et al. 1999; Suyanto et al. 2002).
Fruit bats consists of a single family, Pteropodidae, which includes 44 genera and 166 species widespreadly distributed in sub tropical and tropical areas, from Africa, Madagascar, East Mediterania, Southern Asia, Southeast Asia to Australia, New Caledonia and islands in the Pasific Oceans (Feldhamer et al. 1999; Mickleburgh et al. 1992). Fruit bats are commonly found in agricultural areas with cultivated plant, secondary and primary forest that associated with food resources (Corbet and Hill 1992; Medellin et al. 2000; Fukada et al. 2002; Maryanto et al. 2011). Fruit bats have an important role in ecosystem as seed dispersers and pollinators (Mickleburgh et al. 1992; Maryati et al. 2008). There were 14 families, at least 141 plant species that polinnated with fruit bats (Fujita and Tuttle 1991; Mickleburgh et al. 1992). The distribution and diversity of bats are influenced by food resources, habitat types, elevation and environmental factors such as rainfall, wind, moon phases can all affect number captured and bat activity while bats generally found in the dark moon phases than full moon phases (Barlow 1999; Bork 2006).
In Indonesia, there are 25 genera and 77 species of fruit bats and some of them are endemic in certain areas (Maryanto and Higashi 2011). Sulawesi island (Indonesia) is remarkable for complex geological history and high biodiversity fauna, especially for fruit bats (Campbell et al. 2007; Maryanto and Higashi 2011). The diversity of fruit bats (Pteropodidae) is higher on Sulawesi than other islands, which 29 species of fruit bats are widespread in various areas with 10.7 % endemism level after Papua (Maryanto and Higashi 2011). Some species were endemic in Sulawesi are Boneia bidens, Cynopterus luzoniensis, Dobsonia exoleta, Harpyionycteris celebensis, Neopteryx frosti, Pteropus pumilus, Rousettus celebensis, Rousettus linduensis, Styloctenium wallacei and Thoopterus suhaniahae (Suyanto et al. 2002).
2
(Megachiroptera) in other parts of Sulawesi remain poorly unknown. Bawakaraeng mountain, Gowa, South Sulawesi is the one highest mountain in South Sulawesi, with peak at 2830 m asl were located 90 km from Makassar (Sumaryono and Dasa 2011). The forest types of mountain Bawakaraeng are lowland forest, secondary forest and primary forest that suspected to have high diversity of fruit bats (Hasnawir and Kubota 2006).
Aims
This study aims to determine the fruit bats composition, including age catagorized and reproduction status of female fruit bats, fruit bats diversity in each habitat types, habitat preferences and relation between captured individual fruit bats with the moon phases in the mountain region of Bawakaraeng.
2
METHODS
Study sites
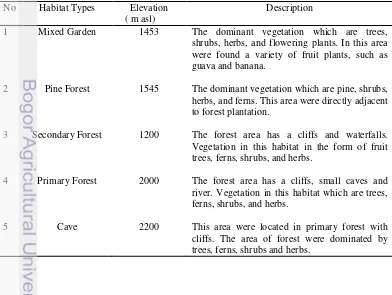
This study was conducted at Bawakaraeng mountain, Gowa and Sinjai Barat, South Sulawesi from September until December 2013. Sampling was done in different habitat types with a various levels of elevation (1200 to 2200 m asl) (Apendixes 1). Five sites were set up at different elevations and habitats : mixed garden (1453 m asl), pine forest (1545 m asl), secondary forest with a river stream (1200 m asl), primary forest with a river stream (2000 m asl) and cave (2200 m asl) (Table 1, Figure 1 and Appendix 1).
Table 1 Habitat types description in Bawakaraeng mountain No Habitat Types Elevation
2 Pine Forest 1545 The dominant vegetation which are pine, shrubs, herbs, and ferns. This area were directly adjacent to forest plantation.
3 Secondary Forest 1200 The forest area has a cliffs and waterfalls. Vegetation in this habitat in the form of fruit trees, ferns, shrubs, and herbs.
4 Primary Forest 2000 The forest area has a cliffs, small caves and river. Vegetation in this habitat which are trees, ferns, shrubs, and herbs.
3
Figure 1 Collecting specimens sites (1) Mixed garden (1453 m asl); (2) Pine forest (1545 m asl); (3) Secondary forest (1200 m asl); (4) Primary forest (2000 m asl) and (5) Cave (2200 m asl). The habitat types description refer to Table 1.
Fruit Bats Sampling
4
Figure 2 Illustration of mist net replacement (Kunz 1988)
Sample Identifications
Samples identification were based on Corbet and Hill (1992), Suyanto (2001) and observed against voucher bats specimen in Indonesian Institute of Science (LIPI). Identification were done until species level, including sex, age category, reproduction status and measurement of external morphology and skull.
External morphology measurement
5
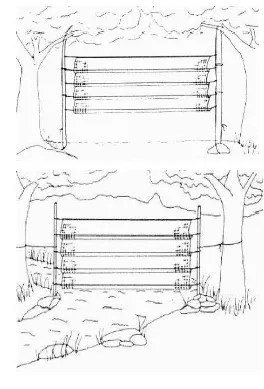
Figure 3 External morphology measurement (Rahman and Abdullah 2010)
Skull Measurement
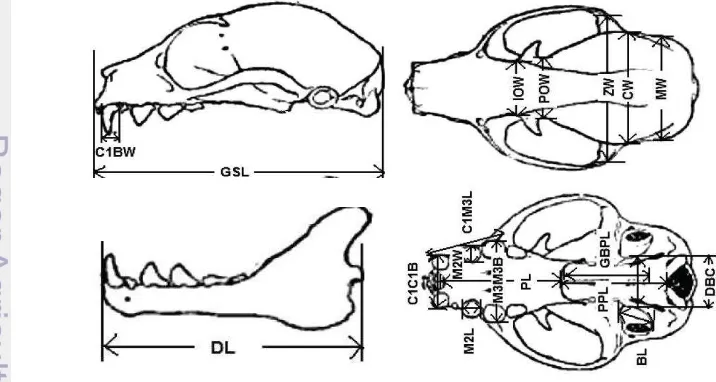
Measurement of skull including Greatest skull length (GLS), Postorbital width (POW), Zygomatic breadth (ZB), Braincase width (BW), Mesopterygoid fossa width (MSF), Bulla length (BL), Dentary length (DL), outside upper canine width (C1-C1), upper canine to third molar (C1-M3), upper third molar widht (M3 -M3), Condilo Canine Length (CCL), Condilo Braincase Length (CBL), Lower first canine (C1-C1), Lower canine to third molar length (C1-M3), Lower molar width (M3-M3) (Figure 4) (Kitchener and Maharadatunkamsi 1999; Helgen 2005).
6 from adults by joints of finger bone, cartilaginous ends of the bones in juveniles appear paler and more translucent than the joints of adults (Cristian and Helversen 2005). Female reproductive condition were divided into four categories, (1) reproductive (R) is a female in adult age, (2) non-reproductive (NR) is a female individual that does not reproduce, (3) pregnant (P) are individual pregnant females, (4) lactating (L) are female individuals that are nursing their baby (Racey 1988). Pregnancy in females could be observed with full and enlarged size in abdomen. Lactating females could be observed by enlarged nipples, which when gently massaged will express milk (Racey 1988).
Fruit Bat Individuals Number in Relation to Moon Phases
Bats activity based on moon phases was determined by calculating effort (number of individuals / night / nets). The data from different nights are pooled according to moon phases (Morrison 1978; Bork 2006). The moon phases were used in this study are based on Bork (2006), they are new moon, wanning crescent, third quarter, waxing gibbous and full moon. The moon phases data among nights were download in www.moonphasesconnection.com.
Data Analysis
7
3
RESULTS AND DISCUSSIONS
Results
Fruit Bats Composition and Identification
Composition bats species were captured during 40 nights included Boneia bidens (Jentink, 1989) (159 individuals; 60%), Thoopterus suhaeniahae Maryanto, et al. 2012 (50 individuals, 18.86%), Rousettus celebensis K. Andersen, 1907 (21 individuals; 7.95%), Thoopterus nigrescens (Gray,1870) (11 individuals; 4.16%), Eonycteris spelaea Jentink, 1888 (9 individuals; 3.40%), Rousettus amplexicaudatus (Geoffroy, 1810) (6 individuals; 2.27%), Dobsonia viridis (Heude, 1896) (4 individuals; 2.27%), Styloctenium wallacei Gray, 1866 (2 individuals, 0.75%), Dobsonia exoleta (2 individuals, 0.75%) and Cynopterus luzoniensis (Peters, 1861) (1 individuals; 0.37%) (Appendix 2). Six of ten fruit bats species were found endemic to Sulawesi which are Boneia bidens, Cynopterus luzoniensis, Dobsonia exoleta, Rousettus celebensis, Styloctenium wallacei and Thoopterus suhaeniahae. Two species were widespreadly distribute in entire Indonesia are Rousettus amplexicaudatus and Eonycteris spelaea.
8
Fruit Bats Estimation Number
Total number of species exist in Bawakaraeng Mountain was estimated based on the number of individuals captured each nights. Total night sampling was 40 nights, where successful trapping were 27 nights. Estimation of bats species number during 27 nights were 12 species (Figure 5). Sampling effort on this study were almost maximal, because only two species estimated was not caught yet, the bats were captured included 10 species from 7 genera (Table 2). The total sampling effort (5112 m2) were resulted 265 individuals of fruit bats representing 10 species (Table 2).
Table 2 Individual number of bats effort mist net/nights captured in different habitat types
Habitat Altitude Bb* Ts* Tn Rc* Ra Es Sw* De* Dv Cl* (m asl)
SF 1200 0.00 3.25 0.00 15.9 0.00 0.00 0.00 0.00 0.00 0.00
MG 1453 2.35 10.29 2.35 55.6 1.76 2.65 0.59 0.59 1.76 0.29
PiF 1545 0.00 11.10 2.06 0.00 0.00 0.00 0.00 0.00 0.00 0.00
PF 2000 55.60 4.40 2.2 4.4 0.00 0.00 0.00 0.00 0.00 0.00
Abbreviations of habitat and species names MG=Mixed Garden, PiF=Pine Forest,
SF=Secondary Forest, PF=Primary Forest; Bb=Boneia bidens, Ts=Thoopterus
suhaniahae, Tn=Thoopterus nigrescens, Rc=Rousettus celebensis, Ra=Rousettus amplexicaudatus, Es=Eonycteris spelaea, Sw=Styloctenium wallacei, De= Dobsonia exoleta, Dv=Dobsonia viridis, Cl=Cynopterus luzoniensis (*= endemic species in Sulawesi and adjacent island).
9
Age Catagorized and Reproduction Status of Female Bats
Based on age catagorized, 13 infants (4.90%), 16 juveniles (6.03%), 17 sub adults (6.41%) and 219 adults (82.64%). The composition of the fruit bats captured by sex was 121 females (45.66%) and 144 males (54.33%). Based on female bats reproduction categories, the composition of female individuals in reproductive period or adult were 73 individuals (60.33%), not reproducing or non-adult were 22 individuals (18.18%). Of the female reproductive category, 6 individuals (4.95%) are pregnant and 20 individuals (16.52%) are nursing. Some species that were found in pregnant and have an infant and nursing are B. bidens, D. viridis, E. spelaea, T. suhaniahae, R. amplexicaudatus, and R. celebensis. The reproductive female were found higher in November and December (Figure 6).
Figure 6 Female reproduction condition each month, R=reproductive, NR=non reproductive, P=pregnant, N=nursing
Diversity of Fruit Bats
10
Table 3 Individuals number of species in each habitat types and variation in both Shannon-Wiener and Simpson indexes for fruit bats diversity
Species
Abbreviations of habitat MG=Mixed Garden, PiF=Pine Forest, SF= Secondary Forest, PF=Primary Forest
Habitat Preferences
11
Figure 6 Principal Component Analysis (PC1, PC2, PC3)
Figure 7 Principal Component Analysis (PC1, PC2, PC3) explained the correlation between fruit bats and habitat
Fruit Bat Individuals Number Captured In Relation To Moon Phases
12
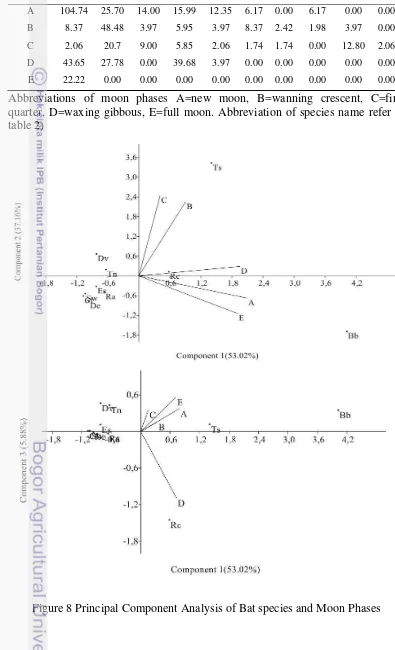
Table 4 Effort of individuals captured mist net/nights based on moon phases
Moon Phases
Individuals number of bats captured
Bb* Ts* Tn Rc* Ra Es Sw* De* Dv Cl*
A 104.74 25.70 14.00 15.99 12.35 6.17 0.00 6.17 0.00 0.00
B 8.37 48.48 3.97 5.95 3.97 8.37 2.42 1.98 3.97 0.00
C 2.06 20.7 9.00 5.85 2.06 1.74 1.74 0.00 12.80 2.06
D 43.65 27.78 0.00 39.68 3.97 0.00 0.00 0.00 0.00 0.00
E 22.22 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00
Abbreviations of moon phases A=new moon, B=wanning crescent, C=first quarter, D=waxing gibbous, E=full moon. Abbreviation of species name refer to table 2)
13
Discussions
Fruit Bats Captured and Distribution
The fruit bats that were found in Bawakaraeng Mountain were also widely distributed in Sulawesi and adjacent island (endemic species). These species included B. bidens, C. luzonensis, D. exoleta, S. wallacei, T. suhaniahae and T. nigrescens (Suyanto et al., 2002), Two species which are D. viridis and R. celebensis were endemic to Sulawesi and Molluccas. Meanwhile, R. amplexicaudatus and E. spelaea are found widely distributed throughout Indonesia (Corbet and Hill 1992; Suyanto et al.2002). R. celebensis was found in Sulawesi, but R. amplexicaudatus were found in entire Indonesia (Corbet and Hill 1992). Rousettus genera entirely distributed in Indonesian islands, but some of the species are endemic in several areas (Corbet dan Hill 1992; Suyanto et al. 2002). Endemic Sulawesi Rousettus is Rousettus linduensis, a new fruit bat that was found in Lore Lindu National Park, Central Sulawesi (Maryanto and Yani 2003). Corbet and Hill (1992) were recorded the distribution of four Rousettus species in Indonesia, they are R. amplexicaudatus (Sumatra, Borneo, Sulawesi, Molluccas, Java, Bali and Lesser Sunda), R. celebensis (Sulawesi), R. leschenaultii (Java), R. spinalatus (Sumatra and Borneo).
Boneia bidens were morphologically resemble with Rousettus genera, but the different are Boneia has larger size than Rousettus (Bergmans and Rozendaal 1988). Boneia bidens were endemic to Sulawesi, this species were recorded in Sulawesi and adjacent island (Suyanto 2001; Suyanto et al. 2002). Cynopterus genera were widespreadly distribute in entire Indonesia. This genera has seven species, they are C. brachyotis (Sumatra, Java, Borneo, Bali, and Molluccas), C. Horsfeldi (Sumatra, Java and Borneo), C. luzoniensis (Sulawesi), C. minutus (Sumatra, Borneo, Java and Sulawesi), C. nusatenggara (Nusa tenggara), C. sphinx (Sumatra, Java, Borneo, Sulawesi) and C. tithaecheilus (Sumatra, Java, Bali, Lombok and Timor) (Kitchener and Maharadatunkamsi 1991; Corbet and Hill 1992; Suyanto et al. 2002).
Dobsonia genera were widespreadly distribute in east Indonesia region. That genera has nine species, they are D. beauforti (West Papua), D. crenulata (Sangihe island, Tongian, Banggai island and Halmahera), D. emersa (West Papua), D. exoleta (Sulawesi), D. magna (West Papua), D. minor (Sulawesi and West Papua), D. moluccensis (Molluccas), D. peroni (Bali and Nusa Tenggara) and D. viridis (Sulawesi and Molluccas) (Bergmans and Rozendaal 1988; Corbet and Hill 1992). Eonycteris genera have two species that were found in all Indonesian, they are E. spelaea (all Indonesia islands) and E. major (Borneo) (Corbet and Hill 1992; Suyanto 2001; Suyanto et al. 2002).
14
found in several areas at Talaud, Wowoni islands and Rorekatimbu (Maryanto et al. 2012).
Fruit Bats Estimation Number
Estimated curve were discuss differences between species number of collected and expected species (Figure 3). Sobs (Mao Tau) is the total number of species that was captured in this study. Jack 1 mean value is the first order Jacknife richness estimator of species number (Gotelli and Colwell 2011). Some factors that suspected causing other bats species were not caught including sampling duration, mist net efficiency, including the placement and regular check and environmental conditions, such as heavy rain, wind and the moon phases (Barlow 1999; Lang et al. 2004; Larsen et al. 2007). In this study, it is possible to get 12 species of fruit bats if adding night sampling was conducted. Mist net position in this study were held in some area, there were near fruit trees, over stream area, but some mist net were placed far from fruit trees. Mist net was not regularly checked each night and environment factor that were observed there was no bats were caught in heavy rain and wind. In addition, in the full moon phases there were only a few individuals of bats captured.
Age Catagorized and Reproduction Status of Female Bats
Age catagorized and pregnant female bats shows the correlation between breeding season and rainfall in Neotropics area (Coates and Estrada 2001). In this study, the most age catagorized were caught are adult and some are pregnant female. Coates and Estrada (2001) reported that flowering peaks occur in dry season, where the fruiting peaks in the rain season. That study shows that the reproductive peaks (adult age catagorized) were coincident with these periods. In this study, a reproductive peaks (adult catagorized) were found in December. In this study, the adult female more dominance than non reproductive, pregnant and nursing. The adult females number mostly found in November and December. Generally, bats were produce one offspring in a year with periods 3-4 month, with nursing period about one year (Suyanto 2001).
Diversity of Fruit Bats
15 agricultural areas. Another study were also found the highest abundance of fruit bats in secondary and primary forest, some habitat types with a river stream with flower and fruit availability (Heideman and Heaney 1989; Wiantoro and Ahmadi 2011).
Some studies reported that the number of fruit bats species also influence with the elevation pattern. Maryanto et al. (2011) reported that the highest diversity of fruit bats were stable in lower mountain association (300 and 1500 m asl) and decreased in upper mountain association (2100 m asl). Bruce et al (1998) in study about elevation in relation among birds, bats, and rats diversity in Amazon mountain, Peru reported that the highest bats diversity were found in 500-1500 m asl and decreased in 2000-3500 m asl. Both studies suggest that species richness decreases with elevation and habitat type that associated with food avaibility.
Habitat Preferences
Fruit bats were commonly found in different habitat types which closely related with fruit and flower availability (Corbet and Hill 1992; Maryanto et al. 2011). Habitat used in fruit bats were vary as roosting habitat and foraging habitat (Mickleburgh et al. 1992), such as agroforestry, mixed garden, forest area (primary and secondary forest) and caves (Bergmans and Rozendall 1988; Corbet and Hill 1992; Wijayanti 2011). In this study, all species were found in mixed garden (1453 m asl) (Table 1). The species were simpatrically found in mixed garden, they are C. luzoniensis, D. viridis, D. exoleta, E. spelaea, R. amplexicaudatus and S. wallacei. Another four species which are B. bidens, R. celebensis, T. nigrescens and T. suhaniahae were found simpatrically in primary forest (2000 m asl). Previous study also found Thoopterus suhaniahae has been collected sympatrically with T. nigrescens, R. celebensis and C. luzoniensis (Maryanto et al. 2012). Some species were found sympatrically in the same habitat shows the present of fruit bats were depend on habitat types that closely related with fruit and flower availability (Whitmore 1989; Corbet and Hill 1992).
16
species were found at 1453 m asl, 2000 m asl and 2200 m asl in caves with large colony. celebensis and R. amplexicaudatus. Both species were found in mixed garden, but R. celebensis was found in secondary and primary forest with river flow (1200-2000 m asl). Previous study noted that R. celebensis related to garden location and cave (Bergmans and Rozendall 1988) and lower lowland forest with 1200 m asl (Maryanto et al. 2011).
Species that were specialized nectarivorus, E. spelaea and R. amplexicaudatus were found in mixed garden (1453 m asl), both species were play major in pollination (Maryati et al. 2008; Soegiharto et al. 2011). This species were commonly found in agricultural with flowers availability, but some studies were found this species in some caves, including karst caves with high and low light intensity (Maryanto and Maharadatunkamsi 1991; Kunz and Fenton 2003; Ruczynski et al. 2010; Wijayanti 2011). Altringham (1996) reported that R. amplexicaudatus and E. spelaea were able to used echolocation, that cases suggest that both species were adapted in hight and low condition (Wijayanti 2011). In this study both species were suspected used the caves in Bawakaraeng mountain region as roosting habitat. In addition, E. spelaea and R. amplexicaudatus has a widely foraging area about 38 km, this ability was important in seeds dispersal (Suyanto 2001; Kunz and Fenton 2003). Another species that were found in mixed garden (1453 m asl) are D. exoleta, D. viridis and S. wallacei. Esselsstyn (2007) were found Styloctenium near guava trees in Phillipines, while Maryanto et al. (2011) recorded S. wallacei at 600-1800 m asl in lowlands area. D. exoleta and D. viridis were recorded found in lowland forest at 600-1200 m asl (Maryanto et al. 2011).
Fruit Bat Individuals Number Captured In Relation To Moon Phases
The behaviour and activity of nocturnal animals affected with changing light conditions in relation with lunar cycle (Lang et al. 2005). Morrison (1978) reported that lunar phobia to explain this behaviour, some animals forage less or avoid the bright moon phases in order to reduce predation risk. Lunar phobia have been documented in some animals such as Rodent (Clarke 1983) and Kanggoro rats (Dipodomys spectabilis) (Daly et al. 1992). Bats are classified as nocturnal animals where the activity at night is affected by moon light cycle or moon phases (Lunar phobia) (Barlow 1999; Lang et al. 2005; Bork 2006; Mello et al. 2013). This behavior suggests that flying in moonlight may significantly increase risk of predation by visually oriented predators such as snake and owl (Lang et al. 2005).
17 frugivores bats decrease when full moon or bright moonlight (Morrisson 1978; Lang et al. 2005; Bork 2006; Mello et al. 2013).
All species in this study mostly found in new moon, waxing crescent, third quarter and waxing gibbous, except for B. bidens, that the only species was found in full moon phases (Table 2). Lang et al. (2005) reported that foraging activity bat, Lophostoma silvicolum were higher in new moon phases than full moon phases. Some studies reported that the number individuals captured of Noctilio leporinus (Bork 2006); Artibeus lituratus, Carollia perspicillata, Sturnira lilium (Mello et al. 2013) were found higher in dark moon phases, they are new moon, waning gibbous and waxing gibbous than full moon phases. Bats that were found in full moon phases were more adapted in light moon intensity than other bats (Bork 2006). Mello et al. (2013) reported that during full moon bats move to darker parts to forage B. bidens that were found in full moon phases showed this species was more adapted to the bright moonlight intensity, suspected this species were found in degraded habitat, such as agricultural (Bergmans and Rozendaal 1988).
4 CONCLUSIONS
The highest individual fruit bats captured in Bawakaraeng mountain were Boneia bidens (60%) and Thoopterus suhaniahae (18.86%), while composition of the fruit bats captured by sex are 121 females (45.66%) and 144 males (54.33%). In this study, the most age catagorized were caught are adult (reproductive). The highest diversity of fruit bats in Bawakaraeng Mountain were in mixed garden (1453 m asl), primary forest (2000 m asl) and secondary forest with stream (1200 m asl). Fruit bats abundance in this study were found mixed garden (1453 m asl) and primary forest (2000 m asl) were tightly associated with food availability. Moon phases affected the number of bats individual captured where the number of individual bats captured in the dark moon phase was higher than full moon Sulawesi and some off-lying islands (Mammalia, Megachiroptera). Netherland (NL): Zoologische Verhandelingen.
Bork SK. 2006. Lunar phobia in the greater fishing bat Noctilio leporinus (Chiroptera: Noctilionidae). Revista de Biologial Tropical. 54(4):1117-1123.
18
Campbell P, Scheneider CJ, Zubaid A, Adnan AM, Kunz TH. 2007. Morphological and ecological correlates of coexistence in Malaysian fruit bats (Chiroptera:Pteropodidae). Journal ofMammalogy. 88:105-118. Clarke JA. 1983. Moonlights influence on predator/prey interactions between
short-eated owls (Asio flammeus) and deermice (Peromycus maniculatus). Behavioural Ecology and Sociobiology. 13:205-209.
Coates R, Estrada A. 2001. Species composition and reproductive phenology of bats in a tropical landscape at Los Tuxtlas, Mexico. Tropical ecology. 17:627-646.
Corbet GB, Hill JE. 1992. The Mammals of the Indomalayan Region. A Systematics Review. Oxford (GB) : Oxford Press.
Cristian D, Helversen V. 2005. Illustrated identification key to the bats of Europe. Germany (DE): Electronic Publication.
Daly M, Behrends PR, Wilson MI, Jacobs LF. 1992. Behavioral modulation of predation risk-Moonlight avoidance and crepuscular compensation in a nocturnal desert rodent (Dypodomys merriami). Animal Behaviour. 48:9-18.
Esselstyn JA. 2007. A new species of stripe-faced fruit bats(Chiroptera: Pteropodidae: Styloctenium) from the Philippines. 2007. Mammalogy. 88(4):951-958.
Elangovan V, Marimuthu G. 2001. Effect on moonlight on the foragingbehaviour of a megachiropteran bat Cynopterus sphinx. Zoology. 253:347-350. Fujita MS, Tuttle MD. 1991. Flying foxes (Chiroptera: Pteropodidae): Threatened
animals of key ecological and economic importance. Conservation Biology. 5:454–463.
Fukuda D, Tisen OB, Momose K, Sakai S. 2009. Bat diversity in the vegetation mosaic around a lowland Dipterocarp forest of Borneo. The Raffles Bulletin Of Zooology. 57(1): 213-221.
Feldhamer GA, Drickamer LC, Vessey SH, Merritt JF, Krajewski C. 1999. Mammalogy: Adaptation, Diversity, Ecology 3rd ed. Boston Massachusetts (US): McGraw-Hill Co.
Gotelli NJ, Colwell RK. 2011. Estimating Species Richness. Chapter 4 page 39-54. In AE. Magguran and BJ. McGill, editors. Frontiers in Measuring Biodiversity. New York (US): Oxford University Press.
Hall LS. Grigg GG. Moritz C. Ketol B. Sait I. Marni W. Abdullah MT. 2004. Biogeography of fruit bats in Southeast Asia. Sarawak Museum Journal. 80:191-284.
Hammer O, David AT. Harper, Ryan PD. 2001. Past: paleontological statistics software package for education and data analysis. Paleontologica Electronic. 4:4-9.
Hasnawir OH, Kubota T. 2006. Landslide Disaster at Mt. Bawakaraeng Caldera, South Sulawesi, Indonesia. Kyushu Journal of Forest Research, 59:269-272.
19 Heaney LR, Heideman PD, Rickart EA, Utzurrum RB. Klompen JSH. 1989. Elevation zonation of mammals in the central Phillipines. Tropical Ecology. 5:259-280.
Helgen K.M. 2005. Systematics of the Pasific monkey faced bats (Chiroptera: Pteropodidae), with a new species of Pteralopex and a new Fijian genus. Systematics and Biodiversity 3 (4):433-453.
Hodgkison R, Balding ST, Zubaid A, Kunz TH. 2004. Habitat structure, wing morphology, and the vertical stratification of Malaysian fruit bats (Megachiroptera: Pteropodidae). Tropical Ecology. 20:667-673.
Kitchener DJ, Maharadatunkamsi. 1991. Description of new species of Cynopterus (Chiroptera: Pteripodidae) from Nusa Tenggara, Indonesia. Western Australia Museum. 15:119-173.
Kunz TH. 1988. Ecological and Behavioural Methods for the Study of Bats. Washington (US): Smithsonian Institution Press.
Kunz TH, Fenton MB. 2003. Bat Ecology. Chicago (US): The University Of
Chicago Press.
Larsen JR, Begler KA, Genoways HH, Masefield WP, Kirsch RA, Pedersen SC. 2007. Mist netting bias, species accumulation curves and the rediscovery of two bats on Montserrat (Lesser Antiles). Acta Chiropterologica. 9(2): 423–435.
Lang AB, Weise CD, Kalko EKV and Roemer H. 2004. The bias of bats netting. Bat Research News. 45:235–236.
Lang AB, Elizabeth K, Kalko V, Romer H. 2005. Activity levels of bats and katydids in relation to the lunar cycle. Behavioral Ecology.
Maguran AE. 2004. Measuring biological diversity. Malden (GB): Blackwell Publishing.
Maryati AP, Kartono P, Maryanto I. 2008. Kelelawar pemakan buah sebagai polinator yang diidentifikasi melalui polen yang digunakan sebagai sumber pakannya di kawasan Sektor Linggarjati. TN. Ciremai. Jurnal Biologi Indonesia. 4(5):335-348.
Maryanto I, Yani M. 2003. The new species of the Rousettus bat from Lore Lindu National Park Central Sulawesi, Indonesia. Mammal Study. 28:111-120. Maryanto I, Yani M, Priyono SN, Wiantoro S. 2011. Altitudinal distribution of
fruit bats in Lore Lindu National Park, Central Sulawesi, Indonesia. Hystrix, Italian Journal of Mammalogy. 22(1):167-177.
Maryanto I, Yani M, Priyono SN, Wiantoro S. 2012. A new species of fruit bat (Megachiroptera: Pteropodidae: Thoopterus) from Sulawesi and adjacent islands, Indonesia. Records Of The Western Australian Museum. 27:068– 084.
Maryanto I, Higashi S. 2011. Comparison of zoogeography among rats, fruit bats and insectivorous bats on Indonesian islands. Treubia. 38:33-52.
Medellin RA. Equihua AM. Amin MA. 2000. Bats diversity and abundance as indicators of disturbance in Neotropical rainforest. Conservation Biology. 14:1666-1675.
20
Mickleburgh PS, Anthony MH, Paul AR. 1992. Old world fruit bats. Switzerland (CH):IUCN/SSC Chiroptera Specialist Group.
Morrison DW. 1978. Lunar phobia in a Neotropical fruit bats Artibeus jamaicensis (Chiroptera; Phyllostomidae). Animal Behaviour. 26:286-288. Rahman M, Abdullah M. 2010. Morphological variation in the dusky fruit bat,
Penthetor lucasi, in Sarawak, Malaysia. Tropical Natural History 10(2): 141-158.
Racey, PA. 1988. Reproductive assessment in bats, pp 31-43. In Ecological and Behavioural Methods for Study of Bats. Washington (US): Smithsonian Institution Press.
Ruczyński I, Nicholls B, Macleod CD, Racey PA. 2010. Selection of roosting habitats by Nyctalus noctula and Nyctalus leisleri in Białowieża Forest – Adaptive response to forest management. Forest Ecology and Management. 259:1633–1641.
Sampaio EM, Kalko EKV, Enrico B, Bernal RH, Charles OH. 2003. A biodiversity assessment of Bats (Chiroptera) in a tropical lowland rainforest of Central Amazonia, including methodological and conservation considerations. Studies on Neotropical Fauna and Environment. 38:17-31.
Storz JF, Bhat H, Kunz TH. 2000. Social structure of a polygonous tent-making bat Cynopterus sphinx (Megachiroptera). Zoology. 251:2.
Sumaryono, Dasa YT. 2011. Simulasi aliran bahan rombakan di Gunung Bawakaraeng, Sulawesi Selatan. Lingkungan dan Bencana Geologi. 2:191– 202.
Suyanto A. 2001. Kelelawar di Indonesia. Bogor (ID) : LIPI.
Suyanto A, Yoneda M, Maryanto I, Maharadatunkamsi Sugardjito J. 2002. Checklist of the Mammals of Indonesia : Scientific Names and Distribution Area Tables in Indonesia Catagories for Conservation. Bogor (ID): LIPI. Soegiharto S, Kartono AP, Maryanto I. 2010. Pengelompokan kelelawar pemakan
buah dan nektar berdasarkan karakteristik jenis pakan polen di Kebun Raya Bogor Indonesia. Biologi Indonesia 6(2):225-235.
Wiantoro S, Achamadi AS. 2011. Keanekaragaman mamalia kecil di Pulau Moti. Ekologi Ternate. 55-68.
Wijayanti F. 2011. Biodiversitas dan pola pemilihan sarang kelelawar: studi kasus di kawasan karst gombong Kabupaten Kebumen Jawa Tengah [Thesis]. Bogor (ID): Institut Pertanian Bogor.
Whitmore TC. 1984. Tropical rain forest of the Far East. Oxford (GB): Claredon Press.
21
22
Appendix 1 Habitat types of Fruit Bats in Bawakaraeng Mountain
Mixed Garden (1453 m asl)
Pine Forest (1545 m asl)
23
Primary forest with stream (2000 m asl)
24
Appendix 2 Morphology and skull of captured fruit bats in Bawakaraeng Mountain
Boneia bidens
Thoopterus suhaniahae
25
Rousettus amplexicaudatus
Rousettus celebensis
Eonycteris spelaea
26
Dobsonia exoleta
Styloctenium wallacei
27 Appendix 3 Measurement (mm) for external morphology and skull (see Methods
section for explanation of characters code) Boneia bidens (Male)
28
Rousettus amplexicaudatus (Female)
31 Thoopterus nigrescens (Female)
32
Eonycteris spelaeae (Female)
35
BIOGRAPHY