RIYANDI
POST GRADUATE SCHOOL BOGOR AGRICULTURE UNIVERSITY
BOGOR 2013
PERNYATAAN MENGENAI TESIS DAN
SUMBER INFORMASI SERTA PELIMPAHAN HAK CIPTA*
Dengan ini saya menyatakan bahwa Tesis berjudul Color Variations of Immature Nypa Worms in The Kapuas Estuary, West Kalimantan adalah benar karya saya dengan arahan dari komisi pembimbing dan belum diajukan dalam bentuk apa pun kepada perguruan tinggi mana pun. Sumber informasi yang berasal atau dikutip dari karya yang diterbitkan maupun tidak diterbitkan dari penulis lain telah disebutkan dalam teks dan dicantumkan dalam Daftar Pustaka di bagian akhir tesis ini.
Dengan ini saya melimpahkan hak cipta dari karya tulis saya kepada Institut Pertanian Bogor.
Bogor, Agustus 2013 Riyandi NRP G352110051
SUMMARY
RIYANDI. Color Variations of Immature Nypa Worms in The Kapuas Estuary, West Kalimantan. Supervised by BAMBANG SURYOBROTO, TRI ATMOWIDI and IIN INAYAT AL HAKIM
Nypa worm (Namalycastis rhodochorde) previously described to the new species, included in Namalycastis abiuma species group with had an unusual shape and had large body. Color variation in N. rhodochorde was obtained in mature. N. rhodochorde had normal color when life was bright pink, while mature were turned red (females) and green (males). But until now there was no report color variation in immature N. rhodochorde. This study had examined color variations of nypa worms in Kapuas Estuary, West Kalimantan.
Photogrammetry is one of the methods used to study the variations in color. This method could be measure color through photos. Samples were photographed on spiral labyrinth glass to limit the movement of the worm and Strobist Macro Photo Studio for light stabilization. Digital images of nypa worm was recorded in RAW format then converted to 8-bit TIFF. Determination of the measured area is by selecting the whole dorsal body seen in TIFF files as ROI. Therefore the values of RGB colour spaces was converted to the corresponding values of CIE Lab colour space. Beside digital photogrammetry, color was described by the eye of author.
Histological analysis was performed observed the anatomical difference between the color groups. This was due to morphological and anatomical changes occurring during maturation N. rhodochorde. Epidermal cells was observed due to contains of secretory cells that function as mucus secretion. Mucus plays an important role in the life of polychaeta. Epidermal preparation of nypa worm was done using paraffin method.
Based on the analysis using photogrammetry, there is no significant difference between males and females colors. By eye, obtained color variations in immature worms categorized as bright pink, dark pink and brown. Most of the worms were bright pink and the least is brown. Bright pink worm was immature whereas dark pink worm was submature. These suggest that color variation related to gametogenesis. From histology of epidermis we obtained three forms of secretory cell within each group. Dark pink had more closed cell, it was suspected that cell more active so that more of mucus in cell. This was coused the nypa worm prepared to maturity. Different with brown worms that was immature had larger cells, it was suspected to be an adaptation to the environment. In this study was not found mature samples. It was suspected when sampling was not a of spawning time. Polychaeta spawning time is strongly influenced by the physical and biological environment conditions.
RINGKASAN
RIYANDI. Variasi Warna Pada Cacing Nipah yang Belum Matang Di Muara Kapuas, Kalimantan Barat. Dibimbing oleh BAMBANG SURYOBROTO, TRI ATMOWIDI dan IIN INAYAT AL HAKIM
Cacing nipah (Namalycastis rhodochorde) sebelum dideskripsikan menjadi jenis baru adalah termasuk dalam kelompok jenis Namalycastis abiuma yang memiliki bentuk yang tidak biasa dan tubuh yang panjang. Variasi warna pada N. rhodochorde didapatkan pada individu yang sudah matang. N. rhodochorde memiliki warna normal merah muda cerah, namun pada waktu matang akan berubah menjadi merah (betina) dan hijau (jantan). Sampai saat ini belum ada laporan mengenai variasi warna N. rhodochorde yang belum matang. Penelitian ini bertujuan mengkaji variasi warna pada cacing nipah yang belum matang di muara Sungai Kapuas, Kalimantan Barat.
Fotogrametri adalah salah satu metode untuk mempelajari variasi warna. Metode ini memungkinkan mengukur warna melalui foto. Sampel difoto di atas spiral labirin kaca untuk membatasi gerak cacing dan Strobist Macro Photo Studio untuk mendapat kondisi cahaya yang stabil. Foto digital cacing nipah diambil dengan menggunakan bentuk RAW yang dikonversi ke bentuk TIFF 8-bit. Bagian yang diukur (ROI) adalah seluruh bagian dorsal yang dipilih dari berkas TIFF. Kemudian nilai RGB dikonversi ke ruang warna CIE Lab. Selain dengan metode fotogrametri, warna juga dideskripsikan menggunakan mata oleh penulis.
Analisis histologis dilakukan untuk melihat perbedaan anatomi dari beberapa kelompok warna. Hal ini disebabkan selama proses kematangan N. rhodochorde akan terjadi perubahan morfologi dan anatomi. Sel epidermis dikaji karena memiliki sel sekresi yang berfungsi menghasilkan lendir. Lendir beperan sangat penting dalam kehidupan polychaeta. Preparat epidermis disiapkan menggunakan metode parafin.
©
Hak Cipta Milik IPB, Tahun 2013
Hak Cipta Dilindungi Undang-Undang
Dilarang mengutip sebagian atau seluruh karya tulis ini tanpa mencantumkan atau menyebutkan sumbernya. Pengutipan hanya untuk kepentingan pendidikan, penelitian, penulisan karya ilmiah, penyusunan laporan, penulisan kritik, atau tinjauan suatu masalah; dan pengutipan tersebut tidak merugikan kepentingan IPB
Thesis
As partial fulfillment of the requirements for Master Degree in Animal Biosciences
RIYANDI
POST GRADUATE SCHOOL BOGOR AGRICULTURE UNIVERSITY
BOGOR 2013
Title : Color Variations of Immature Nypa Worms in The Kapuas Estuary, West Kalimantan
Name : Riyandi
Registration number : G352110051
Certified by, Advisory Committee
Dr. Bambang Suryobroto Chairman
Dr. Tri Atmowidi
Member Dra. Iin Inayat Al Hakim, M.Si. Member
Acknowledged by Coordinator
Major of Animal Biosciences
Dr. Ir. R.R. Dyah Perwitasari, MSc
Dean of Postgraduate School
Dr. Ir Dahrul Syah, MScAgr
PREFACE
Praise and gratitude writer prayed to Allah Subhanahu wa ta'ala for all gifts so that this thesis was completed. Selected titles in a study is the Color Variations of Immature Nypa Worms in the Kapuas Estuary, West Kalimantan.
Authors say thanks to Dr. Bambang Suryobroto, Dr. Tri Atmowidi and Dra. Iin Inayat Al Hakim, M.Si as supervisors, as well as Junardi M.Si that has a lot to give suggestions during this study. Thanks also to Mr. Yuliadi Zamroni M.Si and Andy Darmawan M.Si who have been willing to take the time and suggestions during the study. I also would like to thank Dikti Depdiknas for Postgraduate Scholarships (BU).
In addition, the authors also say thank you to friends BSH 2011 who are always provides motivation and inspiration in completing the study. Also to the “zoo corner” friends who have been willing to sharing during the author completed this studies at BSH. Thanks are also due to the father and mother who always pray and support the whole family. Thanks also to my beloved wife and daughter for their support to author complete this study. Thank you to all who could not mention one by one for the prayers and support. Hopefully this paper useful for all.
CONTENTS
LIST OF TABLE vi
LIST OF FIGURE vi
1. INTRODUCTION 1
2. MATERIAL AND METHOD 2
Study Site and Subjects 2
Identification 2
Colecting Digital Photos 4
Color Analysis 5
Histology 5
3. RESULT 6
Identification 6
Correspondences of Color Determination by Eye and Photogrammetry 9
Sexual Dimorphism in Color Variation 9
Histology of Epidermis
4. DISCUSSION 10
5. CONCLUSION 12
LIST OF TABLE
1. Number of individual with brown, dark pink or bright pink with eye 7 2. Coefficients of linear discriminants in each function 7 3. Statistical Analysis of L* a * b * and chroma for each sex 8
LIST OF FIGURE
1. (A) Mangrove forest in Kapuas Estuary. (B) Sample collection by
manual digging (hand collection) 2
2. Research Map in Kapuas Estuary (Pulau Panjang). Located in the western of Pontianak city (West Kalimantan) and faces Karimata
Strait. 3
3. Spiral labyrinth glass (A) and Strobist Macro Photo Studio (B) 3 4. (A) Normal color was bright pink (Glasby et al. 2007); (B) Dark
pink pattern was color with almost dark in all part of body; (C)
Brown pattern was having patch of dark color (like black). 4 5. Dorsal (A) and lateral (B) view of Namalycastis rhodochorde.
antenae (an); prostomium (pro); peristomium (pr); peristomial cirrus
(Pc); palps (P) 6
6. Lateral view (A) showed that sub-biramus type of 9th, 10th and 11th parapodia. (B) showed heterogomph falciger chaetae. cirrophore (c);
chaetae (ct); blade (b) 7
7. Gametes of nypa worm. (A) male gametes (cluster of
spermatogonia). (B) female gametes (oocyte) 7
8. Visualization the log eyes vs photogrammetry data on the first two discriminant axes. Individual scores are plotted on the first
(horizontal) and second (vertical) discriminant axes. Bright pink (1), dark pink (2) and brown (3). Proportion of trace first function comprises 96.8% and the second function comprises 3.2% of the
variance 8
9. Longitudinal section of epidermis of N. rhodochorde. (A) Bright pink worm. (B) Dark Pink Worm. (C) Brown worm. (cu) cuticle; (ec) epidermis cell; (cm) circular muscle; (cf) collagen fibre; (sc)
1.
INTRODUCTION
Polychaeta is a diverse class (McHugh 2005; Heuer et al. 2010). The high diversity of Polychaeta because it has a high level of evolutionary plasticity (Heuer et al. 2010). Nypa worm (Namalycastis rhodochorde) was include in Family Nereididae Sub-Family Namanereidinae (Glasby et al. 2007). Nypa worm before described to the new species, included in Namalycastis abiuma species group with had an unusual shape and had large body (Glasby et al. 2007). It was indicated that the nypa worm had a unique morphology.
Nypa worm distributed in South-east Asia (Sunda shelf only) including Mekong Delta (Vietnam), West Kalimantan (Indonesia) and Sabah (Malaysia) (Glasby et al. 2007). Nypa worm had a smaller range of habitats which in Nypa frutican forest (Junardi 2008), so there was also possible to be obtained on the area of mangrove forests in the dominance of nypa. Nypa worm is living on a muddy estuary (Glasby et al. 2007; Junardi 2008). This may cause the nypa worm had a high risk to environmental changes. Dynamics of estuarine environments that was given effect for polychaeta such as environment and development (Hamzavi et al. 2012).
Nypa worm is gonochoristic who have one organ sex on the individual. Reproductive pattern of nypa worm is monotelik that spawn only once in the life cycle. In spawning time, nypa worm released sperm into water and fertilize similarly released eggs (ect-aquasperm) (Junardi et al. 2010). After fertilization, the embryo of Nereididae develops in the egg then undergoes cleavage until become larvae. The larvae develop into juvenile to mature individuals and evolved freely in their habitat (Fischer et al. 2010).
Color variation in N. rhodochorde was obtained in mature. N. rhodochorde
generally when life was bright pink, while mature were turned red (females) and green (males) (Glasby et al. 2007). N. rhodochorde in Glasby et al. (2007) was photographed in mangroves of the Kota Kinabalu Wetland Center (Sabah, Malaysia) and there was no color recorded in West Kalimantan. Moreover, until now there was no report color variation in immature N. rhodochorde.
2
2.
MATERIAL
Research has been carried out in December 2012 to May 2013. sampling method was purposive
location by purposive that was
intertidal zone of the Pulau Panjang in Kapuas Province, Indonesia) (Figure
Samples were taken randomly digging (hand collection) (Figure 2B)
soil and midrib of nypa tree from the original
maintained by spraying brackish water (Junardi 20 were taken at the Laboratory of
Sciences Tanjungpura University.
Worm identification and analysis Section of Biosystematic and E
University and Oceanographic Research Center Indonesia LIPI. preserved using alcohol 70% (
Nypa worms were identified in reference to (1999). Identification used
the phylogeny such as prostomium and setae (Fauchald 1977).
having parapodia that uniramou absent but having 2 aciculae (Wu
observing the shape and length of the antenna and the form of pygidium 1999). Description of N. rhodochorde
Figures 1 (A) Mangrove forest in Kapuas Estuary. (B) S by manual digging
MATERIAL AND METHOD
Study Site and Subjects
Research has been carried out in December 2012 to May 2013. purposive random sampling. Determined of sampling location by purposive that was from the mangrove forest (Figure 1A)
Pulau Panjang in Kapuas Estuary (West Kalimantan (Figure 2).
taken randomly in mangrove areas at low tide by manual (Figure 2B). Samples were stored in box filled with of nypa tree from the original habitat. Soil moisture in the
brackish water (Junardi 2008). All images of life worm taken at the Laboratory of Zoology, Faculty of Mathematics and Natural
University.
Identification
dentification and analysis were carried out at the Laboratory of Biosystematic and Ecological of Animal, Bogor Agricultural University and Oceanographic Research Center Indonesia LIPI. Sampel was
alcohol 70% (Wu et al. 1985).
identified in reference to Wu et al. (1985) and Glasby Identification used characters that are commonly used in the assessment o
prostomium, peristomium, eversible pharynx,
(Fauchald 1977). Sub-Family Namanereidinae is characterized by having parapodia that uniramous or sub-biramous, notosetae and notosetae lobe absent but having 2 aciculae (Wu et al. 1985). Genus identification was
the shape and length of the antenna and the form of pygidium
rhodochorde followed Glasby et al. (2007).
(A) Mangrove forest in Kapuas Estuary. (B) Sample collection by manual digging (hand collection)
Research has been carried out in December 2012 to May 2013. The random sampling. Determined of sampling (Figure 1A) at the that are commonly used in the assessment of , parapodia, manereidinae is characterized by ramous, notosetae and notosetae lobe was done by the shape and length of the antenna and the form of pygidium (Glasby
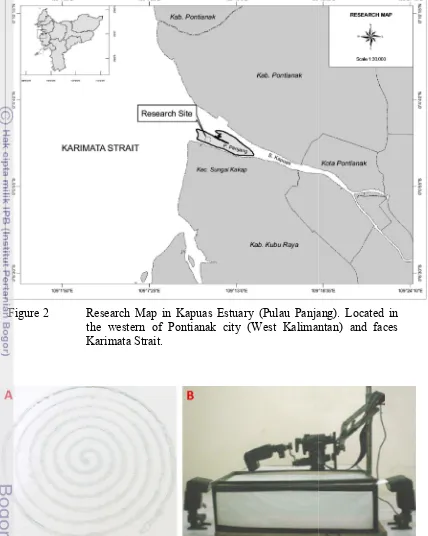
Figure 2 Research Map in Kapuas Estuary (Pulau Panjang). the western of Pontianak city (West Kalimantan) Karimata Strait
Figure 3 Spiral labyrinth glass (A) and Strobist Macro Photo Studio (B) Research Map in Kapuas Estuary (Pulau Panjang). the western of Pontianak city (West Kalimantan) Karimata Strait.
Spiral labyrinth glass (A) and Strobist Macro Photo Studio (B)
3
Research Map in Kapuas Estuary (Pulau Panjang). Located in the western of Pontianak city (West Kalimantan) and faces
4
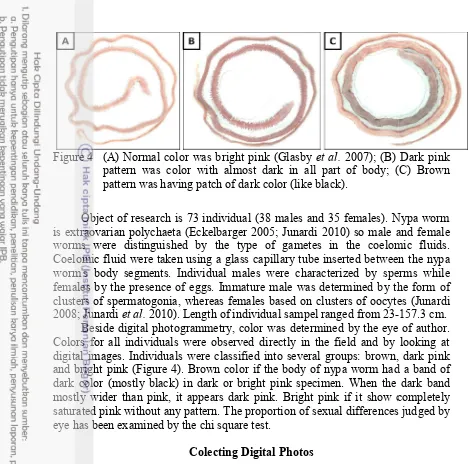
Figure 4 (A) Normal color
pattern was color with almost dark in all part of pattern was having patch of dark color (like black).
Object of research is 73 individual (38 is extraovarian polychaeta (
worms were distinguished by the type of gametes in the coelomic
Coelomic fluid were taken using a glass capillary tube inserted between the nypa worm's body segments. Individual males
females by the presence of eggs. Immature male clusters of spermatogonia, whereas females based 2008; Junardi et al. 2010). Length of
Beside digital photogrammetry, color
Colors for all individuals were observed directly in the field and by looking at digital images. Individuals
and bright pink (Figure 4). Brown color if the body of nypa worm had a band of dark color (mostly black) in dark or bright
mostly wider than pink, it appears dark pink. Bright pink if it show completely saturated pink without any pattern.
eye has been examined by the chi square test
Photogrammetry was used to method to give quantitative data
spiral labyrinth glass to limit the movement of the worm were taken while the worm
Image capture was done using Strobist Macro Photo Studio with external flash for light stabilization using 4 external flash YN 460
YongNuo Photographic Equipment Co. Ltd) (Figure
was adjusted with 18% reflective gray card (Mennon, China).
were set to 6000 K to adapt the ambient light condition at shooting time.
Digital images of nypa worm was recorded with a Canon EOS 1100 D digital SLR camera (12.2 megapixel, fitted with a Canon EF
IS lens) (Canon Inc, Taiwan) in RAW format.
subject recording enabling work to be done at the pixel level processing occurring (Perez 20
using UFRAW (http://ufraw.sourceforge.net/
Normal color was bright pink (Glasby et al. 2007); (B) pattern was color with almost dark in all part of body; (C) pattern was having patch of dark color (like black).
arch is 73 individual (38 males and 35 females). Nypa worm polychaeta (Eckelbarger 2005; Junardi 2010) so male and female distinguished by the type of gametes in the coelomic
were taken using a glass capillary tube inserted between the nypa worm's body segments. Individual males were characterized by sperm
females by the presence of eggs. Immature male was determined by th
, whereas females based on clusters of oocytes (Junardi Length of individual sampel ranged from
23-photogrammetry, color was determined by the eye
Colors for all individuals were observed directly in the field and by looking at digital images. Individuals were classified into several groups: brown, dark pink . Brown color if the body of nypa worm had a band of black) in dark or bright pink specimen. When the
mostly wider than pink, it appears dark pink. Bright pink if it show completely saturated pink without any pattern. The proportion of sexual differences judged eye has been examined by the chi square test.
Colecting Digital Photos
hotogrammetry was used to analyze color. Photogrammetry provides a method to give quantitative data (Linder 2006). Samples were photographed on spiral labyrinth glass to limit the movement of the worm (Figure 3A)
worm was alive to avoid any discoloration of the worm. Image capture was done using Strobist Macro Photo Studio with external flash for light stabilization using 4 external flash YN 460-II Speedlight (ShenZhen YongNuo Photographic Equipment Co. Ltd) (Figure 3B). Ambient light condition was adjusted with 18% reflective gray card (Mennon, China). Color temperature were set to 6000 K to adapt the ambient light condition at shooting time.
Digital images of nypa worm was recorded with a Canon EOS 1100 D camera (12.2 megapixel, fitted with a Canon EF-S 18-55mm f/3.5 ) (Canon Inc, Taiwan) in RAW format. Camera RAW files is a near
recording enabling work to be done at the pixel level without
(Perez 2007). RAW files were converted to 8-bit TIFF files http://ufraw.sourceforge.net/). were taken using a glass capillary tube inserted between the nypa characterized by sperms while determined by the form of mostly wider than pink, it appears dark pink. Bright pink if it show completely differences judged by
Photogrammetry provides a Samples were photographed on A). Images to avoid any discoloration of the worm. Image capture was done using Strobist Macro Photo Studio with external flash for Speedlight (ShenZhen B). Ambient light condition olor temperature were set to 6000 K to adapt the ambient light condition at shooting time.
Digital images of nypa worm was recorded with a Canon EOS 1100 D 55mm f/3.5-5.6 near-to-the-without in camera
5
Color Analysis
Measured area is the dorsal area, which is darker than other parts of the body. Determination of the measured area is by selecting the whole dorsal body seen in TIFF files as ROI (Regions of Interest). Data were processed with ImageJ (http://imagej.nih.gov). Data was average from all pixels measured inside ROI.
The output from the camera (in this case EOS 1100D using CMOS sensor) is interpreted in RGB values. RGB color space is not designed for human vision, but for the physical display devices, for instance computer monitor (Baldevbhai and Anand 2012). CIE L*a*b* color space was recommended by the CIE
(Commission Internationale de l'Eclairage) as a standardized interpretattion based on color matching experiments using principle of trichromatic theory of color vision (Perez 2007). CIE Lab color space describes the human colour vision and independent of the device used as a reference. It give a good interpretation of the color segmentation and most sensitive component to changes in practical imaging conditions (Baldevbhai and Anand 2012). Therefore the values of RGB colour spaces was converted to the corresponding values of CIE colour space.
CIE color space is a 3-dimensional hypothetical space with 3 color axes (Perez 2007). Color is expressed via 3 parameters: L*, a*, and b*. The L* value,
represents the lightness of color responses, L* ranges from 0-100 (black to white);
the a* value from -60 (green) to +60 (red), represents the hue degree of green to
red; and b* value was -60 (blue) to +60 (yellow), represents the hue degree blue to
yellow. If the a* and b* values further away from 0 then the colors would be
brighter. Further, one can calculated the chroma value, which is a plain indicator for the degree of color hue, from the a* and b* values as follows (Perez 2007). The chroma value, ranging 0-85 (from square root of minimum and maximum square of a* and b* value) :
Chroma = √ ∗ + ∗
The sexual differences between males and females has been examined via the Wilcoxon test (Koyabu et al. 2008). The relationship between the combination of color values and the color determined by eye was using linear discriminant analysis on L*, a*, and b* data. Calculation was done using R 2.11.0.
Histology
Histological analysis was performed to observed the anatomical difference between the color groups. This was due to morphological and anatomical changes occurring during maturation N. rhodochorde. Maturation is characterized by changing of body color (red in females and green in males) and having softer body than immature (Junardi 2012). Epidermis of polychaeta is composed of pseudostratified and covered by a cuticle. Collagenous cuticle produced by the epidermis covers the body wall of polychaeta. Epidermal cells consists of secretory and non-secretory cells. Secretory cells play a role to produce mucus in nypa worm (Hausen 2005; Mastrodonato et al. 2006).
6
Calcium Chloride) solution
dehydrated with graded alcohol treatment and cleared using xylol. used for infiltration and embedding
Sample was stained using h
The identification of specimens showed that
rhodochorde (n=73). Characters
prostomium; peristomium and phary
almost uniformly rounded from the anterior to mid towards the posterior. Prostomium cleft anteriorly relatively short subconical (
palpophores and palpostyles spherical peristomium. Having 4 pairs
invisible inside proboscis. dorsal and ventral cirrus. D posteriorly cirrophores were
neuroacicula was present but had only neorochaetae spiniger in supraacicular position
position. Having heterogomph falci teeth blade (Figure 6B).
Identification of males and females was using coelomic fluid to gamets. The observation of coelomic
stages 2 or immature to sub from 25μm - 60μm) (Figure7)
in all samples. This may be because January, while according to
November. There were 38 males and 35 females.
Figure 5 Dorsal (A) and lateral (B) view of
(an); prostomium (pro); peristomium (pr); peristomial cirrus (Pc); palps (P)
solution for ±3 days at room temperature. Sample was dehydrated with graded alcohol treatment and cleared using xylol. Paraffin
embedding. Tissue was cut using a rotary microtome hematoxylin and eosin.
3.
RESULT
Identification
The identification of specimens showed that samples were
haracters used were body shape and colouration; prostomium; peristomium and pharynx; parapodia; chaetae. Body shape
rounded from the anterior to mid-body but slightly flattened Prostomium cleft anteriorly and the shape of antenae relatively short subconical (Figure 5A). Palps biarticulate with
palpostyles spherical (Figure 5B). The eye was not visible inside . Having 4 pairs short peristomial cirrus (Figure 5A-B). Pharynx was
Parapodia type was sub-biramous. Each parapodia ha . Dorsal cirri anteriorly with cylindrical cirrophores, were flattened (leaf-like) (Figure 6A). Notoacicula and neuroacicula was present but had only neorochaetae. Having sesquigomph spiniger in supraacicular position. Lacking spiniger chaetae in subacicular position. Having heterogomph falcigerous chaetae in supraacicular with smooth
Identification of males and females was using coelomic fluid to The observation of coelomic fluid was found that they were
sub-mature in gamet maturity (gamete sizes was ranged (Figure7). The result showed that there was no mature worms
may be because samples were collected in December January, while according to Glasby (2007) spawning time in S
There were 38 males and 35 females.
(A) and lateral (B) view of Namalycastis rhodochorde
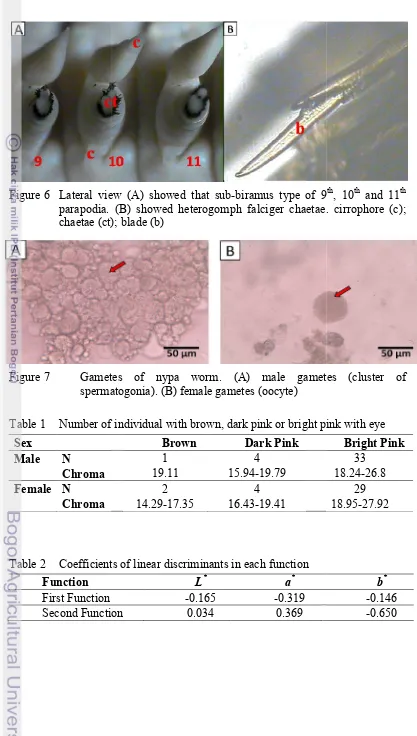
Figure 6 Lateral view (A) showed that parapodia. (B) showed heterogomph chaetae (ct); blade (b)
Figure 7 Gametes
spermatogonia
Table 1 Number of individual with brown, Sex
Male N
Chroma Female N
Chroma
Table 2 Coefficients of linear discriminants Function
Number of individual with brown, dark pink or bright pink with eye
Brown Dark Pink
1 4
19.11 15.94-19.79
2 4
14.29-17.35 16.43-19.41
Coefficients of linear discriminants in each function
L* a*
-0.165 -0.319
Second Function 0.034 0.369
7
biramus type of 9th, 10th and 11th
falciger chaetae. cirrophore (c);
gametes (cluster of
8
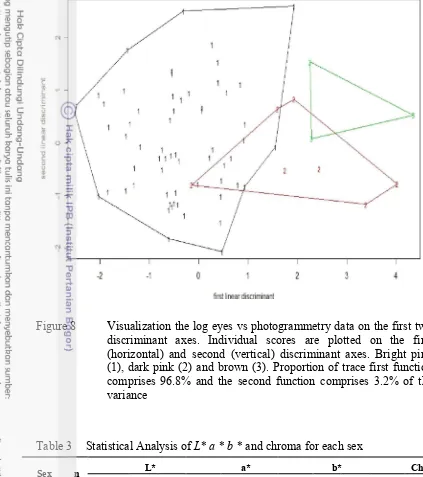
Figure 8 Visualization the log eyes vs photogrammetry data on the first two discriminant axes. Individual scores are plotted on the first (horizontal) and second (vertical) discriminant axes. Bright pink (1), dark pink (2) and brown (3). Proportion of trace first function comprises 96.8% and the second function comprises 3.2% of the variance
Table 3 Statistical Analysis of L* a * b * and chroma for each sex
Sex n L* a* b* Chroma
Mean SD CV Mean SD CV Mean SD CV Mean SD CV
Male 38 50.9 5.58 11 15.7 2.72 17.3 15.8 1.9 12 22.4 2.85 12.7
Female 35 50.7 4.77 9.42 15.6 3.24 20.8 15.4 1.83 11.9 21.9 3.27 14.9
L* a* b* represent luminance, hue degree of red-greeness, hue degree of
9
Correspondences of Color Determination by Eye and Photogrammetry Color of living worms mostly bright pink to dark pink, after preservation turned to pale yellow (Figure 5). Table 1 shows that, using the eye, the bright pink was the dominant color in males and females while the least is brown. The chroma values showed that bright pink was more vivid than dark pink and brown. Based on the analysis using the chi square test ( p > 0.05), there was no difference in proportion of different colors between males and females. Based on the analysis using the Wilcoxon test (p >0.05), there is also no significant difference between males and females colors judged by photogrammetry (Table 3).
The correspondence between color values from photogrammetry and color determined by eye was assesed using discriminant analysis. Coefficients of linear discriminants are in Table 2. Color hues of a* and b* rather than the L* luminance
value were strong determinants of color differentiation. Color hues of a* was the
highest value or the greatest influence in the grouping on discriminant analysis in first function; b* on second function. Figure 8 show that most variation in samples
was grouped by first function (96.8%). In the first function, bright pink and dark pink-brown were separated; the second function (3.2%) separate the groups dark pink and brown.
Sexual Dimorphism in Color Variation
Statisitical analysis for males and females could be seen in the Table 3. The
L* value of 50.9 in males and 50.7 in females showed that they had neutral color
or grey. Value of a* in males was 15.7 and females 15.6, so the color was reddish.
The b* values in males was 15.8 and females 15.4, so they had a yellowish color.
Chroma describes the vividness or dullness of a color. Table 3 showed that chroma value was 22.4 in males and 21.9 in females, it mean the chroma showed the color were dull. All parametric value of male tended to have on average higher than the females. Based on Tabel 3 males were more vivid than females. Canonical discriminant analysis showed that the chroma is an important determinant of color differentiation. Value of a* had the highest value so that red
has a big influence in the grouping.
Histology of Epidermis
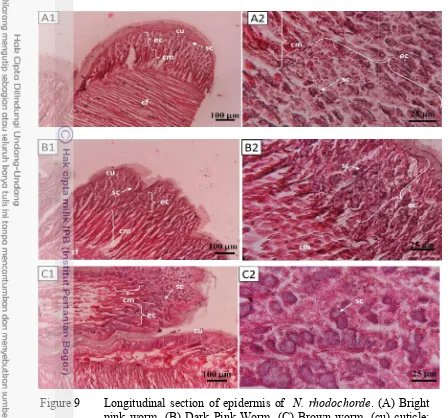
Epidermis of N. rhodochorde was cellular monolayer covered by cuticle. Cuticle were composed of fine fillamentous matrix that usually inhabits collagen fibrils (Figure 9A1-B1-C1). Under epidermis there was circular muscular. Figure 4 showed the muscular fibres. Cuticle and muscle in all of types showed the same composition.
10
Figure 9 Longitudinal section
pink worm. (B) Dark Pink Worm
(ec) epidermis cell; (cm) circular muscle; (cf) collagen fibre; (sc) secretory cell.
Glasby et al. (2007) described that immature pink coloration which turned red (
There is no reports on color
color variations in immature worms brown. Most of the worms were
size differences between colors because the r same. Oocyte size of bright
Longitudinal section of epidermis of N. rhodochorde. (A) Bright pink worm. (B) Dark Pink Worm. (C) Brown worm. (cu) cuticle; (ec) epidermis cell; (cm) circular muscle; (cf) collagen fibre; (sc) secretory cell.
4.
DISCUSSION
. (2007) described that immature N. rhodochorde
turned red (females) and green (males) when matured There is no reports on color variations in immature worms. Present study obtained color variations in immature worms categorized as bright pink, dark pink and of the worms were bright pink and the least is brown. There was no size differences between colors because the ranges of body length were
of bright pink worm were small ranging from 25µm ), while the dark pink were bigger ranging from 35µm - 50
brown worm ranging from 25µm - 30µm (immature). bright pink and brown were in stage of the spermatogonia Dark pink worms were found in the stage of spermato
in smaller amounts. It was suspected that reduction of the . (A) Bright (cu) cuticle; (ec) epidermis cell; (cm) circular muscle; (cf) collagen fibre; (sc)
11
number of cells in the clusters was a step toward maturity (Junardi 2010). These suggest that color variation related to gametogenesis.
Secretory cells have diverse function in polychaeta, for instances they secrete mucus to help the worm to eat (Costa et al. 2006) and to move (Pardo and Amaral 2004). In Syllidae, they used mucus to brood egg (Martin 2005).
Namanereis littoralis member of the same sub-family Namanereidinae as N. rhodochorde, produce abundant secretion to ensure viability of sex products and successful fertilization out of the water environment and protecting the animals and their clutches from desiccation (Ezhova 2011).
From histology of epidermis we obtained three forms of secretory cell within each group. Dark pink had more closed cell, it was suspected that cell more active so that more of mucus in cell. Solid composition of the cell caused the cell density also increased that make the color more darker. In females, this was caused by the dark pink were a color that had toward to maturity (Junardi 2008) which mean the worm was preparing mucus to keep the eggs. Dark pink also found in males. This coused the males also was prepare to maturity.
Different with brown worms that had larger cells, it was suspected to be an adaptation to the environment. This was caused nypa worm had a smaller range of habitats (Junardi 2008). Polychaeta could been secrete mucus for adaptation to environment. Laeonereis acuta (Nereididae) produced mucus to antioxidant defence system of the worm against environmental (Moraes et al. 2006). But on the contrary if there was exposure to pollutant (such heavy metal) it would be interfere with the production of mucus such as Laeonereis acuta that had chronic or acutely exposed to copper, mucus was absence on their body wall (Geracitano
et al. 2004).
In this study was not found mature samples. It was suspected when sampling was not a of spawning time. Polychaeta spawning time is strongly influenced by the physical environment conditions such as temperature, day length, and lunar cycles (Belal 2012). Phytoplankton bloom is one of the biological events that may cause the spawning mechanism as in Platynereis dumerilii (Watson et al 2003). It was also suspected of sampling sites residing on a single location because microhabitat conditions greatly affect the maturity. Individual populations can breed at different conditions, according to local conditions. In addition Nereidae can inhibit the maturation of gametes by promoting growth and regeneration through hormonal mechanisms (Lawrence and Soame 2004; Rouhi et al. 2008).
12
5.
CONCLUSION
All samples included in immature to sub-mature category. Color analysis showed no significant color differences between males and females in immature worm. Through the eyes, obtained variations of color (bright pink, dark pink and brown) with the dominant red in the grouping. In each group were also found variations in secretions cell.
6.
REFERENCES
Alam MA, Gomes A, Sarkar SK, Shuvaeva OV, Vishnevetskaya NS, Gustaytis MA, Bhattacharya BD, Godhantaraman N. 2010. Trace Metal Bioaccumulation by Soft-bottom Polychaetes (Annelida) of Sundarban Mangrove Wetland, India and Their Potential Use as Contamination Indicator. Bull Environ Contam Toxicol. 85:492–496
Baldevbhai PJ, Anand RS. 2012. Color Image Segmentation for Medical Images Using L*a*b* Color Space. IOSRJECE. 1: 24-45
Belal AAM. 2012. Oogenesis and Spawning of Pomatoleious kraussii (Baired, 1865) (Polychaeta: Serpulidae) in Suez Bay. Egypt J Aquati Res. 38: 119–124
Costa PFE, Oliveira RF, Fonseca LCD. 2006. Feeding Ecology of Nereis diversicolor (O.F. Müller) (Annelida, Polychaeta) on Estuarine and Lagoon Environments in the Southwest Coast of Portugal. Panam J Aquat. 1:114-126
Dean HK. 2008. The Use of Polychaetes (Annelida) as Indicator Species of Marine Pollution: A Review. Rev Biol Trop. 56: 11-38
Eckelbarger KJ. 2005. Oogenesis and Oocytes. Hydrobiologia. 535/536:179-198 Ezhova EE. 2011. Spawning and Early Ontogenesis of the Littoral Polychaete
Namanereis littoralis (Grube, 1876) (Nereididae, Namanereidinae). Russ J Dev Biol.42: 160–167
Fauchald K. 1977. The Polychaete Worm: Definition And Keys to the Orders,
Famili and Genera. Los Angeles (US): Natural History Museum of Los
Angeles County
Fischer AHL, Henrich T, Arendt D. 2010. The Normal Development of
Platynereis dumerilii (Nereididae, Annelida). FRONT ZOOL. 7:31
Geracitano LA, Bocchetti R, Monserrat JM, Regoli F, Bianchini A. 2004. Oxidative Stress Responses in Two Populations of Laeonereis acuta
(Polychaeta, Nereididae) after Acute and Chronic Exposure to Copper.
Mar Environ Res. 58: 1–17
Glasby CJ. 1999. The Namanereidinae (Polychaeta: Nereididae): Part 1, taxonomy and phylogeny. Rec Aust Mus, Supplement 25: 1–129
13
Hamzavi SF, Kamrani E, Salarzadeh A, Salarpouri A. 2012. Study of Polychaete Seasonal Changes (Ecological Indices) in Basatyn Estuary Nay band Bay of Bushehr. J Basic Appl Sci Res. 8: 8466-8470
Hausen H. 2005. Comparative Structure of the Epidermis in Polychaetes (Annelida). Hydrobiologia. 535/536: 25-35
Heuer CM, Muller CHG, Todt C, Loesel R. 2010. Comparative Neuroanatomy Suggests Repeated Reduction of Neuroarchitectural Complexity in Annelida. Front Zool. 7:13
Junardi. 2008. Karakteristik Morfologi dan Habitat Cacing Nipah Namalycastis
rhodochorde (Polychaeta: Nereididae: Namanereidinae) di Hutan
Mangrove Sungai Kakap Kalimantan Barat. Jurnal Sains MIPA. 14: 85-89
Junardi, Setyawati TR, Yuwono E. 2010. Gametogenesis Cacing Nipah
Namalycastis rhodochorde (Polychaeta: Nereididae). Jurnal Ilmu
Dasar.11: 39–44
Koyabu DB, Malaivijitnond S, Hamada Y. 2008. Pelage Color Variation of
Macaca arctoides and Its Evolutionary Implications. Int J Primatol
29:531–541
Lawrence AJ, Soame JM. 2004. The Effects of Climate Change on the Reproduction of Coastal Invertebrates. Ibis. 146: 29–39
Linder W. 2006. Digital Photogrammetry: A Practical Course. Second Edition. New York (US): Springer.
Martin GS. 2005. Exogoninae (Polychaeta: Syllidae) from Australia With the Description of a New Genus and Twenty-two New Species. Rec Aust Mus. 57: 39–152
Mastrodonato M, Gherardi M, Todisco G, Sciscioli M, Lepore E. 2006. The Epidermis of Timarete filigera (Polychaeta, Cirratulidae): Histochemical and Ultrastructural Analysis of the Gland Cells. Tissue Cell. 38: 279–284 McHugh D. 2005. Molecular Systematic Of Polychaetes (Annelida).
Hydrobiologia 535/536: 309-318
Moraes TB, Ferreira JLR, Rosa CEd, Sandrini JZ, Votto AP, Trindade GS, Geracitano LA, Abreu PC, Monserrat JM. 2006. Antioxidant Properties of the Mucus Secreted by Laeonereis acuta (Polychaeta, Nereididae): A Defense Against Environmental Pro-Oxidants?. Comp Biochem Physiol. 142: 293–300
Rouhi A, Sif J, Gillet P, Deutsch B. 2008. Reproduction and Population Dynamics of Perinereis cultrifera (Polychaeta: Nereididae) of the Atlantic Coast, El Jadida, Morocco. Cah Biol Mar. 49: 151-160
Watson GJ, Bentley MG, Gaudron SM, Hardege JD. 2003. The Role of Chemical Signals in the Spawning Induction of Polychaete Worms and other Marine Invertebrates. J. Exp Mar Biol Ecol. 294: 169–187
14
CURRICULUM VITAE
Author was born in Sambas on June 18th, 1986 and as the first child of