IDENTIFICATION OF PHYTOCHEMICAL AND ANTIOXIDANT
ACTIVITY IN PEEL AND SEED OF TROPICAL
FRUITS FROM INDONESIA
AINISSYA FITRI
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
DECLARATION OF THESIS AND INFORMATION SOURCES
OF INFORMATION AND PATENT
I, Ainissya Fitri, hereby stated that this thesis entitled Identification of Phytochemical and Antioxidant Activity in Peel and Seed of Tropical Fruits from Indonesia is true of my own work under the supervisor advisory board and that it has not been submitted before in any form to any university. The content of this thesis has been examined by the advising advisory board and external examiner. Sources of information which is derived or cited either from published or unpublished scientific paper from other writers have mentioned in the script and listed in the References at the end part of this thesis.
I hereby handed the copyright of my thesis to Bogor Agricultural University.
Bogor, August 2015
Ainissya Fitri
RINGKASAN
AINISSYA FITRI. Identifikasi Fitokimia dan Aktivitas Antioksidan pada Kulit dan Biji Buah-buahan Tropis dari Indonesia. Dibimbing oleh NAHROWI, ASEP SUDARMAN, dan HIROTOSHI TAMURA.
Oksidatif stress pada ternak dapat berdampak negative terhadap performa dan produktivitas ternak. Untuk mencegah dan atau mengurangi terjadinya oksidatif stress ini diperlukan antioksidan. Sumber antioksidan dapat berupa vitamin E, vitamin C dan polyphenol yang dapat ditemukan dalam buah-buahan dan tanaman. Indonesia memiliki keanekaragaman buah-buahan yang sebagian sudah diketahui sebagai sumber antioksidan namun untuk byproduct dari buah-buahan tersebut belum banyak diketahui aktivitas antioksidannya. Penelitian ini bertujuan untuk menginvetigasi potensi kulit dan biji dari buah tropis sebagai sumber antioksidan dan mengevaluasi ketersediaan antioksidannya secara in vitro. Sampel yang digunakan dalam penelitian ini adalah kulit dan biji buah sirsak, rambai, salak, kapundung, papaya dan matoa. Sampel diekstraksi dengan menggunakan metode QuEChERS. Pelarut yang digunakan adalah acetonitrile. Analisa yang dilakukan dalam penelitian ini terdiri dari tiga macam yaitu (1) analisa aktivitas antioksidan, (2) analisa fitokimia dengan menggunakan HPLC dan (3) analisa ketersediaan antioksidan secara in vitro. Parameter yang diamati untuk analisis pertama dan ketiga meliputi DPPH radical scavenging activity, penghambatan lipid peroksidasi, total phenolik, dan total flavonoid content. Hasil penelitian aktivitas peredaman radikal bebas dengan metode DPPH, penghambatan peroksidasi lemak, total phenolik dan total flavonoid menunjukkan hasil yang berbeda nyata (P<0.05) untuk semua sampel ekstrak yang diuji. Ekstrak kulit salak, kulit matoa dan kulit sirsak memiliki aktivitas antioksidan dan kandungan fenolik yang tinggi dibandingkan dengan sampel ekstrak lainnya. Ketiga sampel ini dianalisa lanjut untuk mengetahui ketersediaanya secara in vitro dan juga diidentifikasi kandungan senyawa fenolik yang terdapat dalam sampel. Hasil identifikasi senyawa fenolik memperlihatkan dominasi caffeic acid di kulit matoa, kulit salak, dan kulit sirsak.
Ketersediaan antioksidan dari kulit matoa, kulit salak dan kulit sirsak setelah dicerna secara in vitro menunjukan perbedaan yang nyata (P<0.05). Ketersediaan antioksidan yang diukur dengan metode DPPH menunjukan bahwa kulit matoa memiliki aktivitas peredaman radikal bebas yang lebih tinggi dibandingkan kulit sirsak dan kulit salak. Kadar peroksidasi lemak pada kulit sirsak lebih rendah dibandingkan dengan kulit salak dan matoa. Kulit sirsak mengandung nilai fenolik yang tinggi dibandingkan dengan kulit matoa dan kulit salak setelah dicerna secara in vitro. Kesimpulan dari penelitian ini adalah kulit matoa dan kulit sirsak memiliki potensi yang tinggi sebagai sumber antioksidan.
SUMMARY
AINISSYA FITRI. Identification of Phytochemical and Antioxidant Activity in Peel and Seed of Tropical Fruits from Indonesia. Supervised by NAHROWI, ASEP SUDARMAN and HIROTOSHI TAMURA.
Tropic condition could stimulate oxidative stress in livestock. To inhibit the occurrence of oxidative stress it is necessary antioxidants as feeds. Indonesia has a lot of diversity of fruits that are already known as a source of antioxidants, but for their byproduct have not been explored yet. The purpose of this study was to investigate the antioxidant activity (via 1,1-diphenyl-2- picrylhydrazyl (DPPH), lipid peroxidation, total phenolic and total flavonoid contents) in peel and seed of six tropical fruits from Indonesia and to evaluate the bioavailability of the antioxidant in in vitro digestion.
Six tropical fruits namely kapundung, matoa, papaya, rambai salak and soursop were used. This study was conducted in two phases. The first phase is screening of antioxidant activity and the second phase is evaluating of the availability of antioxidant activity after an in vitro digestion. In the first phase, the DPPH, lipid peroxidation (as MDA values) and total phenolic content values in all samples were determined to be 6.4 to 281.6 mg/ml, 1.2 to 24.7 nmol/mg of linoleic acid, 47.6 to 317.0 mg/g gallic acid equivalent respectively. The peels of matoa and salak exhibited the lowest scavenging concentration of 50% (SC50) values (6.6 and 6.4 µg/ml, respectively) and total phenolic content (317.0 and 265.5 mg GAE/g dry basis, respectively), while soursop peels (96.4%) had the highest value of inhibition of lipid peroxidation.
Three peels had been chosen for the second phase. Caffeic acid is the most abundant phenolic compound in the matoa, salak and soursop peels. After digestion, DPPH radical scavenging activity in soursop (35.0 to 75.3%) and matoa (70.2 to 81.8%) peel was increased, whereas the value in salak peel (89.1 to 32.5%) was decreased. After digestion lipid peroxidation concentration increased in six tropical fruits except soursop peel that was produced the lowest value of lipid peroxidation (0.65 μmol MDA/mg linoleic acid). Increased total phenolic contents were found in matoa (7.5 to 18.6 mg GAE/g sample) and soursop (2.0 to 14.2 mg GAE/g sample) peel, whereas decreased total phenolic contents were detected in salak peel. These results concluded that peels from matoa and soursop could serve as potential sources of antioxidant as feed supplement.
© Copyright of Bogor Agricultural University, 2015
Protected by the laws
Any unauthorized quotation of all contents or any part thereof is strictly prohibited. Citation is only for educational purpose, research, scientific writing, reports writing, critique and problem analysis; and citations would not give any disadvantage on behalf of Bogor Agricultural University.
A Thesis submitted for the Degree Programs of Master of Science in Animal Feed and Nutrition Science
IDENTIFICATION OF PHYTOCHEMICAL AND ANTIOXIDANT
ACTIVITY IN PEEL AND SEED OF TROPICAL
FRUITS FROM INDONESIA
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
2015
FOREWORD
First and foremost, I would humbly distinguish the Most Gracious Allah SWT, all praises to Allah for the gifts and His blessing in completing this thesis with title Identification Phytochemical and Antioxidant Activity in Peel and Seed of Tropical Fruits from Indonesia. This thesis submitted for the Degree Programs of Master of Science in Master of Science of Animal Nutrition and Feed Science.
I would like to sincerely deliver my greatest gratitude to my advisor: Prof Dr Ir Nahrowi, MSc, Dr Ir Asep Sudarman, MRurSc, Prof Hitotoshi Tamura for their advice, expertness, encouragement, and support. My sincere thanks also to Assit. Prof Lina Yonekura for advices and insightful comments. I would like to express my thankful to Prof Dr. Ir Toto Toharmat, MScAgr for the useful advice and support. I would also express my gratitude to Dr Ir Dwierra Evvyernie A, MSc as head of study program and Mr. Supriadi as her Assistant who have been very kindly taking care of the paper work concerning the process of study at this department.
My appreciations were also extended to Japan Student Services Organization (JASSO) Scholarship for granting scholarship during the study and experiment in Kagawa University and also for SUIJI-JDP (Six University Initiative Japan-Indonesia Joint Degree Program) who allowed me to expand my knowledge and experiences in Japan. This master thesis would not have been possible unless the funding of Indonesian Government scholarship (Beasiswa Unggulan).
I also addressed my gratitude to all staff and class mate in Animal Nutrition and Feed Science, Graduate School Bogor Agricultural University. And I would like to take this moment to deeply express my thankful feeling to my family members who have been praying, loving and supporting as always. Finally, I hope this thesis can give information about antioxidant sources and their bioavailability for enhancing livestock production in Indonesia.
Bogor, August 2015
Ainissya Fitri
TABLE OF CONTENT
LIST OF TABLES xii
LIST OF PICTURES xii
LIST OF APPENDIXS xii
1 Introduction 1
Objectives 2
Hypothesis 2
2 Materials and Methods 2
Plant materials 3
Preparation of the plant extract 3
Analysis of DPPH free radical scavenging activity 4
Lipid peroxidation (TBARS Assay) 5
Total phenolic (TP) 5
Total Flavonol (TF) 6
Phytochemical screening 6
The Bioavailability of antioxidant in In Vitro digestion 6
Statistical analysis 7
3 Result and Discussion 7
The Yield of extract of peel and seed in tropical fruits 7
The DPPH radical scavenging activity 8
Lipid peroxidation 11
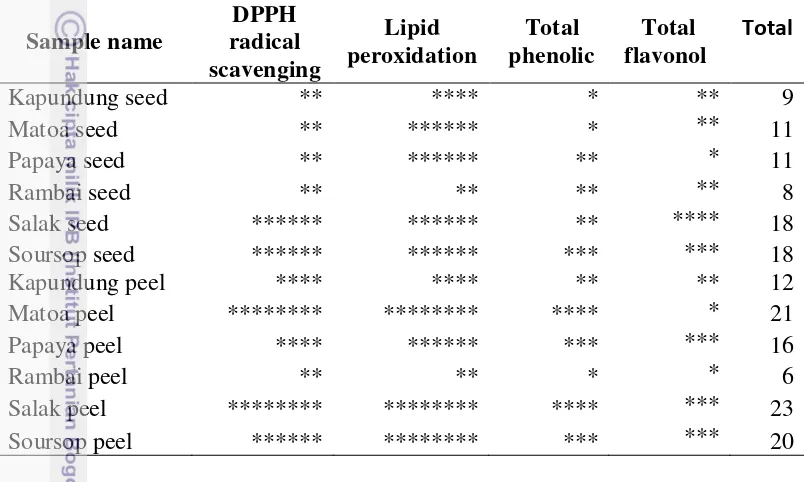
Total phenolic (TP) and total flavonol (TF) 14 The bioavailability of antioxidant in in vitro digestion 16 Identification and quantification of phenolic compound in matoa peel, salak peel and soursop peel
18
4 Conclusions 22
Recommendation 22
5 References 23
LIST OF TABLES
1 Yield of extract of peel and seed in tropical fruits in Indonesia 8 2 DPPH Radical Scavenging Activity of By-product Fruits 10 3 Lipid peroxidation (LP), total phenolic (TP), total flavonol (TF) of peel
and seed of tropical fruits
14 4 The matrix of the result of antioxidant activity, lipid peroxidation, total
phenolic and total flavonoid
16 5 DPPH radical scavenging activity, lipid peroxidation inhibition, and
total phenolic in vitro digestion
17 6 Phenolic compound in samples before and after in vitro digestion 19
LIST OF PICTURES
1 All samples fruits in this experiment 3
2 Flow chart of experiment 4
3 The mechanism of stabilization of DPPH radical with antioxidant 9
4 SC50 value of DPPH Scavenging Activity 11
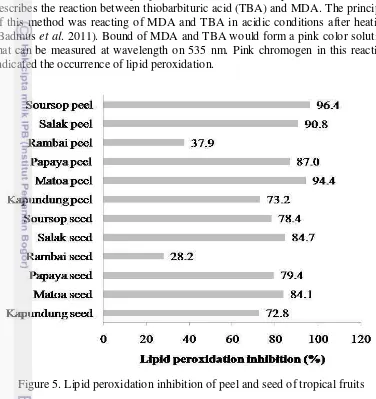
5 Lipid peroxidation inhibition of peel and seed of tropical fruits 12
6 Mechanism of Lipid Peroxidation 13
7 HPLC chromatogram of soursop peel prior and after in vitro digestion 21 8 HPLC chromatogram of salak peel prior and after in vitro digestion 21 9 HPLC chromatogram of matoa peel prior and after in vitro digestion 22
LIST OF APPENDIXES
1 ANOVA of lipid peroxidation in peel and seed of tropical fruits 28 2 Duncan test of lipid peroxidation in peel and seed of tropical fruits 28 3 ANOVA of SC50 value of DPPH Scavenging Activity in peel and seed
of tropical fruits
28 4 Duncan test of SC50 value of DPPH Scavenging Activity in peel and
seed of tropical fruits
fruits
13 ANOVA of lipid peroxidation undigested and digested tropical fruit peels
31 14 Duncan test of lipid peroxidation undigested of tropical fruits 31 15 Duncan test of lipid peroxidation digested of tropical fruits 32 16 ANOVA of lipid peroxidation between undigested and digested of
tropical fruits
32 17 ANOVA of SC50 value of DPPH Scavenging Activity between
undigested and digested of tropical fruits
32 18 ANOVA of SC50 value of DPPH Scavenging Activity undigested and
digested tropical fruit peels
33 19 Duncan test of SC50 value of DPPH Scavenging digested of tropical
fruits (concentration 10 µg/ml)
33 20 Duncan test of SC50 value of DPPH Scavenging undigested of tropical
fruits (concentration 10 µg/ml)
33 21 Duncan test of SC50 value of DPPH Scavenging digested of tropical
fruits (concentration 50 µg/ml)
33 22 Duncan test of SC50 value of DPPH Scavenging undigested of tropical
fruits (concentration 50 µg/ml)
34 23 Wavelength detection (λ), retention time (rT), and linear regression
data of phenolic compounds
34
INTRODUCTION
Indonesia is a tropical country with temperatures ranging from 23 to 33o C and humidity of 45-97% (BMKG, 2013). This condition for animals such as laying hen, broiler, cattle, dairy cow, goat and sheep, can stimulate the release of heat which is generated during the metabolism process in the body and leads to stress. It might cause the formation of free radicals which can easily react with other substances (proteins, fats, and DNA). Free radicals can give damages to cell walls and impair the function of organs that play crucial roles in the body's metabolic system. An imbalance between the generated free radicals and the endogenous antioxidant systems leads to oxidative stress (Yoshikawa and Naito 2002). Oxidative stress in animals can inhibit growth rate, decrease appetite, decrease nutrient digestibility, impair the function of the immune system, decrease the product quality, and increase animal mortality (Sugito et al. 2010; Rajani et al.
2011; Hashemi et al. 2012).
An antioxidant is required to prevent oxidative stress from free radicals. It could stabilize free radicals with complete electrons lack and inhibit the further of chain reaction of free radical formation. Diets, containing antioxidants such as vitamin E, vitamin C and secondary phytochemical compounds for animals that exposed oxidative stress could enhanced serum antioxidant status and daily gain in beef cattle (Wang et al. 2011), reduce mortality and the MDA (malondyaldehyde) product in meat of broilers (Rajani et al. 2011), and improve milk quality of dairy goat (Mardalena et al. 2011). These antioxidant properties can be found in fruit (Balasundram et al. 2006; Poudel et al. 2008).
Fruits contained essential nutrients and micronutrients such as minerals, fibers, vitamins and secondary phytochemical compounds (Ribeiro da Silva et al.
2014). Their combination seems to be responsible for reducing the level of oxidative stress and protecting cells from damage. In addition, non-edible part of fruits is regarded as potential resources of antioxidant. For example, resources such as citrus peel, carrot skin, grape skin and seed, tomato pomace, olive pomace, red beet skin and pomace, and onion skins (Schieber et al. 2001; Balasundram et al. 2006; Poudel et al. 2008; Galanakis 2012) have been reported to have multiple biological effects, including antioxidant activity. Ayala-Zavala et al. (2011) stated that byproduct mass of fruits have economic and environmental impacts, dietary additives, new food and pharmaceutical resources, providing of nutraceutical supplement and contributing to the recovery of agro-industrial process waste.
Indonesia has a lot of kinds of biodiversity of fruits. As a tropical country, Indonesia placed third in production quantity of fresh tropical fruit worldwide, followed by India and Philippines (FAO, 2012). Some fruits have already been identified as sources of antioxidant, however for the by-product of those plant have not been explored yet. The unexplored antioxidant sources are, for example, the peels and seeds of soursop (Annona muricata L.), papaya (Carica papaya L.), matoa (Pometia pinnata), rambai (Baccaurea motleyana), kapundung (Baccaurea racemosa Reinw.) and salak (Salacca zalacca). By-products of these fruits can be served as feed additives for animals.
2
taken into consideration (Pavan et al. 2014). Actually, it is important to measure bioavailability of antioxidant to know whether it has effects on health or not. Determination of the bioavailablity of antioxidant can used in vitro digestion procedure. The in vitro digestion has been used often to simulate gastrointestinal conditions since they can be considered relatively simple when compared to the in vivo model, besides being safe and do not present ethical restrictions.
Objectives
The objectives of this study are to determine the potential of peel and seed of tropical fruits as an antioxidant sources and to evaluate the bioavailability of antioxidant activity after digestion.
MATERIALS AND METHODS
Time and place of experiment
This experiment was implemented from May 2014 to March 2015 at the Laboratory of Department of Biochemistry and Food Science, Faculty of Agriculture, Kagawa University, Japan.
Chemicals
3
Plant materials
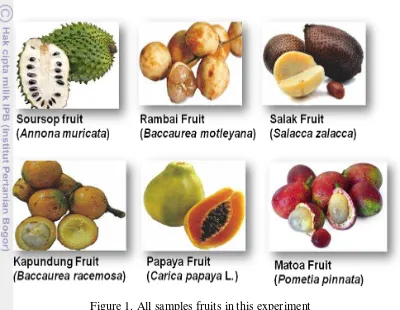
All samples were collected from Bogor, Indonesia (Figure 1). The samples were peels and seeds of Sirsak fruit (Annona muricata L.), Papaya fruit (Carica papaya L.), Matoa fruit (Pometia pinnata), Rambai fruit (Baccaurea motleyana), Kapundung fruit (Baccaurea racemosa Reinw.), Salak fruit (Salacca zalacca). The samples were obtained in February 2014. All samples were dried at 50oC for 12 to 24 hours and were ground to a fine powder.
Figure 1. All samples fruits in this experiment
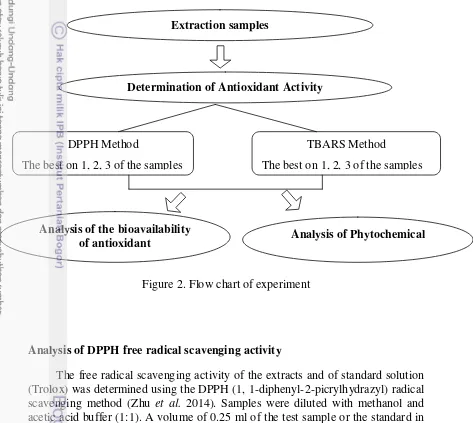
Flow chart of Experiment
This experiment was implemented in three phases. First phase was extraction of the samples, second phase was determination of antioxidant activity with two methods (DPPH method and TBA method), and third phase was analysis of phytochemical and the bioavailability of antioxidant in the samples that resulted high antioxidant activity in the second phase (Figure 2).
Preparation of the plant extract
We used QuEChERS method for extraction method described by Sato et al.
4
Extraction samples
Determination of Antioxidant Activity
DPPH Method
The best on 1, 2, 3 of the samples
TBARS Method
The best on 1, 2, 3 of the samples
Analysis of Phytochemical Analysis of the bioavailability
of antioxidant
4 g anhydrous MgSO4 and this solution was shaked for 1 min. The tube was centrifuged at 3000 rpm for 5 min. The supernatant from the extraction was evaporated and scratched. The extracts were either used immediately for analysis or stored in a freezer. The extracts were analyzed for DPPH free radical scavenging, lipid peroxidation, total phenolic, total flavonoid, and phytochemical analysis with HPLC.
Figure 2. Flow chart of experiment
Analysis of DPPH free radical scavenging activity
The free radical scavenging activity of the extracts and of standard solution (Trolox) was determined using the DPPH (1, 1-diphenyl-2-picrylhydrazyl) radical scavenging method (Zhu et al. 2014). Samples were diluted with methanol and acetic acid buffer (1:1). A volume of 0.25 ml of the test sample or the standard in different concentrations (200, 100, 50, 10, 5, 1 μg/ml) was mixed with 0.25 ml of acetic acid buffer (0.10 M), 0.25 ml of methanol and 0.25 ml of DPPH (0.4 mM in methanol). The reaction mixtures were vigorously mixed and incubated for 30 min at room temperature in the dark. The absorbance of mixtures was measured by a spectrophotometer, JASCO V-520-SR UV−vis spectrometer (JASCO Corp., Tokyo, Japan) at 517 nm and trolox was used as a standard chemical.
The DPPH is expressed as a percentage using the following formula: % DPPH radical scavenging activity (SC) = [(Acontrol - Asample) / Acontrol] x 100
5
activity values. Each sample was done in triplicate. The average of SC50 value was then calculated.
Lipid peroxidation (TBARS Assay)
Lipid peroxidation inhibition was determined by using a thiobarbituric acid (TBA) method (Tamura and Yamagami 1994). The capacity of each sample solution (10 mg/ml) to inhibit lipid peroxidation was evaluated using the modified assay of thiobarbituric acid reactive substances (TBARS). Linoleic acid (5 mg) was mixed with 0.2% SDS Tris-HCl buffer (4.8 ml) and sample solution (100 μl). Then, 20 mM ferrous sulfate aqueous solution (100 μl) was added to oxidize the induction of lipid peroxidation and the mixture was incubated for 16 h at 37oC. The reaction was stopped by adding 1% BHT-EtOH (200 μl).
The production of TBARS, mainly malondialdehyde (MDA), as a secondary product of peroxidation, was measured in the following way. The reaction solution (1 ml) was mixed with 0.05 N HCl (3 ml) and 0.05 M TBA-50% acetic acid (1 ml) and then incubated for 30 min at 100 oC. After cooling the solution to room temperature, n-butanol (4 ml) was added and the mixtures were shaken vigorously and adding EtOH (200 μl) to remove air bubbles. The mixtures were centrifuged (10 min, 2500 rpm) and the absorbance of the n-butanol layer (upper layer) was measured at 535 nm. To make a standard curve, 1,1,3,3-tetraethoxypropane standard solution (0, 2.5, 5, 10, 20, 30, 40, 50, 60, 70, 80 nmol/ml) were measured as described above (after incubating for 16 h at 37 oC). Lipid peroxidation was expressed in nmol of MDA per mg of linoleic acid (nmol MDA/mg linoleic acid).
The percentage of lipid peroxidation inhibition had calculated using the following formula:
Lipid peroxidation inhibition (%) = [(Lcontrol - Lsample) / Lcontrol] x 100
Lcontrol was the result of lipid peroxidation in milli-Q (pure distilled water) , and Lsample was the result of lipid peroxidation of the test sample.
Total phenolic (TP)
Total phenolic was determined with procedure of Folin-Ciocalteu (Asada and Tamura 2012), using gallic acid as a standard phenolic compound. Each sample extract and gallic acid was diluted with methanol at a concentration 1 mg/ml. Then, 20 µ l of solution was mixed with 200 µl of 50% phenol reagent, 200 µl of 10% sodium carbonate aqueous solution, and 800 µl milli-Q water.
The mixture was stored for 1 h in the dark place at room temperature before reading the absorbance at 760 nm with spectrophotometer by 5 mm length of a
quartz cell using an UV−vis spectrophotometer (JASCO V-520-SR). To make a standard curve (y = 0.0009x + 0.0031; R2 = 0.0996), gallic acid standard solution (0, 200, 400, 600, 800, and 1000 μg/ml) were measured in the same method described above. Results of each sample were expressed as mg gallic acid equivalent (GAE)/g dry basis (mg GAE/g dry basis).
6
abs = absorbance; a = slope the line; b = the y-intercept; C = concentration of samples
Total Flavonol (TF)
Total flavonol was determined following the method of Poudel et al. (2008), using quercetin as a standard. Each sample was diluted with 95% ethanol at a concentration 5 mg/mL. A volume of 0.25 mL of the sample or standard was pipetted in a test tube and 0.25 mL 0.1% HCl in 95% ethanol (v/v) and 4.55 mL 2% HCl (v/v) were added. The solution was mixed and allowed to stand for approximately 15 min before reading the absorbance at 360nm with a spectrophotometer (JASCO V-520-SR UV−vis spectrometer). Quercetin dissolved in 95% ethanol was used as standard. Total flavonol content was determined from the calibration curve (y = 2,719x + 0,095; R2 = 0.997) by extrapolating the known concentration of quercetin (1-0.0625 mg/mL) against the absorbance at 360 nm. All the determination were expressed as mg quercetin equivalent (QE)/g dry basis (mg QE/g dry basis).
abs = absorbance; a = slope the line; b = the y-intercept; C = concentration of samples
Phytochemical screening
The HPLC analysis of all samples was carried out on a JASCO HPLC system equipped with JASCO MD 2010 plus photodiode array detector and double JASCO PU-980 pumps. The separation was performed on a Mightysil RP-18 250-4.6 column (5μm). The mobile phases are 10% CH3CN-0.5% TFA as eluent A and 100% CH3CN-0.5% TFA as eluent B. A gradient program is performed: 0% B (0-2 min); 0 to 100% B (2-45 min); 100% B (45-47 min); 100 to 0 % B (47 min). The injection volume is 10 µL. The flow rate was fixed at 0.5 mL/min and the column temperature was set at 40oC. The following individual compounds such as gallic acid, caffeic acid, cinnamic acid, quercetin, catechin, syringic acid, ferulic acid, vanillic acid, rosmarinic acid and chlorogenic acid were used as phenolic standards. Calibration curves for each standard were prepared for quantification. Phenolic compounds were quantified by the peak area of maximum absorption wavelength, respectively.
The Bioavailability of antioxidant in in vitro digestion
7
µg/ml). Briefly, samples was mixed with 5 ml of pepsin buffer solution (1600 unit/ml pepsin, 3.6 mM CaCl2, 12 mM KCl, 1.5 mM MgCl2.6H2O, 49 mM NaCl, 6.4 mM KH2PO4) in a 25 ml conical flask. The mixture was acidified with 2 M HCl until it reached to pH 2.5 and the mixture was placed in a shaker bath at 37°C for 1 hour. Thereafter, intestinal digestion was performed with the addition of 5 ml of pancreatin-bile solution (0.682 g of pancreatin and 0.062 g bile extract in 155 ml of 0.1 M NaHCO3) and the pH of solution was adjusted to 6.8 with 2 M HCl. The mixture was incubated in a shaker bath at 37°C for 2 hour. The digesta was stored at freezer temperature until further analysis.
The analysis for the bioavailability of antioxidant were DPPH radical scavenging activity in concentration of 10 and 50 µg/ml, total phenolic content in concentration of 1000 µg/ml and lipid peroxidation concentration of in 1000
µg/ml. The units of DPPH radical scavenging activity, total phenolic content and lipid peroxidation were %, mg GAE/g sample, and µmol/mg linoleic acid respectively.
Statistical analysis
All data are expressed as mean ± standard deviation (SD) in triplicate. For comparisons among samples, data were analysed by ANOVA and Duncan test (SPSS, version 16.0). A probability of 5% or less was accepted as statistically significant.
The matrix of the result after screening all samples was calculated by using sample quartiles with the lower quartile (Q1) was 25th percentile, second quartile (Q2) was 50th percentile and upper quartile (Q3) was 75th percentile. The DPPH radical scavenging activity and lipid peroxidation were double rank because we consider this is more important in term of antioxidant activities.
RESULT AND DISCUSSION
The yield of extract of peel and seed in tropical fruits
Extraction was done to obtain secondary metabolites in fruits and vegetables. Extraction could carried out by several methods such as maceration, soxhlet extraction, ultrasonic-assisted extraction, microwave assisted extraction, liquid-liquid extraction, solid phase extraction, QuEChERS extraction and so on (Delgado-Zamarreno et al. 2012; Burin et al. 2014). Generally, solvent that used in the extraction method was organic chemical solvents like ethanol, methanol, hexane, acetone, acetonitrile and dichloromethane.
8
determine compounds of antioxidant, antiallergic, antifungal and isoflavones compounds in food samples (Delgado-Zamarreno et al. 2012; Brosnan et al. 2014; Burin et al. 2014; Sato et al. 2014; Sato et al. 2015).
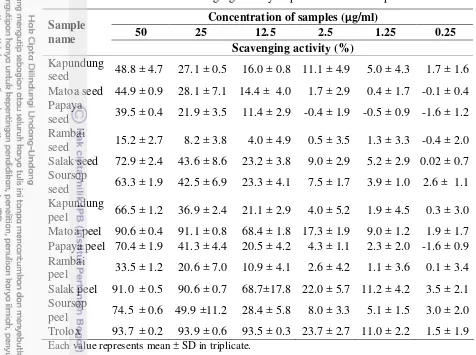
All samples form after extracted was paste, but the form of matoa peel after extracted was powder. Table 1 showed the yield of extraction from the peels and seeds of tropical fruits. Sequences of extract yield of the peel and seed of tropical fruits were as following rambai seed > rambai peel > papaya peel > matoa peel > salak seed > kapundung peel > kapundung seed > papaya seed > salak peel > matoa seed > soursop peel > soursop seed. Differences of the extract yield obtained might be affected by the solvent type, the ratio of solvent and sample, particle size of sample and extraction time (Ng et al. 2012). The result of yield of extract exhibited that rambai seed have higher amount than other samples. This is because of the oil component founded in rambai seed extract. Furtheremore, seed fraction in fruits has high oil content compared peel of the fruits.
Table 1. Yield of extract of peel and seed in tropical fruits in Indonesia Sample name Dried powder Yield of extract Yield
(g) (mg) mg/g %
Measurement of the antioxidant activity can be performed using chromogen compound from free radicals. One of these compounds is DPPH (1, 1-Diphenyl-2-picryl-hydrazyl). The DPPH is stable free radicals. The method of DPPH radical scavenging activity was very popular to evaluate the antioxidant activity. In addition, several reasons to use this method are easier, cheap cost and fast to evaluate radical scavenging activity (Sharma and Bhat 2009).
9
purple colorless (Sharma and Bhat 2009). The mechanism of antioxidants with free radicals was shown in Figure 3.
Free radicals removed one hydrogen atom of antioxidants (hydrogen atom transfer) or antioxidants donated electrons to free radicals (electron transfer), so it made free radicals became inactive. In the method of DPPH radical scavenging activity the both mechanisms can occured (Liang and Kitts 2014; Villano et al. 2007).
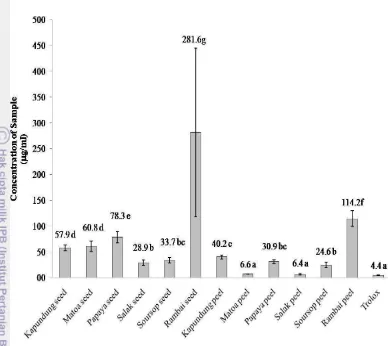
Table 2 and Figure 4 presented the DPPH radical scavenging activity in the peel and seed of tropical fruits. Five different concentrations were done in this study and we got a percentage of scavenging from this assay. At 50 μg/ml concentration, all samples showed mean of percent scavenging 15-92%. Salak peel and matoa peel at 50 and 25 μg/ml concentrations had similar percent scavenging to trolox (standard). However, in 12.5 μg/ml concentration the percent inhibition of matoa peel (68.7%) and salak peel (68.4%) was lower than trolox (93.5%).
Figure 3. The mechanism of stabilization of DPPH radical with antioxidant (Liang and Kitts 2014).
10
Table 2. The DPPH radical scavenging activity of peel and seed of tropical fruis Sample Each value represents mean ± SD in triplicate.
Kanlayavattanakul et al. (2013) reported that the SC50 values of DPPH scavenging radical activity on ethyl acetate fraction of the salak peel (2.93 μg/ml) had high antioxidant activity compared with of 70% ethanol fraction (8.87 μg/ml), and aqueous (4.12 μg/ml). In this study, the SC50 value of salak peel had in between the SC50 value of 70% ethanol fraction and ethyl acetate fraction of Kanlayavattanakul et al. (2013) studied. The differences of SC50 values of DPPH scavenging radical activity might be caused by differences solvent used, cultivation and varieties of fruit. Rambai seed wasnot dissolved perfectly in methanol solvent, it caused the DPPH result wasnot good.
11
Figure 4. The SC50 values of DPPH scavenging activity (n=3)
The DPPH radical scavenging activity is the good way to determine of antioxidant capacities in the sample. However, only one method to determine of antioxidant activity is not enough. Furthermore, the biggest limitation of DPPH assay is that is not related to specific free radicals that have physiological relevance (Liang and Kitts 2014). Based on this statement, the lipid peroxidation had been done in this present study.
Lipid peroxidation
The major component of cell membranes is lipid. The types of these lipids are polyunsaturated fatty acids such as linoleic acid, linolenic acid and arachidonic acid. Those lipids were susceptible targets as oxidative substrates. Oxidized of lipid or lipid peroxidation could be used as one of the markers of oxidative stress that lead to several pathogenesis of diseases. Hence in lipid peroxidation process would generate some radical from lipid such as hydroxyl radical (HO·), the hydroperoxyl radical (HOO·), the lipid peroxyl radical (LOO·), and the alkoxyl radical (LO·) (Yoshikawa and Naito 2002).
12
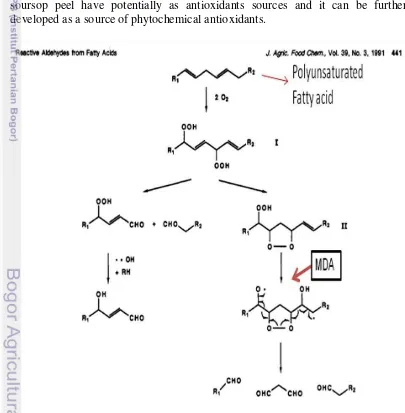
oxidized to form dihydroperoxide intermediate (phase 1) where as it would generate 4-HN (4-hydroxy-2-nonenal) formation. When 4-HN is oxidized, it would form 13-hydroxy-10, 12-cyclic intermediate peroxide which would generate hexanal. Malondialdehyde (MDA) was also formed as a result of hexanal formation (phase 2) through the formation of the cyclic peroxide intermediate (Tamura et al. 1991). MDA is a dangerous product that ascribed as cytotoxic.
The measurement of lipid peroxidation can be done by TBARS method that describes the reaction between thiobarbituric acid (TBA) and MDA. The principle of this method was reacting of MDA and TBA in acidic conditions after heating (Badmus et al. 2011). Bound of MDA and TBA would form a pink color solution that can be measured at wavelength on 535 nm. Pink chromogen in this reaction indicated the occurrence of lipid peroxidation.
13
This assay determined the ability of all sample to inhibit lipid peroxidaton present in linoleic acid to evaluate their antioxidant potential. The process of inhibition is more sensitive and taken a long time. Soursop peel had high ability to inhibit lipid peroxidation. The inhibition ability from soursop skin was almost 30-folds from control. This is due a presence of phenolic compounds in the samples tested. In the soursop peel, it might have aromatic compounds with vicinal methoxyhydroxy or dihidroxy groups (Tamura and Yamagami 1994).
Flavonoids, tannins, saponins and alkaloids were known to neutralize free radicals by donating their electrons to free radical that made an oxidation in lipid. Sources of antioxidant like α-tocopherol, (+) cathechin, BHT (butylated hydroxytoluene), and anthocyanin had an activity to suppress lipid peroxidation (Tamura and Yamagami 1994). In addition, the other factors that affected in lipid peroxidation inhibition were lipophilicity and location within the membrane (Heijnen et al. 2002).
Based upon the results of this study on the antioxidant activity by DPPH method and TBARS method of TBA proved that salak peel, matoa peel and soursop peel have potentially as antioxidants sources and it can be further developed as a source of phytochemical antioxidants.
14
Total phenolic (TP) and total flavonol (TF)
Phenolic compounds are secondary metabolites in fruits or plants. Those are derivatives of the pentose phosphate, shikimate, and phenylpropanoid pathways in plants (Randhir et al. 2004). Phenolic compounds had a great chemical structure to against free radical and showed antioxidant activity effectively in in vitro study. Antioxidant activity of phenolic compounds was remarkable in high reactivity with free radicals by (1) donating of hydrogen or electron, (2) stabilizing and removing the unpaired electrons by the ability of derived polyphenols radical, (3) chelating with metal ions such as iron, cupper, zinc and so on. Many studies showed that phenolic compounds were involved in scavenging of hydrogen peroxide on the vegetables and fruits.
The total phenolic values in the peels were higher than those in the pulps. Phenolic compounds might tend to accumulate in the dermal tissues of the plant body due to their potential role in protecting against ultraviolet radiations, acting as attractants in fruit dispersal, and as defence chemicals against pathogens and predators (Contreras-Calderón et al. 2011).
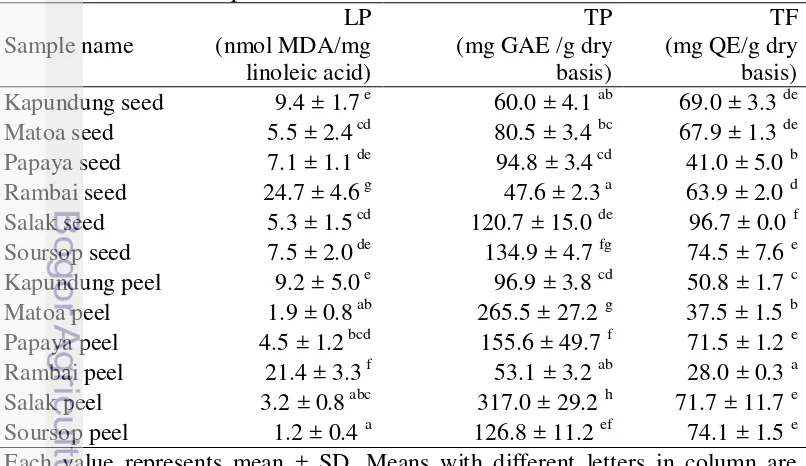
Table 3 presented the results of the TP in the peels and seeds of tropical fruit. The peels had a greater phenolic content than the seeds. The TP in all samples ranged between 47-317 mg GAE/g dry powders. In the peels, salak was the highest value of TP, followed by matoa, papaya, soursop, kapundung and rambai. In the seeds, soursop had a higher value of TPC than others. Based on the data of TP in this study, salak peel contained the highest phenolic content (317 mg GAE/g dry basis), followed by matoa peel (265.5 mg GAE/g dry basis), soursop seed (134.9 mg GAE/g dry basis) and soursop peel (126.8 mg GAE/g dry basis). Table 3. Lipid peroxidation (LP), total phenolic (TP), total flavonol (TF) of peel
15
The phenolics concentration of fruits evaluated in this work was higher than that found by other authors. Samonte and Trinidad (2013) reported that total phenolic on soursop peel was 75.04 mg GAE/g sample. Silva et al. (2014) found the TP value of by-product of soursop and papaya was 14.4 and 7.8 mg GAE/g dry basis, respectively. The TP value in soursop peel was similar to Surinam cherry byproduct from Brazil (Silva et al. 2014). These differences might be because of several factors such as type of cultivation, climate, fruit variety, geographic origin and environment stress (Deng et al. 2010).
The values of total phenolics in this study have a linear correlation with the DPPH radical scavenging activity. The higher of total phenolic value resulted, the greater of antioxidant activity on against free radicals.
Flavonoids are low molecular weight compound containing 15 carbon atoms (C6-C3-C6 structure). More than 4000 derived flavonoids from plants that have been identified. Flavonol is one of the flavonoid groups. The best-known flavonols are quecertin and kaempferol, which are most common among fruits and vegetables. Several groups of flavonoids are known to have a biological activity like antiallergy, antioxidant, anticancer and anti-inflammatory (Balasundram et al. 2006).
In this study, a standard chemical that was used to measure total flavonol is quecertin. Quecertin is one of flavonoid compound in flavonols group. Quecertin was reported that it had a great antioxidant activity and it is abundant in fruit and vegetables. The meant value of flavonols contained in the peel and seeds of tropical fruit were 28.0-96.7 mg QE/g dry basis (Table 3). All samples tested showed significantly difference to the total flavonol values (P<0.05). Salak seed (96.7 mg QE/g dry basis) had the highest flavonol content, followed by soursop peel (74.1 mg QE/g dry basis), soursop seed (74.5 mg QE/g dry basis), papaya peel (71.5 mg QE/g dry basis) and salak peel (71.7 mg QE/g dry basis). Rambai peel had the lowest flavonols content in the study. Several types of flavonols are found in the peels and seeds of fruits are quecertin, rutin, kaempferol, catechin and myricetin (Li et al. 2013).
16
The high activity of DPPH radical scavenging activity and lipid peroxidation inhibition in salak peel, matoa peel and soursop peel might be caused by flavonoids and phenolic compound in these samples. To indentify the phenolic compounds contained in these samples, we did characterization of phenolic compounds using HPLC (High-performance liquid chromatography).
Table 4. The matrix of the result of antioxidant activity, lipid peroxidation, total phenolic and total flavonoid in peel and seed of tropical fruits
Sample name
The bioavailability of antioxidant in in vitro digestion
Polyphenols are major plant compounds with antioxidant activity, although they are not the only ones. Polyphenolic compounds affect the functional and nutritional values of vegetable proteins, reducing the nutritional values of foodstufs, and contributing to the sensory and organoleptic properties of fruits and vegetables (colour, taste, astringency).
The in vitro digestion has been used often to simulate gastrointestinal conditions since they can be considered relatively simple when compared to the in vivo model, besides being safe and do not present ethical restrictions. Many studies determined the antioxidant capacities of fruits but the antioxidant activity in gastrointestinal system was not taken into consideration.
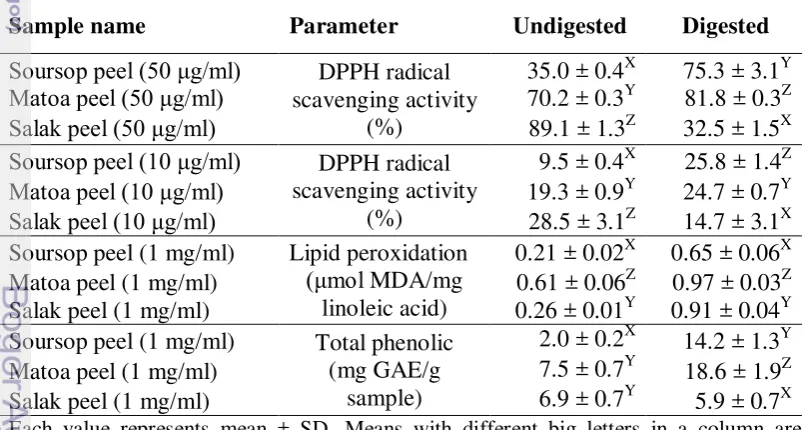
The result from the screening of antioxidant activity, lipid peroxidation and total phenolic exhibited that matoa peel, salak peel and soursop peel had a high antioxidant activity. Thus, these three samples were evaluated the bioavailability of antioxidants after in vitro digestion. Table 5 presented that there was significant (P<0.05) difference between the undigested and digested values of the tropical fruit peels, regarding to the DPPH radical scavenging activity, lipid peroxidation, total phenolic content.
17
in the digested peels. Matoa itself (81.8%) showed higher DPPH radical scavenging activity than soursop peel (75.3%) and salak skin (32.5%). Then, the matoa peel (24.7%) and soursop peel (25.8%) had higher the DPPH radical scavenging activity than salak skin (14.7%) at a concentration 10 μg/ml. Pavan et al. (2014) stated that pH and enzymatic interactions during digestion affected the result of antioxidant activity.
The lipid peroxidation of soursop peel and salak peel in the undigested
was the lowest concentration 0.21 and 0.26 μmol/mg linoleic acid, respectively
(Table 5). The inhibition of lipid peroxidation in matoa peel was a 1.18 fold increase compared with blank. After in vitro digestion all fruit peels showed a significant increase in the lipid peroxidation and soursop peel (0.65 μmol MDA/mg linoleic acid) resulted the lowest concentration for lipid peroxidation compared matoa peel and salak peel. Dietary 10% pomace grape to chick that containing total phenolic (0.19 g GAE/100 g dry matter) resulted the lowest lipid peroxidation value 0.055 μg MDA/g meat after storage for 1 days compared control (Chamorro et al. 2015). The MDA content in this present study is still in the range of 0.356 dan 1.14 µmol MDA/mg linoleic acid as Gorelik et al. (2005) and Hur et al. (2009) had reported before. After in vitro digestion of present experiement, MDA content by the lipid peroxidation was increased, compared to that before digestion as Hur et al. has reported in 2009. Furthermore, addition of antioxidant compounds like catechin has been proven to be lower MDA amount than without addition (Gorelik et al. 2005).
Table 5. DPPH radical scavenging activity, lipid peroxidation, and total phenolic undigested and digested tropical fruit peels
Sample name Parameter Undigested Digested
Soursop peel (50 μg/ml) DPPH radical
Soursop peel (1 mg/ml) Lipid peroxidation
(μmol MDA/mg linoleic acid)
0.21 ± 0.02X 0.65 ± 0.06X
Matoa peel (1 mg/ml) 0.61 ± 0.06Z 0.97 ± 0.03Z
Salak peel (1 mg/ml) 0.26 ± 0.01Y 0.91 ± 0.04Y
Soursop peel (1 mg/ml) Total phenolic (mg GAE/g significantly different (P < 0.05) between the samples tested. DPPH radical scavenging activity (n=3), lipid peroxidation (n=6); total phenolic, Total phenolic (n= 4); MDA = Malondialdehyde; GAE = gallic acid equivalent
18
compounds in these samples was decrease after digested (Pavan et al. 2014). The TP values after in vitro digestion showed that matoa peel (18.6 mg GAE/g sample) had the highest value followed by soursop peel and salak peel. Total phenolic contents were found increasing in ileal and excreta of chick after feeding 10% pomace grape (Chamorro et al. 2015).
The increasing of total phenolic after in vitro digestion could be caused by releasing of polyphenols and changing their structural form which has affects their chemical and functional properties (Bhatt and Patel 2013). We suspected that the matrix obtained from samples contains other substances that were not analyzed in this study, including non-phenolic substances involved in changed of antioxidant activity value after the in vitro digestion process. These compounds, such as amino acids, peptides, were released during digestion or were changed (Pavan et al. 2014).
Prior to in vitro digestion, salak peel was the most potent antioxidant containing fruit peel of all samples analysed. However, after in vitro digestion process the antioxidant activity in salak peel was reduced. Soursop peel and matoa peel have a good result after in vitro digestion. This study concluded that soursop peel and matoa peel have high potent bioavailability of antioxidant activity and these could be used as feed addictive for animals. For bioavailability test, absorption rate of chemicals which are detected after in vitro digestion should be considered well finaly.
Identification and quantification of phenolic compound in matoa peel, salak peel and sorsop peel
Phenolic compounds in fruits and plants reported had a pharmacological activities including antioxidant, antitumor, antiallergic and anticarcinogenic properties. The phenolic compounds found in plants constitute a complex mixture, and only a small number of plants have been examined systematically for biologically active phenolic compounds. For the antioxidant activity of phenolic compounds, it depends on the structure, in particular, the number and positions of the hydroxyl groups and the nature of substitutions on the aromatic rings (Balasundram et al. 2006).
Phenolics display a vast variety of structures and include simple phenols, phenolic acids (both benzoic and cinnamic acid derivatives), coumarins, flavonoids, stilbenes, hydrolysable and condensed tannins, lignans, and lignins. Generally, phenolic acids may occur in edible plants as esters or glycosides conjugated with other natural compounds such as flavonoids, alcohols, hydroxyl fatty acids, sterols, and their glucosides (Balasundram et al. 2006).
19
acid, o-coumaric acid, cinnamic acid and chlorogenic acid), and flavonoids (rutin, catechin, and quercetin).
Table 6. Phenolic compounds in samples undigested and digested in vitro
digestion Compound
Matoa peel Salak peel Soursop peel undigested Digested undigested Digested undigested Digested
(mg/g dry basis) (mg/g dry basis) (mg/g dry basis)
In this study, we tried to identify and quantify chemical compounds in those extracts before and after in vitro digestion by HPLC. Generally, a qualitative identification is usually required retention time data of each ingredient detected. In the present case, we identified each selected peak in those chromatograms by comparing UV spectra and retention time. The retention time of the reference standard had been determined in parallel as to compare the retention times of certain candidated peaks in tested plant chromatograms.
Table 6 showed the phenolic compounds in the samples. All of those reference standards appeared in the treated and untreated extracts from salak peel, matoa peel and soursop peel. However, the amounts of each compound were different. Matoa peel, salak peel and soursop peel had high amounts of caffeic acid. Then, the quantity of caffeic acid in matoa peel (18.49 mg/g dry basis) was higher than those of salak peel (16.93 mg/g dry basis) and soursop peel (7.19 mg/g dry basis). Actually, caffeic acid is the most common chemical as plant metabolites and accounted for up to 70% of total hydroxycinnamic acids in fruits (Lafay and Gil-Izquierdo 2007).
20
The phenolic compound in matoa peel, salak peel and soursop peel in undigested and digested was quite different both in compound and the quantity (Table 6, Figure 7-9). All standard phenolic compound in undigested of matoa peel, salak peel, and soursop peel could be detected, but after digestion just some of phenolic compound could be detected in this present study. Gallic acid and procatechuic acid were detected in the digested of matoa peel. In the salak peel after digestion, phenolic compounds detected were catechin, gallic acid, and protocatechuic acid. Then, gallic acid, protocatechuic acid and syringic acid were found in soursop peel digested.
The amounts of each compounds after digestion were changed. Change of a number of phenolic compounds is possible. Factors affecting polyphenol bioavailability will be the amount of polyphenols in food, pH conditions and enzymes involved in polyphenol metabolism (Scalbert and Williamson 2000; Chen et al 2014). Decreased of phenolic compounds after digestion such as gallic acid in matoa peel, catechin and protocatechuic acid in salak peel could be caused by alkaline condition (pancreatic phase). It might also cause lower antioxidant activity.
When polyphenol in plant extracts were exposed to alkaline condition, a proportion of the polyphenol compounds are degraded or transformed into different structural forms with different chemical properties, and different bioaccessibility, bioavailability and biological activity. Chen et al. (2014) reported dehydrodimers of flavan-3-ols can be formed from the oxidation of (+)-catechin
and (−)-epicatechin and also some putative dimmers or polymers and phenolic compounds can be derived from anthocyanidins.
The reason of phenolic compound increased after digestion might be because of enzymatic process during digestion and the form of polyphenol in peel samples. Gallic acid in salak peel and soursop peel after digestion showed increasing in value compared to undigested salak peel and soursop peel. This is because there is a possibility the form of phenolic compounds in these samples was complex form like tannin, alkaloid and saponin (Fitrianingsih et al. 2014). Tannic acid after digestion would be converted into gallic acid. In the strong acid condition, hydrolysable tannins can be converted to gallic acid and the core polyol (Hagerman 2010). Tannin after digestion in salak peel and soursop peel might show highly contribution to synthesis of gallic acid, compared to matoa peel. The enzymatic digestion condition has potentical for increase of protocatechuic acid in matoa peel and soursop peel and also for degradation of cyanidin in these samples (Karaya 2004).
21
Figure 7. HPLC chromatogram of soursop peel undigested (a) and digested (b)
Figure 8. HPLC chromatogram of salak peel undigested (a) and digested (b) a
b
a
22
Figure 9. HPLC chromatogram of matoa peel undigested (a) and digested (b)
CONCLUSIONS
In this present study, antioxidant activities (DPPH radical scavenging, lipid peroxidation inhibition and total phenolic content) of peel and seed of six tropical fruits are determinated. Soursop peel, matoa peel and salak peel have a high potential of antioxidant sources. The major phenolic compounds in soursop peel, matoa peel and salak peel is caffeic acid. Their antioxidant bioavailability have been evaluated. The antioxidant activity after digestion is increased in matoa peel and soursop peel, but decreased in salak peel. It means that matoa peel, a new antioxidant source that was found in this experiment, is the best sources of antioxidant of all sample analyzed.
RECOMENDATION
It is needed to analysis of others phenolic compounds like tannin, proantocyanidin and their metabolisms. It is important that research aims to provide biologically relevant information in an in vivo test.
23
REFERENCES
Arimboor R, Kumar KS, Arumughan C. 2008. Simultaneous estimation of phenolic acids in sea buckthorn (Hippophae rhamnoides) using RP-HPLC
with DAD. J Pharm Biomed Anal
47(1):31-38.doi:10.1016/j.jpba.2007.11.045.
Asada T, Tamura H. 2012. Isolation of bilberry anthocyanidin 3-glycosides bearing ortho-dihydroxyl groups on the B ring by forming an aluminum complex and their antioxidant activity. J Agric Food Chem 60(42):10634-10640.doi:10.1021/jf302476n.
Ayala-Zavala JF, Vega-Vega V, Rosas-Domínguez C, Palafox-Carlos H, Villa-Rodriguez JA, Siddiqui MW, Dávila-Aviña JE, González-Aguilar GA. 2011. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Research International 44(7):1866-1874.doi:10.1016/j.foodres.2011.02.021.
Badmus JA, Adedosu TO, Fatoki JO, Adegbite VA, Adaramoye OA, Odunola OA. 2011. Lipid peroxidation inhibition and antiradical activities of some leaf fractions of mangifera indica. Acta Poloniae Pharmaceutica n Drug Research. 68 (1): 23-29.
Balasundram N, Sundram K, Samman S. 2006. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential
uses. Food Chemistry
99(1):191-203.doi:10.1016/j.foodchem.2005.07.042.
[BMKG] Badan Metereolog, Klimatologi dan Geofisika. 2011. Prakiraan cuaca Indonesia.
http://www.bmkg.go.id/BMKG_Pusat/Meteorologi/Prakiraan_Cuaca_Indo nesia.bmkg [13 Februari 2014]
Bhatt A, Patel V. 2013. Antioxidant activity of garlic using conventional extraction and in vitro gastrointestinal digestion. Free Radicals and Antioxidants. 3: 30-34. doi:10.1016/j.fra.2013.03.003
Brosnan B, Coffey A, Arendt EK, Furey A. 2014. The QuEChERS approach in a novel application for the identification of antifungal compounds produced by lactic acid bacteria cultures. Talanta 129:364-373.doi:10.1016/j.talanta.2014.05.006.
Burin VM, Ferreira-Lima NE, Panceri CP, Bordignon-Luiz MT. 2014. Bioactive compounds and antioxidant activity of Vitis vinifera and Vitis labrusca grapes: Evaluation of different extraction methods. Microchemical Journal 114:155-163.doi:10.1016/j.microc.2013.12.014.
Chamorro S, Viveros A, Rebole A, Rica BD, Arija I, Brenes A. 2015. Influence of dietary enzyme addition on polyphenol utilization andmeat lipid oxidation of chicks fed grape pomace. Food Research Intl. 73: 197-203. http://dx.doi.org/10.1016/j.foodres.2014.11.054.
24
Contreras-Calderón J, Calderón-Jaimes L, Guerra-Hernández E, García-Villanova B. 2011. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Research International 44(7):2047-2053.doi:10.1016/j.foodres.2010.11.003.
Crozier A, Jaganath IB, Clifford MN. 2009. Dietary phenolics: chemistry, bioavailability and effects on health. Nat. Prod. Rep. 26: 1001–1043. Delgado-Zamarreno MM, Perez-Martın L, Bustamante-Rangel R,
Carabias-Martınez R. 2012. A modified QuEChERS method as sample treatment
before the determination of isoflavones in foods by ultra-performance liquid chromatography–triple quadrupole mass spectrometry. Talanta
100:320-328.doi:10.1016/j.talanta.2012.07.070.
Deng S, West BJ, Jensen CJ. 2010. A quantitative comparison of phytochemical components in global noni fruits and their commercial products. Food
Fitrianingsih SP, Lestari F, Aminah A. 2014. Uji Efek Antioksidan Ekstrak Etanol Kulit Buah Salak [Salacca Zalacca (Gaertner) Voss] Dengan Metode Peredaman DPPH. Seminar NAsional Penelitian dan PKM Sains, Teknologi dan kesehatan [Prosiding]. ISSN 2089-3582.
Galanakis CM. 2012. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends in Food Science & Technology 26(2):68-87.doi:10.1016/j.tifs.2012.03.003.
Gorelik S, Lapidot T, Shaham I, Granit R, Ligumsky M. Kohen R, Kanner J. 2005. Lipid Peroxidation and Coupled Vitamin Oxidation in Simulated and Human Gastric Fluid Inhibited by Dietary Polyphenols: Health Implications. J. Agric. Food Chem.53: 3397-3402
Hagerman A. 2010. Hydrolyzable Tannin Structural Chemistry. http://www.users.miamioh.edu/hagermae/Hydrolyzable%20Tannin%20Str uctural%20Chemistry.pdf [30 Juli 2015]
Hashemi SR, Zulkifli I, Davoodi H, Zunita Z, Ebrahimi M. 2012. Growth performance, intestinal microflora, plasma fatty acid profile in broiler chickens fed herbal plant (Euphorbia hirta) and mix of acidifiers. Animal
Feed Science and Technology
178(3-4):167-174.doi:10.1016/j.anifeedsci.2012.09.006.
Heijnen CGM, Haenen GRMM, Minou Oostveen R, Stalpers EM, Bast A. 2002. Protection of Flavonoids Against Lipid Peroxidation: The Structure Activity Relationship Revisited. Free Radical Research 36(5):575-581.doi:10.1080/10715760290025951.
Hur SJ, Lim BO, Park GB, Joo ST. 2009. Effects of Various Fiber Additions on Lipid Digestion during In Vitro Digestion of Beef Patties. J. of Food Sci. 74: c653-c657.
25
injury in rabbits. European Journal of Cardio-thoracic Surgery 16: 458-463.
Karaya S. 2004. Bioavailability of Phenolic Compounds. Critical Rev. in Food
Sci. & Nut. 44(6): 453-464.
http://dx.doi.org/10.1080/10408690490886683
Kanlayavattanakul M, Lourith N, Ospondpant D, Ruktanonchai U, Pongpunyayuen S, Chansriniyom C. 2013. Salak plum peel extract as a safe and efficient antioxidant appraisal for cosmetics. Biosci Biotechnol Biochem 77(5):1068-1074.doi:10.1271/bbb.130034.
Kurniawati A. 2011. Kajian karakter biomassa, kadar dan profil derivat xanthone serta potensi antioksidan kulit buah manggis (garcinia mangostana l.) pada berbagai aspek agronomi [disertasi]. Bogor (ID): Institut Pertanian Bogor.
Lafay S, Gil-Izquierdo A. 2007. Bioavailability of phenolic compound.
Phytochem Rev. 7: 301-311. DOI 10.1007/s11101-007-9077-x
Li F, Li S, Li H-B, Deng G-F, Ling W-H, Wu S, Xu X-R, Chen F. 2013. Antiproliferative activity of peels, pulps and seeds of 61 fruits. Journal of Functional Foods 5(3):1298-1309.doi:10.1016/j.jff.2013.04.016.
Liang N, Kitts DD. 2014. Antioxidant property of coffee components: assessment of methods that define mechanisms of action. Molecules 19(11):19180-19208.doi:10.3390/molecules191119180.
Mardalena, Warly L, Nurdin E, Rusmana WSN, Farizal. 2011. Milk quality of dairy goat by giving feed supplement as antioxidant source. J.Indonesian Trop.Anim.Agric. 36 (3): 205-212.
Ng L, Ang Y, Khoo H, Yim H. 2012. Influence of different extraction parameters on antioxidant properties of Carica papaya peel and seed. Research Journal of Phytochemistry 6(3):61-74.
Pavan V, Sancho RAS, Pastore GM. 2014. The effect of in vitro digestion on the antioxidant activity of fruit extracts (Carica papaya, Artocarpus heterophillus and Annona marcgravii). LWT - Food Science and improved performance and meat shelf-life in broilers using feed adjuncts with presumed antioxidant activity. Animal Feed Science and Technology
170(3-4):239-245.doi:10.1016/j.anifeedsci.2011.09.001.
Randhir R, Lin Y-T, Shetty K. 2004. Phenolics, their antioxidant and antimicrobial activity in dark germinated fenugreek sprouts in response to peptide and phytochemical elicitors. Asia Pacific Journal of Clinical Nutrition 13(3):295-307.
26
Samonte PAL, Trinidad TP. 2013. Dietary fiber, phytonutrients and antioxidant activity of common fruit peels as potential functional food ingredient.
Journal of Chemistry and Chemical Engineering 7(1):70.
Sato A, Shinozaki N, Tamura H. 2014. Secoiridoid Type of Antiallergic Substances in the Pomace, Wasting Materials in Three Varieties of Japanese Olive (Olea europaea), J. Agric. Food Chem., 62(31): 7787-7795. doi: 10.1021/jf502151b
Sato A, Zhang T, Yonekura L, Tamura H. 2015. Antiallergic activities of eleven onions (Allium cepa) were attributed to quercetin 4′-glucoside using QuEChERS method and Pearson's correlation coefficient. Journal of Functional Foods 14:581-589.doi:10.1016/j.jff.2015.02.029.
Scalbert A, Williamson G. 2000. Dietary Intake and Bioavailability of Polyphenols. American Society for Nutritional Sciences. 2073S-2084S. Schieber A, Stintzing FC, Carle R. 2001. By-products of plant food processing as
a source of functional compounds — recent developments. Trends in
Food Science & Technology
12(11):401-413.doi:http://dx.doi.org/10.1016/S0924-2244(02)00012-2.
Sharma OP, Bhat TK. 2009. DPPH antioxidant assay revisited. Food Chemistry
113(4):1202-1205.doi:10.1016/j.foodchem.2008.08.008.
Silva LMR, Figueiredo EAT, Ricardo NMP, Vieira IGP, Figueiredo RW, Brasil IM. 2014. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 143: 398-404.
Sugito, Manalu W, Astuti D, Handharyani E, Chairul. 2010. Morfometrik Usus dan Performa Ayam Broiler yang Diberi Cekaman Panas dan Ekstrak
n-Heksana Kulit Batang ―Jaloh"(Salix tetrasperma Roxb). Media Peternakan-Journal of Animal Science and Technology 30(3).
Tamura H, Kitta K, Shibamoto T. 1991. Formation of reactive aldehydes from fatty acids in a iron (2+)/hydrogen peroxide oxidation system. Journal of agricultural and food chemistry 39(3):439-442.
Tamura H, Yamagami A. 1994. Antioxidative activity of monoacylated anthocyanins isolated from Muscat Bailey A grape. Journal of Agricultural and Food Chemistry 42(8):1612-1615.
Villano D, Fernandez-Pachon MS, Moya ML, Troncoso AM, Garcia-Parrilla MC. 2007. Radical scavenging ability of polyphenolic compounds towards
DPPH free radical. Talanta
71(1):230-235.doi:10.1016/j.talanta.2006.03.050.
Wang HF, Yang WR, Wang YX, Yang ZB, Cui YH. 2011. The Study on the Effects of Chinese Herbal Mixtures on Growth, Activity of Post-Ruminal Digestive Enzymes and Serum Antioxidant Status of Beef Cattle.
Agricultural Sciences in China 10 (3): 448-455. doi:10.1016/S1671-2927(11)60024-2
Yonekura L, Sun H, Soukoulis C, Fisk I. 2014. Microencapsulation of Lactobacillus acidophilus NCIMB 701748 in matrices containing soluble fibre by spray drying Technological characterization, storage stability and survival after in vitro digestion. Journal of Functional Foods 6:205-214.doi:10.1016/j.jff.2013.10.008.
27
Zhu F, Asada T, Sato A, Koi Y, Nishiwaki H, Tamura H. 2014. Rosmarinic acid extract for antioxidant, antiallergic, and alpha-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from Perilla leaves. J Agric Food Chem 62(4):885-892.doi:10.1021/jf404318j.
28
APPENDIXES
Appendix 1. ANOVA of lipid peroxidation in peel and seed of tropical fruits
Sum of Squares df Mean Square F Sig.
Between Groups 3554.160 11 323.105 50.955 .000
Within Groups 380.458 60 6.341
Total 3934.619 71
Appendix 2. Duncan test of lipid peroxidation in peel and seed of tropical fruits Sample name N
Subset for alpha = 0.05
1 2 3 4 5 6 7
Soursop peel 6 1.2
Matoa peel 6 1.9 1.9
Salak peel 6 3.2 3.2 3.2
Papaya peel 6 4.5 4.5 4.5
Salak seed 6 5.3 5.3
Matoa seed 6 5.5 5.5
Papaya seed 6 7.1 7.1
Soursop seed 6 7.5 7.5
Kapundung peel 6 9.2
Kapundung seed 6 9.4
Rambai peel 6 21.4
Rambai seed 6 24.7
Appendix 3. ANOVA of SC50 value of DPPH Scavenging Activity in peel and seed of tropical fruits
Sum of Squares Df Mean Square F Sig.
Between Groups 17409.358 10 1740.936 51.351 .000
Within Groups 745.865 22 33.903
Total 18155.223 32
Appendix 4. Duncan test of SC50 value of DPPH Scavenging Activity in peel and seed of tropical fruits
Sample name N Subset for alpha = 0.05
1 2 3 4 5
Trolox 3 4.4
29
Appendix 5. ANOVA of total phenolic content in peel and seed of tropical fruits Sum of Squares Df Mean Square F Sig. Between Groups 305919.185 11 27810.835 74.064 .000
Within Groups 13517.995 36 375.500
30
Appendix 9. ANOVA of total phenolic undigested and digested tropical fruit peels Sum of
Appendix 10. Duncan test of total phenolic undigested of tropical fruits
Sample name N Subset for alpha = 0.05
1 2
Soursop peel 4 2
Salak peel 4 6.875
31
Appendix 11. Duncan test of total phenolic digested of tropical fruits Sample name
Appendix 13. ANOVA of lipid peroxidation undigested and digested tropical fruit peels
Appendix 14. Duncan test of lipid peroxidation undigested of tropical fruits
Sample name N Subset for alpha = 0.05
1 2 3
Soursop peel 6 205.48
Salak peel 6 257.95
32
Appendix 15. Duncan test of lipid peroxidation digested of tropical fruits
Sample name N Subset for alpha = 0.05
1 2 3
Soursop peel 9 649.23
Salak peel 9 909.93
Matoa peel 9 973.04
Appendix 16. ANOVA of lipid peroxidation between undigested and digested of tropical fruits Within Groups 27584.69 13 2121.899
Total 736457.6 14
Matoa peel
Between Groups 487623.6 1 487623.6 248.217 0 Within Groups 25538.58 13 1964.506
Total 513162.1 14
Salak peel
Between Groups 1530275 1 1530275 1335 0 Within Groups 14905.05 13 1146.542
33
Appendix 19. Duncan test of SC50 value of DPPH Scavenging digested of tropical fruits (concentration 10 µg/ml) tropical fruits (concentration 10 µg/ml)
Sample name N
Appendix 21. Duncan test of SC50 value of DPPH Scavenging digested of tropical fruits (concentration 50 µg/ml)
34
1 2 3
Salak peel 3 32.4667
Soursop peel 3 75.3333
Matoa peel 3 81.8000
Appendix 22. Duncan test of SC50 value of DPPH Scavenging undigested of tropical fruits (concentration 50 µg/ml)
Sample name N Subset for alpha = 0.05
1 2 3
Soursop peel 3 35.0333
Matoa peel 3 70.2067
Salak peel 3 89.1200
Appendix 23. Wavelength detection (λ), retention time (rT), and linear regression data of phenolic compounds
Compound wavelenght rT Linear regression formula
Cinnamic acid 270 18.3 y = 798,7x + 0,311
Caffeic acid 310 10.2 y = 31,17x + 2,500
Catechin 280 7.6 y = 55,53x + 0,811
Chlorogenic acid 320 10.0 y = 512,4x - 5,254
Ferulic acid 310 13.0 y = 386,9x + 9,395
Gallic acid 270 3.8 y = 353,2x + 6,566
o-Coumaric acid 270 14.7 y = 420,8x - 0,908
Protocatechuic acid 260 5.6 y = 269,2x + 5,577
p-Coumaric acid 280 12.1 y = 439,3x + 7,069
Quercetin 340 16.9 y = 188,9x + 2,722
Rosmarinic acid 330 14.5 y = 289,9x - 2,606
Rutin 340 12.1 y = 212,5x - 0,357
35
Appendix 24. Phenolic compound in this experiment
Cinnamic acid
Caffeic acid Catechin
Chlorogenic acid
Ferulic acid Gallic acid
o-Coumaric acid Protocatechuic acid p-Coumaric acid
Quecertin Rosmarinic acid Rutin