Cite this:New J. Chem.,2015,
39, 3043
Synthesis, characterization and antioxidant
activity of organoselenium and organotellurium
compound derivatives of chrysin
†
Sergio F. Fonseca,a
David B. Lima,a
Diego Alves,a
Raquel G. Jacob,a
Gelson Perin,a
Eder Joa˜o Lenarda˜o*a
and Lucielli Savegnago*b
Herein we describe the results on the synthesis and the evaluation of the antioxidant activity of several organochalcogen-containing chrysin derivatives (Se and Te). The semi-synthetic compounds were easily synthesized in good to excellent yields by the reaction of 7-(2-bromoethoxy)-chrysin with nucleophilic organoselenium and organotellurium species. The antioxidant properties of Se- and Te-containing chrysin derivatives were evaluated by three differentin vitro assays, the 1,1-diphenyl-2-picryl-hydrazyl (DPPH) and 2,20-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radical scavenging activities and
ferric ion reducing antioxidant power (FRAP). Compounds4i(which contains the butyltellurium moiety) and 4j (which contains a 4-phenyltellurium moiety) exhibited higher activities than chrysin and the selenium analogues, with compound4ibeing the most potent antioxidant.
Introduction
A complex natural and non-natural enzymatic anti-oxidant defense system present in the human body is able to oppose damage that free radicals and other oxidants may cause in an organism.1 Several diseases such as cancer,2 Alzheimer’s disease3 and Parkinson’s disease4 can be promoted by free radicals. An efficient way to prevent the effects of free radicals could involve an ample intake of dietary antioxidants.5,6
In this way, there has been an increase in the number of reports regarding flavonoids and its derivatives due to their well-known biological activities.7These activities can be explained by the presence of aromatic moieties and many oxygenated groups in their structure.
Flavonoids are natural polyphenolic phytochemicals that can be found in several fruits and vegetables and are commonly present in the average human diet.8They are a class of poly-phenolic secondary metabolites in plants that comprises about 6500 natural compounds.9
Chrysin (5,7-dihydroxyflavone) is a flavonoid commonly found in several plant extracts, honey, fruits and vegetables, such as passion fruit (Passiflora edulis),10propolis,11among others.
Just like other flavonoids, chrysin has been reported to exhibit many biological activities such as anti-viral,12 anticancer,13 anti-bactericidal,14 anti-inflammatory,15 anti-allergic,16 anti-mutagenic,17anti-anxiolytic18and antioxidant19effects. In recent times, several research groups around the world have been modifying the profile of chrysin and found that some chrysin derivatives can present diverse biological activities.20,21
Chemical modification of natural products has attracted great interest of many research groups nowadays, who aim to improve their original biological activities. Among the several modifications that can be performed in natural compounds’ structure, which include cyclization, dehydration, reduction and oxidation reactions, is the insertion of organochalcogen moieties. The chemical properties of organochalcogen compounds are widely described, being used as very attractive synthetic targets due to their selective reactions,22their use in asymmetric catalysis,23 as intermediates in the synthesis of several natural products24and also due to their biological activities.25
The antioxidant activity of selenium derivatives was recently reviewed and reported by several authors in papers and book chapters.26 The role of organoselenium and organotellurium moieties in the antioxidant activity of phenolic derivatives has been recently studied.27,28 In this sense, Engman and co-workers prepared a series of chalcogen-containing butylated hydroxyanisole (Ch-BHA)27and 3-pyridinols (Ch-Py)28and eval-uated their antioxidant properties. The insertion of an organo-selenium or organotellurium group in natural compounds has shown to enhance several of their biological and pharma-ceutical properties, such as antibacterial, antifungal29 and aLaborato´rio de Sı´ntese Orgaˆnica Limpa - LASOL - CCQFA - Universidade Federal
de Pelotas - UFPel, CEP 96010-900, Pelotas, RS, Brazil.
E-mail: [email protected]; Fax:+55 (53) 3275-7533; Tel:+55 (53) 3275-7533 bGrupo de Pesquisa em Neurobiotecnologia - GPN, CDTec, Universidade Federal de
Pelotas, UFPel, Pelotas, RS, Brazil. E-mail: [email protected] †Electronic supplementary information (ESI) available. See DOI: 10.1039/ c4nj02329c
Received (in Porto Alegre, Brazil) 17th December 2014,
Accepted 5th February 2015
DOI: 10.1039/c4nj02329c
www.rsc.org/njc
NJC
antioxidant activities.30This enhancement can be attributed to their ability to stabilize free radicals.25–30
Therefore, based on the considerations above, and in con-tinuation of our studies on the synthesis of semi-synthetic organochalcogenium compounds and aiming to combine the bioactive properties of chrysin with those of organochalco-genium, we present here our results on the synthesis of new Se- and Te-containing chrysin derivatives and their antioxidant activitiesin vitro(Scheme 1).
Results and discussion
ChemistryThe first step in the synthesis of organochalcogen derivatives of chrysin involves the preparation of the key intermediate 7-(2-bromoethoxy)-5-hydroxy-2-phenyl-4H-chromen-4-one2using a synthetic route adapted from Hu and co-workers31(Scheme 2). Starting from the readily available chrysin1, 7-(2-bromoethoxy)-5-hydroxy-2-phenyl-4H-chromen-4-one2was obtained after reaction with 1,2-dibromoethane in the presence of potassium carbonate as a base and acetone as solvent. The brominated chrysin 2 was obtained as a yellow solid after purification by flash chromato-graphy and used in the next step of the synthesis.
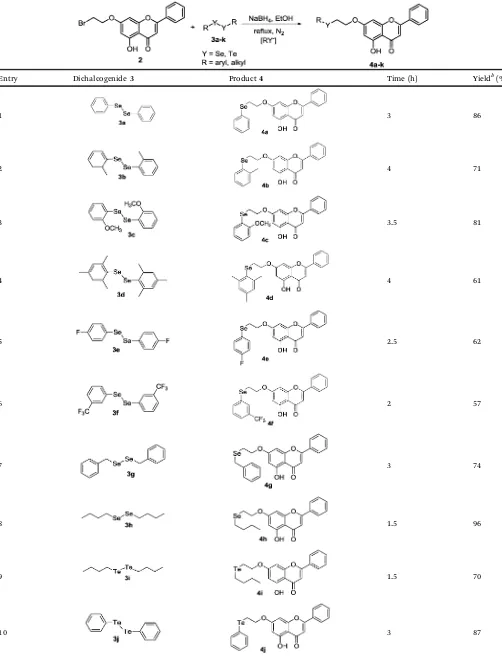
With compound 2 in hand, it was reacted with different organochalcogenolate anions, which were generated in situ from the respective diorganyl dichalcogenides (3a–k)32 in the presence of sodium borohydride and ethanol as solvent, to give the desired products4a–k(Scheme 3 and Table 1).
The reaction procedure is very simple. For example, to generate the phenylselenolate anionin situ, a solution of diphenyl diselenide3a(0.5 mmol) in ethanol (15.0 mL) was reacted with NaBH4 (1.25 mmol) under a nitrogen atmosphere.32 After few minutes, the yellowish solution turned colorless, indicating that the diselenide was reduced. At this point, the previously pre-pared bromo-containing chrysin2(1.0 mmol) was added at room
temperature under vigorous stirring. After refluxing for 3 h, the desired Se-containing chrysin4awas isolated in 86% yield as a white solid (Table 1, entry 1). By using this protocol, several other diorganyl dichalcogenides were used and in all tested examples, the respective selenium- and tellurium functionalized chrysin derivatives were obtained in good yields (Table 1).
As can be seen in Table 1, this approach is applicable for both, diorganyl diselenides and diorganyl ditellurides, containing neutral, electron-withdrawing and electron donating groups. As expected for a SN2 mechanism, weaker nucleophiles afforded lower yields of the substituted product4. This was the case when 3e (R = 4-fluorophenyl, entry 5, 62%) and 3f (R = 3-(trifluoro-methyl)phenyl, entry 6, 57% yield) were used as chalcogenium nucleophiles. In contrast, when dibutyl diselenide3hwas used, the respective Se-chrysin4hwas obtained in 96% yield only after 1.5 h (Table 1, entry 8). Accordingly, bis-2-tolyl diselenide (3b), bis-2-anisyl diselenide (3c) and dibenzyl diselenide 3g reacted with2to afford, respectively4b,4cand4gin 71%, 81% and 74% yields after 3–4 h (Table 1, entries 2, 3 and 7). The results presented in Table 1 also indicate that steric factors are impor-tant in this reaction. Thus, when the highly hindered dimesityl diselenide 3d was used as the nucleophile, the respective Se-chrysin4dwas obtained in 61% yield, in spite of the presence of three electron donating groups (Table 1, entry 4).
The method developed here was also useful for the prepara-tion of tellurium-containing chrysin derivatives (Table 1, entries 9–11). Good yields of Te-chrysin 4i (R = butyl, 1.5 h, 70%) and4j(R = phenyl, 3 h, 87%) were obtained from dibutyl ditelluride3iand diphenyl ditelluride3j, respectively (Table 1, entries 9 and 10). The electronic effect was remarkable when 3k (R = 4-chlorophenyl) was used, with the respective Te-functionalized chrysin4kbeing isolated in 57% yield after 6 h. Antioxidant activity.In order to verify the effect caused by the presence of the organochalcogenium moiety in the new molecules, the antioxidant activity of compounds4a,4b,4c,4g, 4h,4iand4jwas evaluated by differentin vitromethods and compared to the parent, unmodified chrysin1(Tables 2–4).
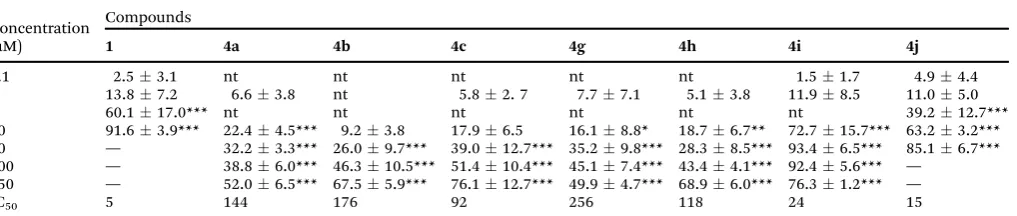
Radical scavenging activity.As can be seen in Table 2, among the tested compounds, 4i, which contains the butyltellurium moiety, demonstrated to be very potent in the DPPH radical scavenging activity, with a IC50value (concentration required to inhibit 50% of the radicals) of 36mM. Butyltellurium-chrysin4i showed a higher radical scavenging activity than the phenyl-tellurium analog4j(IC50 value of 79mM). On the other hand, selenium-containing compounds (4b, 4c, 4g and 4h) did not present IC50because they were not able to scavenge 50% of the DPPH radical, while compound4adid not present any effect.
The results obtained in the DPPHassay are in agreement with several reports in the literature, in which tellurium-containing Scheme 1 General scheme of the present work.
Scheme 2 Synthesis of key intermediate2.
Table 1 Synthesis of selenium and tellurium-containing chrysin derivatives4a–ka
Entry Dichalcogenide3 Product4 Time (h) Yieldb(%)
1 3 86
2 4 71
3 3.5 81
4 4 61
5 2.5 62
6 2 57
7 3 74
8 1.5 96
9 1.5 70
compounds have shown to be very effective antioxidant agents, even more than their selenium analogues.26–28,33–35
Although Sim and co-workers36 have demonstrated that chrysin1presents low antioxidant activity in the DPPH assay, (6.4% and 8.5% inhibitory effect, at 100 and 1000mM, respec-tively), in our hands, chrysin did not present any significant activity in all the assays performed (data not shown).
In Table 3 is presented the ABTS+ radical scavenging activity of chalcogen-containing compounds 4a, 4b, 4c, 4g, 4h, 4i and4j and the unmodified chrysin 1. Similar to that
observed in the DPPHassay, the tellurium-derivatives4iand4j presented superior scavenging activities (IC50of 24 and 15mM respectively) than the selenium analogues, being comparable to that of chrysin1(IC50of 5mM).
The most common spectrophotometric methods to deter-mine the antioxidant activity of organic compounds are based on DPPHand ABTS+, which react directly with the antioxidant species under evaluation.37The principle of the DPPH assay involves an electron transfer reaction and a hydrogen-atom abstraction. Thus, the assay is based on the measurement of Table 1 (continued)
Entry Dichalcogenide3 Product4 Time (h) Yieldb(%)
11 6 57
a
Reaction conditions:2 (1.0 mmol) was added to a mixture of3(0.5 mmol) and NaBH4(1.25 mmol) in EtOH (15.0 mL) and the resulting suspension was refluxed for the time indicated.bYields after purification by column chromatography on silica gel.
Table 2 DPPH radical scavenging of compounds4b–cand4g–j
Concentration (mM)
Compound
4b 4c 4g 4h 4i 4j
1 nt nt nt nt 0.60.6 1.60.9
10 0.10.1 1.51.4 0.10.1 0.40.7 32.81.2*** 9.13.6
50 3.91.0 4.41.5 0.50.5 2.11.9 81.012.7*** 39.319.4**
100 9.13.8** 8.82.7** 0.90.6 6.53.6* 93.42.8*** 62.015.6***
250 16.42.7*** 17.02.8*** 4.62.9* 16.13.9*** 94.92.2*** 92.32.6***
IC50 — — — — 36.0 79.0
Data are presented as meanSD (n= 3). The values are expressed in percentage of inhibition in relation to control. IC50= concentration of compound required for 50% scavenging. The asterisks represent significant difference (*)po0.05; (**)po0.01; (***)po0.001 when compared with the control sample using the Student–Newman–Keuls test forpost-hoccomparison. nt = not tested.
Table 3 ABTS radical scavenging activities of compounds1,4a,4b,4c,4g,4h,4iand4j
Concentration (mM)
Compounds
1 4a 4b 4c 4g 4h 4i 4j
0.1 2.53.1 nt nt nt nt nt 1.51.7 4.94.4
1 13.87.2 6.63.8 nt 5.82. 7 7.77.1 5.13.8 11.98.5 11.05.0
5 60.117.0*** nt nt nt nt nt nt 39.212.7***
10 91.63.9*** 22.44.5*** 9.23.8 17.96.5 16.18.8* 18.76.7** 72.715.7*** 63.23.2*** 50 — 32.23.3*** 26.09.7*** 39.012.7*** 35.29.8*** 28.38.5*** 93.46.5*** 85.16.7*** 100 — 38.86.0*** 46.310.5*** 51.410.4*** 45.17.4*** 43.44.1*** 92.45.6*** —
250 — 52.06.5*** 67.55.9*** 76.112.7*** 49.94.7*** 68.96.0*** 76.31.2*** —
IC50 5 144 176 92 256 118 24 15
the scavenging ability of antioxidants towards the DPPH radical. The ABTS+assay is based on a single electron transfer, and the scavenging of the ABTS+ radical-cation in some cases can be more efficient than that of DPPH.38
The results obtained from radical scavenging revealed that (i) the tellurium-containing compounds 4i and 4j presented higher scavenging efficiency among the new chalcogen-chrysin; (ii) compounds4iand4jpresented DPPHand similar ABTS+ scavenging capacities compared to the parent chrysin1; and (iii) compound 4i, which contains a butyltellurium moiety, presented the best results demonstrating the importance of this group in the antioxidant activity.
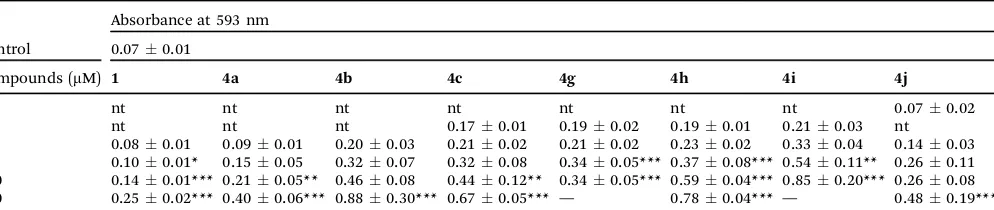
Ferric ion reducing antioxidant power (FRAP). Several reports in the literature have demonstrated that the antioxidant activity might be correlated with the reducing power of a compound.39,40Thus, based on this evidence, the FRAP assay was used to determine the reducing power of compounds4a, 4b,4c,4g,4h,4iand4jto clarify the relationship between the antioxidant effect and the reducing power, comparing them to chrysin1(Table 4).
Compound 4i exhibited the best results and its reducing power was increased by using higher concentrations. This result is in agreement with those found in the ABTS+assay, confirming that the antioxidant activity of 4i and 4j could involve the electron transfer by the tellurium atom. Similar to that recently observed for alkyltelluro-pyiridinols,28 the high reactivity of telluro-chrysin4i–jwould involve a novel, different reaction mechanism compared to that of unmodified flavonoid 1, which is due to the presence of tellurium. In addition, the FRAP assay revealed that all chrysin derivatives show a higher ferric reducing ability than chrysin, with the tellurium-containing compounds presenting higher activity than the selenium ones.
Conclusions
In summary, a new class of chrysin derivatives containing an organochalcogen group in their structure was synthesized and evaluated for their antioxidant activity in vitro. The products were easily obtained in good to excellent yields and in a relatively short period of time using mild reaction conditions. The tellurium-containing derivatives of chrysin presented higher antioxidant activities than the selenium ones, with compound
4i(containing a butyltellurium moiety) being the more active. Other bioassays are currently in progress to verify other possible activities of the new semi-synthetic chalcogen chrysin derivatives as well as the mechanism involved.
Experimental section
Chemistry – general remarksThe reactions were monitored by TLC carried out on Merck silica gel (60 F254) by using UV light, iodine vapor and 5% vanillin in 10% H2SO4and heat as developing agents.1H NMR and13C NMR spectra were recorded using Bruker DPX 300 and 75 (300 and 75 MHz respectively) instruments using CDCl3as solvent and calibrated using tetramethylsilane as an internal standard. Coupling constants (J) are reported in Hertz.
High-resolution mass spectra (HRMS) were obtained for all compounds on a LTQ Orbitrap Discovery mass spectrometer (Thermo Fisher Scientific). This hybrid system meets the LTQ XL linear ion trap mass spectrometer and an Orbitrap mass analyzer. The experiments were performedviadirect infusion of the sample (flow: 10mL min 1) in positive-ion mode using electrospray ionization. Elemental composition calculations for comparison were executed using the specific tool included in the Qual Browser module of Xcalibur (Thermo Fisher Scientific, release 2.0.7) software. Mass spectra (MS) were recorded on a Shimadzu GCMSQP2010 mass spectrometer.
Melting point (mp) values were measured using a Marte PFD III instrument with 0.11C precision.
Synthesis of 7-(2-bromoethoxy)-5-hydroxy-2-phenyl-4H
-chromen-4-one 2.The bromo-containing chrysin2was prepared according to Hu and co-workers, with little modifications.30
To a solution of chrysin (0.500 g, 1.96 mmol) in acetone (45.0 mL) in a 100 mL two necked round-bottomed flask, equipped with a reflux condenser and under a N2atmosphere, 1,2-dibromoethane (3.0 mL, 35.0 mmol) and potassium carbo-nate (0.544 g, 3.93 mmol) were added. The reaction was stirred under reflux temperature for 21 h, until the total consumption of the starting material (followed by TLC). The solution was cooled at room temperature, diluted with ethyl acetate (30 mL) and washed with water (330.0 mL). The organic phase was separated, dried over anhydrous MgSO4 and concentrated under vacuum. The product was obtained as a yellow solid, Table 4 Ferric ion reducing antioxidant power (FRAP) of compounds
Absorbance at 593 nm
Control 0.070.01
Compounds (mM) 1 4a 4b 4c 4g 4h 4i 4j
0.1 nt nt nt nt nt nt nt 0.070.02
1 nt nt nt 0.170.01 0.190.02 0.190.01 0.210.03 nt
10 0.080.01 0.090.01 0.200.03 0.210.02 0.210.02 0.230.02 0.330.04 0.140.03 50 0.100.01* 0.150.05 0.320.07 0.320.08 0.340.05*** 0.370.08*** 0.540.11** 0.260.11 100 0.140.01*** 0.210.05** 0.460.08 0.440.12** 0.340.05*** 0.590.04*** 0.850.20*** 0.260.08 250 0.250.02*** 0.400.06*** 0.880.30*** 0.670.05*** — 0.780.04*** — 0.480.19***
mp 161–1621C (lit 38: 157–1581C). Yield: 0.672 g (95%). The spectral data of the obtained compound are in perfect agreement with those reported in the literature.30
General procedure for the synthesis of compounds 4a–k.In a 25 mL two necked round-bottomed flask, equipped with a reflux condenser containing a solution of diorganyl dichalco-genide3 (0.5 mmol) in ethanol (15.0 mL) under a N2 atmo-sphere, sodium borohydride (0.047 g, 1.25 mmol) was added at room temperature under vigorous stirring. Gas evolution was observed during addition. The reaction mixture was stirred under N2 until it became colorless. Then, the 7-bromoethoxy chrysin 2 (0.361 g, 1.0 mmol) was added and the resultant mixture was refluxed (during 1.5–6.0 h, see Table 1) until all the starting material was transformed (followed by TLC). After that, the reaction mixture was cooled at room temperature, diluted with ethyl acetate (15.0 mL) and washed with water (315.0 mL). The organic phase was separated, dried over anhydrous MgSO4 and concentrated under vacuum. The crude products were purified by column chromatography on silica gel using initially hexanes as an eluent to remove the remaining diorganyl dichal-cogenide and then a mixture of hexanes/ethyl acetate (2/8) to afford the desired products4a–k. The spectral data and physical properties of all synthesized compounds are presented below.
5-Hydroxy-2-phenyl-7-[2-(phenylselanyl)ethoxy]-4H
-chromen-4-one (4a).Yield: 0.377 g (86%); white solid; mp 155–1561C. 1H NMR (300 MHz, CDCl 163.8, 162.0, 157.6, 133.2, 131.8, 131.1, 129.2, 129.0, 127.5, 126.2, 105.7, 98.5, 92.9, 77.4, 77.0, 76.6, 67.9, 25.5. MS (relative intensity)m/z: 439 (M + 1, 3); 438 (M+, 10); 281 (5); 254 (22); 185 (100); 157 (69); 77 (27). HRMS: calculated to C23H18O4Se+ [M + H] 439.0443, found 439.0434.
5-Hydroxy-2-phenyl-7-[2-(o-tolylselanyl)ethoxy]-4H
-chromen-4-one (4b).Yield: 0.321 g (71%); light yellow solid; mp 123.9– 127.41C.1H NMR (300 MHz, CDCl3)d12.70 (s, 1H); 7.87–7.84 (m, 2H); 7.53–7.50 (m, 4H); 7.26–7.10 (m, 3H); 6.64 (s, 1H); 6.40 (d,J= 2.2 Hz, 1H); 6.28 (d,J= 2.2 Hz, 1H); 4.23 (t,J= 7.2 Hz, 2H); 3.21 (t,J= 7.2 Hz, 2H); 2.45 (s, 3H). 13C NMR (75 MHz, CDCl3)d182.4, 164.2, 163.9, 162.1, 157.6, 139.9, 132.3, 131.8, 131.2, 130.2, 129.8, 129.0, 127.4, 126.7, 126.2, 105.8, 98.6, 93.0, 77.4, 77.0, 76.6, 67.7, 24.4, 22.5. MS (relative intensity)m/z: 452 (M+, 7); 281 (6); 254 (28); 236 (7); 199 (90); 185 (7); 171 (65); 91 (88); 77 (11); 57 (100). HRMS: calculated to C24H21O4Se+[M + H] 453.0600, found 453.0615.
5-Hydroxy-7-{2-[(2-methoxyphenyl)selanyl]ethoxy}-2-phenyl-4H-chromen-4-one (4c). Yield: 0.378 g (81%); grey solid; mp 132.9–135.61C.1H NMR (300 MHz, CDCl3)d12.65 (s, 1H); 7.85– 7.82 (m, 2H); 7.51–7.44 (m, 4H); 7.25 (ddd,J= 8.1, 7.4 and 1.6 Hz, 1H); 6.91–6.85 (m, 2H); 6.61 (s, 1H); 6.40 (d, J = 2.2 Hz, 1H); 6.27 (d,J= 2.2 Hz, 1H); 4.26 (t,J= 7.3 Hz, 2H); 3.88 (s, 3H); 3.23 (t,J = 7.3 Hz, 2H).13C NMR (75 MHz, CDCl3) d182.3, 164.9, 164.3, 163.9, 162.2, 158.3, 157.7, 132.4, 131.7, 131.2, 128.9, 128.5, 128.6, 126.2, 121.4, 117.9, 110.8, 105.8, 105.7, 98.6, 93.1, 77.4, 77.0, 76.6, 68.1, 55.8, 22.9. MS (relative intensity)
m/z: 468 (M+, 6); 281 (5); 254 (9); 236 (7); 215 (44); 187 (32); 107 (33); 77 (11); 57 (100). HRMS: calculated to C24H21O5Se+[M + H] 469.0549, found 469.0562.
5-Hydroxy-7-[2-(mesitylselanyl)ethoxy]-2-phenyl-4H
-chromen-4-one (4d). Yield: 0.289 g (60%); white/pink solid; mp 135.7–138.4 1C. 1H NMR (300 MHz, CDCl3) d 12.70 (s, 1H); 7.86–7.84 (m, 2H); 7.53–7.52 (m, 3H); 6.95 (s, 2H); 6.63 (s, 1H); 6.35 (d,J= 2.2 Hz, 1H); 6.23 (d,J= 2.2 Hz, 1H); 4.12 (t,J= 7.0 Hz, 2H); 2.99 (t,J= 7.0 Hz, 2H); 2.55 (s, 6H); 2.26 (s, 3H).13C NMR (75 MHz, CDCl3) d 182.3, 164.9, 164.2, 163.9, 162.1, 157.6, 143.1, 138.6, 131.8, 131.2, 129.0, 128.6, 126.4, 126.2, 105.8, 105.7, 98.4, 93.0, 77.4, 77.0, 76.6, 67.9, 25.2, 24.5, 20.9. MS (relative intensity)m/z: 480 (M+, 6); 361 (1); 281 (12); 254 (35); 227 (60); 199 (100); 119 (98); 77 (10). HRMS: calculated to C26H25O4Se+[M + H] 481.0913, found 481.0918.
7-{2-[(4-Fluorophenyl)selanyl]ethoxy}-5-hydroxy-2-phenyl-4H
-chromen-4-one (4e).Yield: 0.282 g (62%); white solid; mp 171.1– 173.71C.1H NMR (300 MHz, CDCl3)d12.70 (s, 1H); 7.87–7.84 ethoxy}-4H-chromen-4-one (4f). Yield: 0.289 g (57%); white solid; mp 154.1–157.71C. 1H NMR (300 MHz, CDCl Hz) 105.8, 105.7, 98.4, 93.0, 67.8, 25.9. MS (relative intensity) m/z: 506 (M+, 17); 281 (5); 253 (100); 225 (87); 145 (14); 77 (12); 69 (36). HRMS: calculated to C24H18F3O4Se+[M + H] 507.0317, found 507.0326.
7-[2-(Benzylselanyl)ethoxy]-5-hydroxy-2-phenyl-4H
-chromen-4-one (4g).Yield: 0.336 g (74%); green solid; mp 115.4–118.21C. 1H NMR (300 MHz, CDCl
3)d12.71 (s, 1H); 7.87–7.84 (m, 2H); 7.52–7.50 (m, 3H); 7.32–7.25 (m, 5H); 6.63 (s, 1H); 6.41 (d,J= 2.2 Hz, 1H); 6.28 (d,J= 2.2 Hz, 1H); 4.13 (t,J= 7.0 Hz, 2H); 3.89 (t, J= 6.7 Hz, 2H). 13C NMR (75 MHz, CDCl3)d182.3, 164.2, 163.8, 162.1, 157.6, 138.8, 131.8, 131.1, 129.0, 128.8, 128.6, 126.9, 126.2, 105.7, 105.7, 98.5, 93.0, 77.4, 77.0, 76.6, 68.7, 27.7, 21.4. MS (relative intensity)m/z: 452 (M+, 1); 285 (6); 255 (9); 171 (11); 77 (5); 69 (75); 55 (100). HRMS: calculated to C24H21O4Se+[M + H] 453.0605, found 453.0603.
7-[2-(Butylselanyl)ethoxy]-5-hydroxy-2-phenyl-4H-chromen-4-one
4.25 (t,J= 7.2 Hz, 2H); 2.91 (t,J= 7.2 Hz, 2H); 2.69 (t,J= 7.4 Hz, 2H); 1.69 (qui,J= 7.5 Hz, 2H); 1.44 (sex,J= 7.5 Hz, 2H); 0.94 (t,J= 7.3 Hz, 3H).13C NMR (75 MHz, CDCl3)d182.3, 164.3, 163.9, 162.1, 157.7, 131.8, 131.2, 129.0, 126.2, 105.8, 105.2, 98.5, 93.0, 77.4, 77.0, 76.6, 68.9, 32.7, 24.5, 22.9, 21.3, 13.5. MS (relative intensity)m/z: 418 (M+, 5); 361 (18); 254 (31); 165 (100); 77 (9). HRMS: calculated to C21H23O4Se+[M + H] 419.0756, found 419.0758.
7-[2-(Butyltellanyl)ethoxy]-5-hydroxy-2-phenyl-4H-chromen-4-one
(4i).Yield: 0.327 g (70%); yellow solid; mp 79.5–81.41C.1H NMR (300 MHz, CDCl3) d 12.70 (s, 1H); 7.87–7.84 (m, 2H); 7.53–7.50 (m, 3H); 6.64 (s, 1H); 6.46 (d,J= 2.2 Hz, 1H); 6.32 (d,J= 2.2 Hz, 1H); 4.32 (t,J= 7.6 Hz, 2H); 2.96 (t,J= 7.6 Hz, 2H); 2.75 (t,J= 7.0 Hz, 2H); 1.77 (qui,J= 7.0 Hz, 2H); 1.40 (sex,J= 7.0 Hz, 2H); 0.93 (t,J= 7.0 Hz, 3H).13C NMR (75 MHz, CDCl
3)d182.3, 164.2, 163.8, 162.1, 157.6, 131.8, 131.1, 129.0, 126.2, 105.7, 105.7, 98.6, 93.0, 77.4, 77.0, 76.6, 70.8, 34.3, 25.0, 13.4, 3.6. MS (relative intensity)m/z: 468 (M+, 3); 411 (19); 254 (100); 215 (8); 187 (11); 77 (9); 57 (93). HRMS: calculated to C21H23O4Te+[M + H] 469.0653, found 469.0554.
5-Hydroxy-2-phenyl-7-[2-(phenyltellanyl)ethoxy]-4H
-chromen-4-one (4j).Yield: 0.407 g (87%); yellow solid; mp 128–1291C. 1H NMR (300 MHz, CDCl3) d12.69 (s, 1H); 7.86–7.79 (m, 4H);
7.53–7.50 (m, 3H); 7.33–7.21 (m, 3H); 6.63 (s, 1H); 6.37 (d,J= 2.2 Hz, 1H); 6.26 (d,J= 2.2 Hz, 1H); 4.34 (t,J= 7.6 Hz, 2H); 3.19 (t,J= 7.6 = Hz, 2H).13C NMR (75 MHz, CDCl3)d182.3, 164.1, 163.8, 162.0, 157.7, 138.8, 131.8, 131.1, 129.3, 129.0, 128.1, 126.2, 105.7, 105.6, 98.6, 93.0, 77.4, 77.0, 76.6, 69.9, 5.9. MS (relative intensity)m/z: 488 (M+, 5); 330 (100); 254 (35); 207 (46); 77 (81). HRMS: calculated to C23H19O4Te+ [M + H] 488.0267, found 488.0274.
7-{2-[(4-Chlorophenyl)tellanyl]ethoxy}-5-hydroxy-2-phenyl-4H
-chromen-4-one (4k). Yield: 0.297 g (57%); yellow solid; mp 140.3–1431C.1H NMR (300 MHz, CDCl3)d12.70 (s, 1H); 7.87– 7.84 (m, 2H); 7.71 (d,J= 8.4 Hz, 2H); 7.53–7.51 (m, 3H); 7.20 (d,J= 8.4 Hz, 2H); 6.64 (s, 1H); 6.38–6.37 (d,J= 2.4 Hz, 2H); 6.27– 6.26 (d,J= 2.1 Hz, 2H); 4.34 (t,J= 7.5 Hz, 2H); 3.19 (t,J= 7.5 Hz, 2H). 13C NMR (75 MHz, CDCl3) d 182.3, 164.9, 164.0, 163.9, 162.1, 157.6, 140.2, 134.8, 131.8, 131.1, 129.6, 129.0, 126.2, 108.1, 105.8, 98.5, 93.1, 77.4, 77.0, 76.6, 69.7, 6.5. MS (relative intensity) m/z: 522 (M+, 6); 364 (100); 281 (6); 254 (32); 241 (41); 237 (19); 111 (19); 77 (12). HRMS: calculated to C23H18ClO4Te+[M + H] 522.9950, found 522.9766.
Antioxidant activity assays
The antioxidant properties of these new compounds were evaluated by three different methodsin vitro: DPPH and ABTS+ radical scavenging activity and ferric ion reducing antioxidant power (FRAP). The results obtained were compared with the results obtained with chrysin.
All drugs were dissolved in dimethyl sulfoxide (DMSO). However, compounds 4d, 4e, 4f and 4k were not evaluated because they were not soluble in this solvent, even after irradia-tion with an ultrasonic bath equipment.
Radical scavenging activity
To determine if compounds4a,4b,4c,4g,4h,4iand4jpresent in vitroantioxidant activity against free radicals, we evaluated
the DPPH and ABTS+scavenging activity of these compounds at different concentrations.
The stable radical DPPH has been widely used for determining the hydrogen- or electron-donating capacity of pure anti-oxidant compounds, plant and fruit extracts and food materials.41,42It is a stable free radical that is commonly used as a substrate to evaluatein vitroanti-oxidant activity. The scavenging activity of compounds 4a, 4b, 4c, 4g, 4h, 4i and 4j (0.1–250 mM) was determined in accordance with the method of Choiet al.43with some modifications.
The ABTS method is based on the ability of anti-oxidants to quench the long-lived ABTS radical cation, a blue/green chromophore with characteristic absorption at 734 nm. The ABTS radical scavenging activity was determined according to the method described by Reet al.44 with some modifications. Different concentrations of compounds (0.1–250 mM) were mixed with the ABTS+solution, and the decrease in the absor-bance at 734 nm was recorded.
The values are expressed as the percentages of radical inhibition absorbance (I%) in relation to the control values, as calculated by the following equation:
I% = [(Ac As/Ac)100]
Ac is the absorbance of the control excluding the test compounds, andAsis the absorbance of the tested compounds.
Ferric ion reducing anti-oxidant power (FRAP)
The FRAP method is based on a redox reaction in which an easily reduced oxidant (Fe3+) is used in excess, and the antioxidants act as reducing agents. The ferric ion (Fe3+) reducing anti-oxidant power (FRAP) method was used to measure the reducing capacity of the compounds. The assay was performed as described by Stratilet al.45 with slight modifications. Different concentrations of compounds 4a,4b,4c,4g,4h,4iand4j(0.1–250mM) and the FRAP reagent were added to each sample, and the mixture was incubated at 371C for 40 min in the dark. The absorbance of the resulting solution was measured at 593 nm using a spectrophotometer.
Statistical analysis
The experimental results were given as the means standard deviation (SD) to show the variations among the groups. The statistical analysis was performed using one-way ANOVA followed by the Newman–Keuls multiple comparison test when appropriate. The differences were considered statistically significant at a prob-ability of less than 5% (po0.05). All tests were performed at least three times in duplicate. The IC50values (the concentration of the sample required to scavenge 50% of the free radicals) were calculated from the graph of the scavenging effect percentage versusthe compound concentration.
Acknowledgements
Notes and references
1 B. Halliwell and J. M. C. Gutteridge,Free Radicals Biol. Med., Clarendon Press, Oxford, 4th edn, 2006.
2 V. L. Kinnula and J. D. Crapo,Free Radical Biol. Med., 2004, 36, 718–744.
3 M. A. Smith, C. A. Rottkamp, A. Nunomura, A. K. Raina and G. Perry,Biochim. Biophys. Acta, 2000,1502, 139–144. 4 J. L. Bolton, M. A. Trush, T. M. Penning, G. Dryhurst and
T. J. Monks,Chem. Res. Toxicol., 2000,13, 135–160. 5 D. H. Hyun, S. S. Emerson, D. G. Jo, M. P. Mattson and R. de
Cabo,Proc. Natl. Acad. Sci. U. S. A., 2006,103, 19908–19912. 6 M. N. Alam and M. N. J. B. Rafiquzzaman,Saudi Pharm. J.,
2013,21, 143–152.
7 K. E. Heim, A. R. Tagliaferro and D. J. Bobilya, J. Nutr. Biochem., 2002,13, 572–584.
8 P. C. Lv, T. T. Cai, Y. Qian, J. Sun and H. L. Zhu,Eur. J. Med. Chem., 2011,46, 393–398.
9 J. B. Harborne and C. A. Williams,Phytochemicals, 2000,55, 481–504.
10 K. Dhawan, S. Dhawan and A. Sharma,J. Ethnopharmacol., 2004,94, 1–23.
11 V. Bankova, M. Popova, S. Bogdanov and A. Sabatini, Z. Naturforsch., 2002,57c, 530–533.
12 E. A. Bae, M. J. Han, M. Lee and D. H. Kim,Biol. Pharm. Bull., 2000,23, 1122–1124.
13 C. Han,Cancer Lett., 1997,114, 153–158.
14 J. M. T. Hamilton-Miller, Antimicrob. Agents Chemother., 1995,39, 2375–2377.
15 E. J. Kim, K. J. Kwon, J. Y. Park, S. H. Lee, C. H. Moon and E. J. Baik,Brain Res., 2002,941, 1–10.
16 N. Matsuo, K. Yamada, K. Yamashita, K. Shoji, M. Mori and M. Sugano,In Vitro Cell. Dev. Biol., 1996,32, 340–344. 17 J. Yamada and Y. Tomita, Biosci., Biotechnol., Biochem.,
1994,58, 2197–2200.
18 C. Wolfman, H. Viola, A. Paladini, F. Dajas and J. H. Medina,Pharmacol., Biochem. Behav., 1994,47, 1–4. 19 S. Miura, J. Watanabe, M. Sano, T. Tomita, T. Osawa,
Y. Hara and I. Tomita,Biol. Pharm. Bull., 1995,18, 1–4. 20 M. V. Veselovskaya, M. M. Garazd, A. S. Ogorodniichuk,
Y. A. L. Garazd and V. P. Khilya,Chem. Nat. Compd., 2008, 44, 704–711.
21 X. Q. Zou, M. P. Sheng, P. H. Chang, F. T. Li, Y. Qiong, W. D. Han and J. L. Yuan,Bioorg. Med. Chem., 2010,18, 3020–3025. 22 G. Perin, E. J. Lenarda˜o, R. G. Jacob and R. B. Panatieri,
Chem. Rev., 2009,109, 1277–1301.
23 A. L. Braga, D. S. Ludtke, F. Vargas and R. C. Braga,Synlett, 2006, 1453–1466.
24 G. Zeni, R. B. Panatieri, E. Lissner, P. H. Menezes, A. L. Braga and H. A. Stefani,Org. Lett., 2001,3, 819–821. 25 L. Savegnago, V. C. Borges, D. Alves, C. R. Jesse, J. B. T.
Rocha and C. W. Nogueira,Life Sci., 2006,79, 1546–1552.
26 See, for example: (a) M. Iwaoka, Antioxidant Organoselenium Molecules, in Organoselenium Chemistry: Between Synthesis and Biochemistry, ed. C. Santi, Bentham Science, e-book, 2014, DOI: 10.2174/97816080583891140101; (b) S. Santoro, J. B. Azeredo, V. Nascimento, L. Sancineto, A. L. Braga and C. Santi,RSC Adv., 2014,4, 31521–31535; (c) V. Nascimento V, E. E. Alberto, D. W. Tondo, D. Dambrowski, M. R. Detty, F. Nome and A. L. Braga, J. Am. Chem. Soc., 2012, 134, 138–141; (d) I. J. Kade and J. B. T. da Rocha, Biokemistri, 2012,24, 1–14.
27 R. Amorati, G. F. Pedulli, L. Valgimigli, H. Johansson and L. Engman,Org. Lett., 2010,12, 2326–2329.
28 S. Kumar, H. Johansson, T. Kanda, L. Engman, T. Mu¨ller, H. Bergenudd, M. Jonsson, G. F. Pedulli, R. Amorati and L. Valgimigli,J. Org. Chem., 2010,75, 716–725.
29 F. N. Victoria, C. S. Radatz, M. Sachini, R. G. Jacob, D. Alves, L. Savegnago, G. Perin, A. S. Motta, W. P. Silva and E. J. Lenarda˜o,Food Control, 2012,23, 95–99.
30 F. N. Victoria, D. M. Martinez, M. Castro, A. M. Casaril, D. Alves, E. J. Lenarda˜o, H. D. Salles, P. H. Schneider and L. Savegnago,Chem.-Biol. Interact., 2013,205, 100–107. 31 K. Hu, W. Wang, H. Cheng, S. Pan and J. Ren,Med. Chem.
Res., 2011,20, 838–846.
32 M. J. Dabdoub, A. C. M. Baroni, E. J. Lenarda˜o, T. R. Gianeti and G. R. Hurtado,Tetrahedron, 2001,57, 4271–4276. 33 L. Engman, T. Kanda, A. Gallegos, R. Williams and G. Powis,
Anti-Cancer Drug Des., 2000,15, 323–330.
34 V. C. Borges, L. Savegnago, S. Pinton, C. R. Jesse, D. Alves and C. W. Nogueira,J. Appl. Toxicol., 2008,28, 839–848. 35 E. Wiedander, L. Engman, E. Suensjo¨, M. Erlansson,
U. Johansson, M. Linden, C. M. Andersson and R. Brattsand, Biochem. Pharmacol., 1998,55, 573–584.
36 G.-S. Sim, B.-C. Lee, H. S. Cho, J. W. Lee, J.-H. Kim, D.-H. Lee, J.-H. Kim, H.-B. Pyo, D. C. Moon, K.-W. Oh, Y. P. Yun and J. T. Hong,Arch. Pharmacal Res., 2007,30, 290–298. 37 I. Gu¨lçin,Chem.-Biol. Interact., 2009,179, 71–80.
38 Y. Soong and P. J. Barlow,Food Chem., 2004,88, 411–417. 39 R. L. Prior, X. Wu and K. Schaich,J. Agric. Food Chem., 2005,
53, 4290–4303.
40 B. Sultana, F. Anwar and R. Przybylski,Food Chem., 2007, 104, 1106–1114.
41 D. Barreca, E. Bellocco, C. Caristi, U. Leuzzi and G. Gattuso, Food Res. Int., 2011,44, 2190–2197.
42 C. W. Chen and C. T. Ho,J. Food Lipids, 1995,2, 35–46. 43 C. W. Choi, S. C. Kim, S. S. Hwang, B. K. Choi, H. J. Ahn,
M. Y. Lee, S. H. Park and S. K. Kim,Plant Sci., 2002,163, 1161–1168.
44 R. Re, N. Pellegrini, A. Proteggente, M. Pannala, M. Yang and C. Rice-Evans, Free Radical Biol. Med., 1999, 26, 1231–1237.