PEPTIDES HYDROLYSATE DERIVED FROM COLLAGEN OF SNAKEHEAD MURREL(Channa striata) SKIN DEMONSTRATE
ANTI-OXIDANT AND ANTI-CHOLESTEROL ACTIVITIES
WENNY SILVIA LOREN BR SINAGA
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
STATEMENT LETTER OF MANUSCRIPT AND SOURCE OF
INFORMATION*
I declare that this manuscript entitled Peptides hydrolysate derived from collagen of snakehead murrel (Channa striata) skin demonstrate anti-oxidant and anti-cholesterol activities is my own work with guidance of the advisors and has not been submitted in any form at any college, except at Bogor Agricultural University. Sources of information derived and quoted from published and unpublished works of other authors mentioned in the text and included in the reference chapter.
Herewith I bestow the copyright of this manuscript to Bogor Agricultural University.
Bogor, April 2015
Wenny Silvia Loren Br Sinaga
RINGKASAN
WENNY SILVIA LOREN BR SINAGA. Hidrolisat Peptida yang Dihasilkan dari Kolagen Kulit Ikan Gabus (Channa striata) yang Memiliki Aktivitas Anti-oksidan dan Anti-kolesterol. Dibimbing oleh MAGGY T. SUHARTONO dan RAYMOND R. TJANDRAWINATA.
Anti-kolesterol dan anti-oksidan memiliki peranan penting untuk menghambat penyakit kardiovaskular, terkait dengan adanya gangguan pada arteri yang disebabkan oleh kolesterol yang teroksidasi. Beberapa tahun belakangan ini sejumlah riset peptida bioaktif memperlihatkan aktivitas sebagai anti-kolesterol dan anti-oksidan. Pada penelitian ini, Acid Soluble Collagen di ekstrak dari kulit ikan gabus dan digunakan sebagai inducer untuk menghasilkan kolagenase oleh Bacillus licheniformis F11.4.
Kolagenase yang dihasilkan di murnikan menggunakan AKTA Purifier (ion exchange, DEAE column) dan mendapatkan fraksi D dan F. Fraksi enzim D dan F
kemudian digunakan untuk membuat hidrolisat peptida dari acid soluble collagen. Hidrolisat peptida yang dihasilkan fraksi D menunjukkan aktivitas inhibitor HMG-CoA sebanding dengan pravastatin dan sedikit aktivitas antioksidan. Sementara, hidrolisat peptide oleh F memiliki aktivitas antioksidan yang lebih sedikit dibandingkan dengan BHT (2mM), vitamin C (2mM) and vitamin E (2mM), tetapi sedikit aktivitas penghambatan HMG-CoA.
SUMMARY
WENNY SILVIA LOREN BR SINAGA. Peptides Hydrolysate Derived from Collagen of Snakehead Murrel (Channa striata) Skin Demonstrate Anti-oxidant and Anti-cholesterol Activities. Supervised by MAGGY T. SUHARTONO dan RAYMOND R. TJANDRAWINATA.
Anti-cholesterol and anti-oxidant play crucial role to combat cardiovascular disease (CVD) related to formation of arterial plagues from oxidation of cholesterol. In the past decades, research on bioactive peptides demonstrating anti-cholesterol and anti-oxidant activities have been reported as the alternative drugs. In this study, acid soluble collagen was extracted from the skin of snakehead murrel and employed to induce production of collagenase by Bacillus licheniformis F11.4. The collagenases secreted were in turn purified through AKTA Purifier (ion exchange, DEAE column) and used to produce peptides hydrolysate.
The purified enzymes were grouped in two distinct collagenase fractions, designated as fraction D and F. Peptides hydrolysate produced by the fraction D was found to demonstrate HMG-CoA inhibitor activity comparable to pravastatin and limited anti-oxidant activity. Meanwhile, peptides hydrolysate generated using the fraction F demonstrated anti-oxidant activity comparable to BHT (2mM), vitamin C (2mM) and vitamin E (2mM), but limited HMG-CoA activity. Combination of the fraction D and F resulted in substantial HMG-CoA inhibition and anti-oxidant activities.
© COPYRIGHT IPB, 2015
All Rights Reserved by Law
It is probihited to quote part or whole of this scientific writing without making citation. Quotations should only be made for the benefits of eduction, research, scientific writing, report writing critical writing or solving a specific problem. Such quotations should not demean the image of IPB.
Thesis
As a requirement to obtain Magister Science degree
in
Food Science Study Program
PEPTIDES HYDROLYSATE DERIVED FROM
COLLAGEN OF SNAKEHEAD MURREL (
Channa striata
)
SKIN DEMONSTRATE OXIDANT AND
ANTI-CHOLESTEROL ACTIVITIES
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
2015
PREFACE
Praise to the God almighty for thy mercy, blessing, and guidance through out the research and finishing this manuscript. The research entitled “Peptides hydrolysate derived from collagen of snakehead murrel (Channa striata) skin demonstrate anti-oxidant and anti-cholesterol activities” was carried out in Bogor Agricultural University and DLBS from January 2013 until February 2014.
By completion of this research and manuscript, the author would like to express great appreciation and sincere thanks to:
1. Prof. Dr. Ir. Maggy T Suhartono, as my supervisor, for her guidance, spirit, help, and advices to see the world during completing this research and manuscript.
2. Raymond R. Tjandrawinata, PhD, MS, MBA as co supervisor for his time, help, advice and opportunity to finish my research in DLBS.
3. Dr. Wangsa Tirta Ismaya as expert trainer in DLBS, for his correction in this thesis, his comment, his help during the research, and time answering my question.
4. Dr. Puspo Edi Giriwono as examiner, for his correction, help and comment. 5. My parents (Robinson Sinaga and Rehulina Br. Simbolon)and broher (Zohdy
Gokta Sinaga) for their Prayer, Believing and support during the study of Food Science and Technology, the research, and manuscript completion.
6. Dexa Laboratories for Biomoleculer Science for the time and chance to do my research was. Ibu Debbie S. Retnoningrum who held responsible in incentive Sinas funding and also for the DLBS members (Bu Henny, Pak Bambang, Mba Hayu, Ka Lolen, Ka Frans, Mas Yogi, Kak Lia, Apry, Bang Madan, Apryani Rahma, Utha, Tia, Putri, Pak Dokter, Mba Aini dan Mba Chandra) For their guidance and help in the laboratory.
7. Food science student (Rahma, Mba Diana Nur Afifah, Silvie, Novan, mba Ino, Diana Lestari, Ibu Wati, Adi, Rahayu, Risma, Nisa, Reny, Septi otong, Ella, Pak Rinto, Ka Becky, Ayu, Evelyn and others) and for Bu Ika for their support, valuable friendship, and unforgettable moments during the study and completion of this research.
8. Special Thanks to my Cikuci (DJ Calvien H) for his prayer, believing, loquaciousness, advice, patient, time, support, lend a hand and shoulder when I cry and need a help and guidance during the study and finish this thesis.
9. GSP IPB and Psaltrio singer (Devide, Silvia, Ka Jems, Arif, Nas, Merry, Yuang, Sars, Roto, Mas Deka, Pak John, Ka Elga, Ka Tasha & Bang Anton, Mba Rini, Ka Tere, Kinan and Mas Ari) with their happiness shared.
10. PP YN Crew (Dessy Cenion and Minion, Btari Sisters, Situmorang Sisters, Monce, Erti, Risma, Risty, Kristin, Mele and the ganks, Risvan, Dira and Others) for their support, long lasting friendship, waiting me when insomnia catch me and precious moments shared during this manuscript written.
Last but not least, hopefully this manuscript is useful for the readers and gives a contribution in food science development.
Bogor, August 2015
CONTENT
LIST OF FIGURE vi
LIST OF APPENDIX vi
1 INTRODUCTION 1
Background 1
Problem Statement 2
Objective 2
Significance of study 2
2 LITERATURE REVIEW 2
3 RESEARCH METHODOLOGY 5
Organisms and materials 5
Production and partial purification of collagenases 5 Collagenase assay and protein concentration determination 6
SDS PAGE and zymogram 6
Preparation of bioactive peptides hydrolysate 6
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity assay 7
HMG-CoA reductase assay 7
4 RESULT AND DISCUSSION 7
Partial purification and characterization of collagenases 7
Anti-oxidant activity 12
Anti-cholesterol activity 12
5 CONCLUSSION AND RECOMMENDATION 14
Conclusion 14
Recommendation 14
Acknowledgements 14
REFFERENCE 14
AUTHOR BIOGRAPHY 24
APPENDIX 19
LIST OF FIGURE
Figure 1 (A) Graphic from AKTA Purifier IEC(DEAE) by using linear gradient salt elution (sample injection: 2mL, 45 CV, Flow rate: 5ml/mnt, Elution: 5ml). (B) Zymography analysis of the enzymes after purification. C: crude enzyme, As: after ammonium sulphate fractionation, 8-50: fractions of enzyme 8 Figure 2 Zymography analysis of enzyme from purification upon
challenge with proteases inhibitors. C: crude enzyme, As: after ammonium sulphate fractionation, and 8-50: fractions of enzyme. Fractions 26-34 are D whilst fractions 40 and 50 are F. Notation E and P refers to EDTA and PMSF, respectively. 9 Figure 3 (A). Graphic from AKTA Purifier by using stepwise salt elution
(sample injection: 65mL, 40 CV, Flow rate: 0.8ml/mnt, Elution: 2ml). (B).Zymography analysis of the enzymes after purification on anion exchanger column. C: crude enzyme, As: after ammonium sulphate fractionation, 18-95: fractions of
enzymes. 10
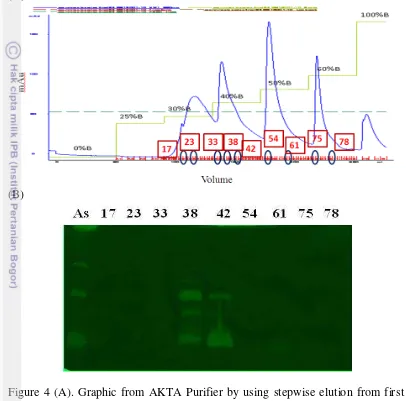
Figure 4 (A). Graphic from AKTA Purifier by using stepwise elution from first purification from collected fraction 48-80 (Figure 3A), (sample injection: 2mL, 45 CV, Flow rate: 0.8ml/mnt, Elution: 2ml). (B).Zymography analysis of the enzymes after purification on anion exchanger column. As: after ammonium sulphate fractionation, 17-78: fractions of enzymes. 11
Figure 5 Antioxidant Activity 12
Figure 6 Inhibition of HMG-CoA reductase 13
LIST OF APPENDIX
Appendix1 Reagents for Bradford Assay 19
Appendix2 Composition of standard solution for Bradford assay 20 Appendix4 Standard Curve of Bovine Serum Albumine (BSA) 21
Appendix5 Reagents for SDS-PAGE analysis 22
Appendix6 Composition of separating and stacking gels for SDS-PAGE and Zymography analysis
1
INTRODUCTION
Background
Bioactive peptide (BP) is derived from proteins through acid or proteolytic activity (Senevirathne and Kim, 2012). BP has been developed into anti-hypertension, anti-oxidant, anti-thrombotic, and hypo-cholesterolemic drugs, which are potent to prevent degenerative diseases, such as cardiovascular disease (CVD). Protein for the source of BP can be from plants, meat, milk (Korhonen and Pihlanto, 2003; Korhonen and Pihlanto, 2006 ) and fish (Senevirathne and Kim, 2012). An example of anti-oxidative BP is lunasin, which is identified in soybean and other plants (Galvez and Lumen, 1999). This peptide is already commercialized in the US and reported to decrease low density lipoprotein (LDL) and cholesterol in the blood (Galvez, 2012).
Recent studies have shown that anti-oxidative BP can be released from casein through enzymatic hydrolysis or during fermentation of milk using protease-producing lactic acid bacteria (Korhonen and Pihlanto, 2003). The anti-oxidative BP displays free radical scavenging activities and inhibits enzymatic and non-enzymatic lipid peroxidation, most likely for being a preferred target over fatty acid free radicals (Rival et al., 2001). Consumption of anti-oxidative BP derived from goat is reported to show anti-atherogenicity by prolonging resistance of the lipoprotein fraction to oxidation (Kullisaar et al., 2003).
Snakehead murrel is one of the freshwater fishes from Channidae family
with high collagen content in their skin. The skin of fish has been considered as waste in the fish processing. In this study, collagen was extracted from fish skin by acid treatment and found that the peptides hydrolysate derived from collagen was able to inhibit the activity of HMG-CoA reductase, the key enzyme for cholesterol biosynthesis. Anti-oxidant activities are detected in this peptides hydrolysate. The peptides were produced by proteolytic digestion using collagenases from B. licheniformis F11.4, a mutant of B. licheniformis from
Indonesia. This bacterium has previously been shown to demonstrate high proteolytic and collagenolytic activities (Waldek et al., 2006), secreting collagenases of 124 kDa and 26 kDa when grown in the presence of water-soluble collagen derived from milkfish skin (Baehaki et al., 2012; Baehaki et al., 2014).
2
Problem Statement
Bioactive peptide is known to have anti-cholesterol and anti-oxidant activities. Non-enzymatic method to make bioactive peptide can produce unspecific peptides while enzymatic protein degradation can work specifically to degrade protein more effectively. In previous study, Bacillus licheniformis F11.4
was found to produce collagenase. Purified enzyme is needed to apply and find the good characteristics of this peptides hydrolysate. In this study Snakehead murrel (Channa striata) skin was extracted by using Acid Solluble Collagen
(ASC) method and used it as inducer to get collagenase from Bacillus licheniformis F11.4 and applied the enzyme to promote peptides hydrolysate and
analyze the bioactivity.
Objective
The aim of this research was: 1) to purify collagenase from B. licheniformis
F11.4 and apply the enzyme for production of collagen bioactive peptide hydrolysate. 2) to characterize peptides hydrolysate specifically their anti-cholesterol and anti-oxidant activities.
Significance of study
This study paves foundation for further enzyme purification and identification of the bioactive peptides and structural-function study of the enzymes.
2
LITERATURE REVIEW
Several proteases from microorganisms have been reported. Collagenase-producing Bacillus reported include: Bacillus licheniformis N22 (Asdornnithee et. al. 1994), Bacillus subtilis FS-2 (Nagano and To 1999), B. subtilis CN2 (Tran and Nagano 2002), Bacillus sp. MO-1 (Okamoto et. al. 2001), B. subtilis AS1.398 (Riu et. al. 2009), Bacillus pumilus CoI-J (Wu e.t al. 2010), Streptomyces sp.
Strain 3B (Petrova et. al. 2006) and Streptomyces parvulus (Sakurai et. al. 2009).
Serine collagenase is often characterized with their molecular weight of 24-36 kDa (Roy et. al. 1996), while metallo-collagenase is 30 to 150 kDa (Harris and
Vatar 1982). Some metallo-collagenases contain zinc and require calcium for stability (Stricklin et. al. 1977).
B. lichenifromis F11 show high protease (collagenase) activity but lack
chitinase activities. B. licheniformis F11.4 displayed rough colony morphology.
Microscopic investigation revealed form motile rods of equal sizes (2.9 by 0.75 μm). According to the physiological and microscopic tests, B. licheniformis F11 is suggested to be representatives of the B. licheniformis species (Waldeck et. al.
3 obtained from sequencing the 16S rRNA gene, including the hypervariable regions V1 to V3; 100% identity to B. licheniformis DSM13/ATCC 14580, which was totally sequenced only recently, was found (Waldeck et. al. 2006). By
targeted deletion of the polyglutamate operon (pga) in B. licheniformis F11, a
derivative form, F11.1 (pga), was obtained, along with lacking polyglutamate
(PGA) formation and, enhanced proteolytic activities. The phenotypic properties were maintained in a strain in which the chiBA operon was additionally deleted: F11.4 (chiBA pga) (Hoffmann et. al. 2010).
Collagen is partly responsible for toughness in red meat and used as tenderizers in the food industry (Cronlund and Woychik 1987). Collagenases have applications in fur and hide tanning to help ensure a uniform dying of leathers (Goshev et. al. 2005; Kanth et. al. 2008). However, the most common uses of these enzymes appear to be in medicine. They are used to treat burns and ulcers (Agren et. al. 1992), to eliminate scar tissue (Shmoilov et. al. 2006) and play an
important role in the successful transplantation of specific organs (Klock et. al.
1996; Kin et. al. 2007). Chung et. al. (2004) found catalytic domain which have
Zn in their active site bind with collagen. Collagenase will loose a rigid triple-helix of collagen. Bond of Gly 775– Ile 776 amino acid were cleave into the typical three-quarter and one-quarter fragments.
Bioactive peptides are proteins synthesized in the cell in the form of large prepropeptides, which are then cleaved and modified to give active products. Milk proteins are a rich source of biologically active peptides such as antihypertensive, antithrombotic, immune-stimulating, antimicrobial, mineral carrying and cholesterol lowering-peptides (Shah 2000). Many researchers are interested to solve the question about the importance of bioactive foods are as food constituents or as drugs and it needs careful examination. ACE inhibitory peptides, immunomodulating peptides, and caseinophosphophopeptides are the most favorite bioactive peptides for application to food stuffs formulated to provide specific health benefits. Casein derived peptides have already found interesting applications as dietary supplements and as pharmaceutical preparations such as tablets, toothpaste, and dental filling material. The efficacy and safe conditions of use of these peptides in animals and in humans remain yet to be proven.
The importance of oxidation in the body and in food stuffs has been widely recognized. Oxidative metabolism is essential for survival of cells. A side effect of this dependence is the production of free radicals and other reactive oxygen species that cause oxidative changes. When an excess of free radicals is formed, they can overwhelm protective enzymes like superoxide dismutase, catalase and peroxidase which cause destructive and lethal cellular effects (e.g. apoptosis) by oxidizing membrane lipids, cellular proteins, DNA, and enzymes thus shutting down cellular process. Recent studies have shown that anti-oxidative peptides can be released from caseins in hydrolysis by digestive enzymes and in fermentation of milk with proteolytic lactic acid bacteria strains (Korhonen and Pihlanto, 2003).
4
oxidized LDL, 8-isoprostanes and the glutathione redox reaction, and enhancing total antioxidative activity. Therefore, it is hypothesized that low antioxidant levels may increase coronary heart disease. More research is needed to elucidate the role of antioxidative peptides in the protective functions in human. Not only from fermented milk, some research which have antioxidant activity have been reported such as soy protein, pig meat protein, seeds protein, and some fish.
The 3-hydroxy-3-methylglutarylcoenzyme A (HMG-CoA) reductase inhibitors or statins, are potent inhibitors of cholesterol biosynthesis that are extensively used in the treatment of patients with hypercholesterolemia. Statins impair cholesterol synthesis by inhibiting the activity of the enzyme HMG-CoA reductase. Inhibiton of cholesterol biosynthesis is accompanied by an increase in low-density lipoprotein (LDL) receptors in the liver, leading to increase uptake and clearance of cholesterol from plasma (Borghi et. al. 2002).
Once a crude collagenase extract is recovered, it can be purified by their physicochemical properties such as ion charge, size exclusion, hydrophobic interaction or affinity. ion-exchange chromatography will separate ions and polar molecules according to charge of protein. Separation can be achieved based on the natural isoelectric point of the protein. At a given pH most proteins have an overall negative or positive charge depending on their isoelectric point (pI), hence these proteins interact with an oppositely charged resin packed in a column. Proteins are retained according to their charge. There are two commonly used types of columns: (a) Sephadex Diethylaminoethyl (DEAE) cellulose for binding to negative charge of proteins and (b) Carboxymethyl (CM) for binding to net positively charged proteins (Kaufman et. al. 1995). At optimal pH levels (near
7.0), most of collagenases bear an overall negative charge and anionic exchange column should be employed. Anion exchange chromatography based on Diethylaminoethyl (DEAE) cellulose or agarose is by far the most common ion exchange technique that has been used by a number of researchers to partially purify collagenase (Kim et. al. 2002).
Gel filtration is known as size exclusion or molecular sieve chromatography. It separates molecules based on size. It is a physical separation where the column packing material contains pores that only molecules within a particular size or mass range can enter and be retained. Many commercial gel matrixes are used such as Sephadex (10, 25, 50, 75, 100 and G-200), Sepharose (6B, 4B, 2B), Sephacryl (S-200, S-300, S-400), and Bio-Gel (10, 30, 60, P-100, P-150, P-200, P-300, A 0.5-50). These different materials have different protein size-exclusion ranges. Proteins can be separated by passing them through the appropriate column. Several researchers performed collagenase purification using gel-filtration chromatography. Ohyama and Hashimoto (1977) used Sephadex G-150 to purify collagenase of human skin. Indra et. al. (2005) used
gel-filtration chromatography on Sephadex G-100 to obtain purified collagenase from hepatopancreas of the marine crab and land snail, respectively. Several gel-powders have been used including: Polyacrylamide and mixtures of polyacrylamide and dextran (Sakurai et. al. 2009). Regardless of support material,
5 HIC makes use of surface hydrophobicity interaction of protein and column packing material in the presence of salt concentrations. Because amino acids have different hydrophobicity, HIC can be used to separate proteins and enzymes with different compositions. This technique is similar to Reverse Phase (RP) chromatography (HPLC application) where non-polar structures, such as alkyl ligands, are attached to a support and form reversible interactions with the solutes. With HIC, however, the interactions are much weaker so that proteins are not strongly retained and can be recovered with polar solvents with varying salt concentrations (Queiroz et. al. 2001). Kristjansson et. al. (1995) used a column of
phenyl-substituted agarose to partially purify collagenase. Sakurai et. al. (2009)
used this technique but with a more hydrophobic butyl substitute.
Affinity chromatography uses a specific interaction between a substrate and a biologically active substance and is the most powerful method for protein purification. This method is based on the high affinity of some proteins to specific chemical groups (ligands) covalently attached to a chromatographic column packing material (Kaufman et. al. 1995). The interaction may be very specific,
with a substrate bonded to the stationary phase that only the enzyme of interest can interact with such as a collagen-collagenase pairing. Stationary phases with collagen as the ligand are not commercially available but a number of research groups have prepared their own packing materials to make use of this very specific interaction (Tyagi and Cleutjens 1996). More general interactions involve a ligand bound to the stationary phase that interacts with a number of molecules that contain certain structures.
3
RESEARCH METHODOLOGY
Organisms and materials
Bacillus licheniformis F11.4 was kindly provided by the Indonesian
government agency for assessment and application of technology (BPPT). The bacterial mutant is derived from B. licheniformis F.11, which was discovered during research collaboration between BPPT and Munster University - Germany under the Indo-German Biotechnology scheme. Collagen from snakehead fish skin was prepared according to Singh et. al. (2011). Chemicals were purchased from Merck, Sigma, or Oxoid, through local distributors, except when specifically mentioned.
Production and partial purification of collagenases
B. licheniformis F11.4 was grown in a medium containing 1% NaCl, 0.5%
tryptone, 0.25% yeast extract, and 5% collagen, for 24 hours at 37°C with agitation speed of 120 rpm. Collagenase secreted into the medium was harvested by cold centrifugation (4°C) for 15 minutes at 7500 g prior to ammonium sulphate
6
(GE Healthcare Life Science, Pittsburg – PA, USA) that had previously been equilibrated with 0.02 M phosphate buffer, pH 8.0. The column was eluted with a gradient of 0-1M NaCl in 0.02 M phosphate buffer, pH 8.0, at a flow rate of 0.5 ml/min. The purification was performed on Äkta purifier system (GE Healthcare Life Science, Pittsburg – PA, USA) at room temperature (25 oC). supernatant was mixed with 0.4 M Na2CO3, followed by addition of Follin reagent
at a ratio of 1:2. The mixture was further incubated at 37°C for 20 minutes. The amount of amino acid produced was measured at 578 nm. One unit of enzyme activity (U) is defined as the amount of enzyme required to produce 1 μmol of amino acid per minute under specific conditions. Protein concentration was determined according to Bradford (1976) using bovine serum albumin (BSA) as the standard.
SDS PAGE and zymogram
Molecular weight of proteins was estimated using SDS-PAGE (Laemmli 1970). 80 μL samples were mixed with 20 μL buffers (contained Tris-HCl, glycerol, SDS, β-mercaptoethanol, and bromphenol blue) and it was heated in boiling water for 5 minutes. The samples were injected into the gel which contained 12% acrylamide for the separating gel and 4% acrylamide for the stacking gel (Appendix 5). The electrophoresis process was run at 70 Volt and 50 mA for 2.15-2.31 hours. After that, the gel was stained in staining solution for several hours and it was destained with destaining solution until the blue bands were contrast with the gel. Composition of reagents for SDS-PAGE analysis can be seen in (Appendix 6). High molecular weight markers were used to estimate the molecular weight of protein.
Enzymes activity in situ was demonstrated in a zymogram gel (Choi et. al.
2000), using 10% non-denaturing PA gel containing 0.1% (w/v) collagen. The methodology was same as SDS PAGE. The differentiation was the acrilamide contain and the samples were not boiled in water.
Preparation of bioactive peptides hydrolysate
One ml of partially purified enzymes (0.4-0.7 mg/ml) was added into 50 ml collagen solution (0.5-1 mg/ml) and mixed with 40 ml phosphate buffer 500 mM. The mixture was incubated for 120 minutes at 40oC. The enzymatic hydrolysis was stopped by an addition of 250 mM TCA. The peptide solution was recovered after centrifugation at 4,000 g in 4ºC and stored at -20oC. For inhibition studies,
7 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity assay
The anti-oxidant measurement was performed according to Li et. al. (2007). Briefly, 500 µl of peptide solution was mixed with 500 µL of 99.5% ethanol and 125µL 0.02% DPPH in 99.5% ethanol. The mixture was kept in the dark for one hour at room temperature. Degradation of DPPH was measured at 517 nm. Radical scavenging activity (RSA) was calculated as a percentage of the activity of the control (absence of antioxidant).
HMG-CoA reductase assay
HMG-CoA reductase assay was performed according to Perchellet et. al. (2009). Prior to the assay, a spectrophotometer was conditioned at 37°C and the measurement was carried out at 340 nm with and interval reading of 15 seconds for five minutes. The reaction mixture contained 5 μl Pravastatin or peptides hydrolysate (the assay buffer as the blank), NADPH, HMG-CoA substrate, and HMG-CoA Reductase (HMGR) (the assay buffer for the negative control and blank). The reaction mixture was thoroughly mixed prior to measurement in the spectrometer. The specific activity of enzyme was calculated according to the following equation:
Where:
12.44 = εmM - the extinction coefficient for NADPH at 340 nm is 6.22 mM–1cm–1. 12.44 represents the 2 NADPH consumed in the reaction.
TV = Total volume of the reaction in ml (ml) V = Volume of enzyme used (ml)
0.6 = Enzyme concentration (mg/ml) LP = Light path in cm (1 cm)
One unit of enzyme is defined as the amount of enzyme required to convert one milli mole of NADPH to NADP+ per minute at 37°C.
4
RESULT AND DISCUSSION
Partial purification and characterization of collagenases
8
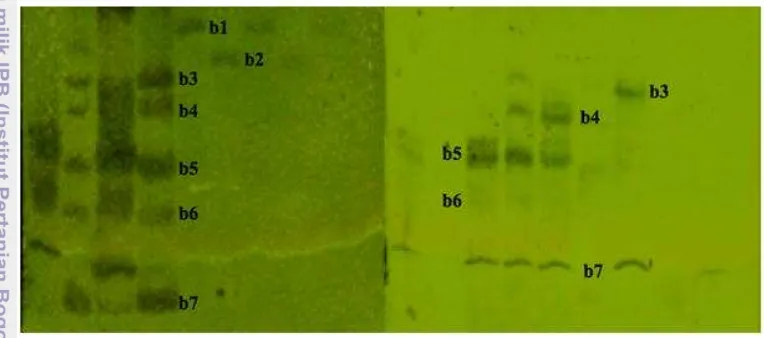
Reason for the popularity of IEC (ion-exchange chromatography) include its (1) high resolving power, (2) high protein-binding capacity, (3) versatility (there are several types of ion exchangers, and the composition of the buffer and pH can be varied over a mile range), (4) straightforward separation principle (primarily according to differences in charge), and (5) ease of performance. First fractions collected during linear elution of DEAE anion exchanger column with NaCl at a gradient concentration of 0-1 M, showed the presence of at least seven collagenases (b1-b7) secreted by B. licheniformis F11.4 (Figure 1B). This first
initiation method used to find the best column for our protease. The result was DEAE is the best column to separate our enzyme (collagenase). The collagenases were classified by means of protease inhibitors such as, PMSF (Phenyl methyl sulfonyl fluoride) for serine protease and EDTA (Ethylene diamine tetra acetic
(A)
(B)
9 acid) for metallo-protease (Figure 2). Interestingly, the inhibition profile identified b1 as serine protease, b2 as metallo-protease, and neither of both (b3-b7). The zymography assay may indicate the presence of possible dimer, trimer, or other oligomeric enzyme states, which are undetectable in SDS PAGE under denaturing conditions. Further, b7 was discovered to actually contain likely two species, which are neither serine nor metallo-protease (27) and a serine protease (40, and 50). The b7 enzyme (Figure 1B) was probably a very small enzyme or an enzyme fragment that still contains the active site of collagenase. Unfortunately, this molecular information cannot yet be disclosed. Nevertheless, the characterization of partially purified enzyme indicated the presence of more than one collagenase with different characteristics. Namely, a serine protease inhibited by PMSF and a metallo-protease inhibited by EDTA.
The enzyme mixture from the first purification using Ion Exchange Chromatography (DEAE 5ml) with NaCl (0-1M) gradient elution (Figure 1A) was not separated clearly. Another method to get better separation of enzyme fraction is stepwise salt elution (0%, 25%, 35%, 60%, 100% of NaCl (1M)) and 8CV (Column Volume) each step (Figure 3A). Direct sample injection was applied to the column. Around 65mL of protein samples were injected to get the high activity of collagenase fraction.
Figure 3 showed that at 35% of salt concentration collagenase fraction 40 and 50 appeared as different protein. But colagenase fraction 50 and 69 (at 60% salt concentration) were similar. This result implies that the percentage of salt needed to be within smaller range. The collagenases from the first stepwise purification (fraction 49 to 80) were pooled to purify in the second stepwise purification. Before purifying the enzyme, the samples were centrifuged around 5 minutes and 5000rpm until 2mL of collagenase was obtained. Collagenase from the first stepwise purification was purified again to second purification to get the best purified collagenase. Figure 4 shows that the collagenase has been well separated by using stepwise salt elution (0%, 25%, 30%, 40%, 50%, 60%, and Figure 2 Zymography analysis of enzyme from purification upon challenge
10
100% of NaCl). At the 40% salt concentration (Figure 4B) the collagenase was well separated. Maybe at this concentration salt can elute better the collagenase. Collagenase b7 (fraction 73 in Figure 3B and fraction 61 in Figure 4B) were separate from the b1-b6 fractions.
The latter fractions 37-41was designated as fraction D while fractions 61-78 were combined and designated as fraction F (Figure 4B). Henceforth, the collagenase samples used were addressed as the fraction D and F. Peptides hydrolysate from snakehead fish skin collagen was generated through enzymatic hydrolysis using collagenases from B. licheniformis F11.4, a mutant of B. licheniformis 11 from Indonesia (Waldeck et al., 2006). Although SDS PAGE and
zymography analysis were unable to clearly classify all collagenase fractions secreted, the inhibition study clearly shown the presence of serine-, metallo-, and neither of both types of proteases. The previous study using collagen from the skin of milkfish (Chanos chanos) indicated secretion of only two
metallo-(A)
(B)
11 collagenases with apparent molecular weight of 26 kDa and 124 kDa (Baehaki et al., 2012; Baehaki et al., 2014). This study could not yet exclude the presence of various oligomeric or even partial degradation forms of the enzymes. This issue would be solved in a peptide-mass finger printing analysis, which would be the
next experiments to do. In particular, species b3 to b6 demonstrated similar bioactivity during the collagenase assay in situ, during which all were identified as
neither serine nor metallo-protease. This finding is interesting because most of collagenases from Bacillus are characterized as metallo-protease (Baehaki et al., 2012; Baehaki et al., 2014; Liu et al., 2010), serine protease (Nagano and To, 1999), or Ca2+-dependent and with disulfide bonds collagenase (Wu et al., 2009). Also, the finding of species F is very interesting because the enzyme is significantly smaller than the reported collagenases from other strains of Bacillus, (A)
(B)
12
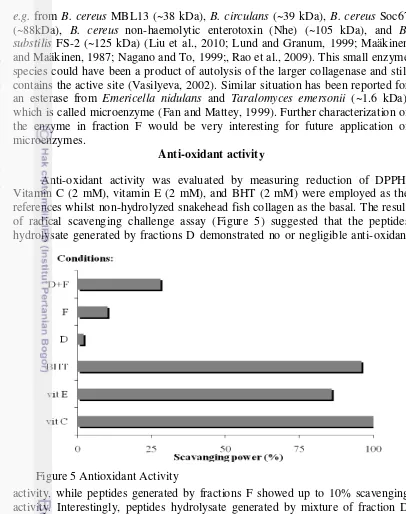
e.g. from B. cereus MBL13 (~38 kDa), B. circulans (~39 kDa), B. cereus Soc67 (~88kDa), B. cereus non-haemolytic enterotoxin (Nhe) (~105 kDa), and B. substilis FS-2 (~125 kDa) (Liu et al., 2010; Lund and Granum, 1999; Maäkinen
and Maäkinen, 1987; Nagano and To, 1999;, Rao et al., 2009). This small enzyme species could have been a product of autolysis of the larger collagenase and still contains the active site (Vasilyeva, 2002). Similar situation has been reported for an esterase from Emericella nidulans and Taralomyces emersonii (~1.6 kDa), which is called microenzyme (Fan and Mattey, 1999). Further characterization of the enzyme in fraction F would be very interesting for future application of microenzymes.
Anti-oxidant activity
Anti-oxidant activity was evaluated by measuring reduction of DPPH. Vitamin C (2 mM), vitamin E (2 mM), and BHT (2 mM) were employed as the references whilst non-hydrolyzed snakehead fish collagen as the basal. The result of radical scavenging challenge assay (Figure 5) suggested that the peptides hydrolysate generated by fractions D demonstrated no or negligible anti-oxidant
activity, while peptides generated by fractions F showed up to 10% scavenging activity. Interestingly, peptides hydrolysate generated by mixture of fraction D and F showed up to 20% scavenging activity. This suggests that components of the two enzyme fractions operate synergistically.
Anti-cholesterol activity
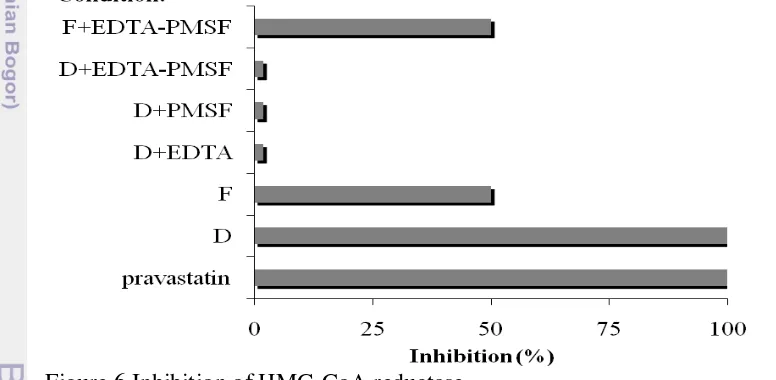
Anti-cholesterol potential of the peptides hydrolysate generated from the snakehead skin collagen by the collagenases of B. licheniformis F11.4 was evaluated by means of inhibition of the activity of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR) (0.5-0.7 mgP/ml) using the well-known anti-cholesterol drug pravastatin as the reference. Samples employed were collagen treated with enzymes of fraction D or F (in the presence and absence of 5 mM PMSF or 5 mM EDTA) and non-hydrolyzed snakehead skin collagen (for the
13 basal activity). The result is presented in Figure 6. Interestingly, peptides hydrolysate generated by fraction D displayed similar inhibition power of HMGR activity to that of pravastatin. Further, treatment of fraction D with PMSF and/or EDTA brought down the inhibition to the basal level. On the other hand, peptides produced by fraction F demonstrated only 50% inhibition to HMG-CoA activity in comparison to pravastatin. However, the activity of fraction F appeared to be sustained upon treatment by both EDTA and PMSF. Fraction D is enigmatic because inhibition of one or few of its enzyme components by EDTA and/or PMSF resulted in impaired bioactive peptides production. Corresponding to the characteristics of collagenases in fraction D, this result suggested that the bioactive peptides were produced by the mixture of serine and/or metallo-proteases, thus b1 and/or b2.
Fractions D and F were challenged to produce the snakehead fish skin collagen peptides hydrolysate for inhibition of HMGR activities and radical scavenging. HMGR is responsible for synthesis of mevalonate, and considered as the key enzyme for biosynthesis of cholesterol and other non-steroidal isoprenoid compounds (Arnaud et al., 2005). Therefore, controlling the HMGR activity may lead to control the synthesis of cholesterol. Peptides hydrolysate produced by both fractions D and F demonstrated full and 50% inhibition to HMGR activity, respectively. However, fraction D was unable to produce the peptides hydrolysate in the presence of EDTA and/or PMSF. On the other hands, fraction F still
hydrolyzed the collagen to produce bioactive peptides hydrolysate in the presence of the protease inhibitors. Combining the fractions D and F to produce bioactive peptides hydrolysate with anti-cholesterol functionality could be an intelligent option. This option may coincide with the finding of higher anti-oxidant activity of peptides hydrolysate recovered from hydrolysis of collagen by the combined fractions D and F. Thus, combined fractions D and F produced bioactive peptides hydrolysate with high anti-cholesterol activity accompanied by anti-oxidant activity, which leads to lower cholesterol level as well as prevention of radical formation in the blood. This circumstance would be ideal to combat development of cardiovascular diseases as functional food and drugs anti-CVD.
14
5
CONCLUSSION AND RECOMMENDATION
Conclusion
Secretion of collagenolytic enzyme from B. licheniformis F11.4 using acid soluble collagen from snakehead fish skin as the inducer resulted in mixture of collagenases with diverse characteristics. The enzymes were clustered into two groups so called the fraction D and F. The two enzyme fractions are capable to produce peptides hydrolysate that inhibits HMG-CoA activity and demonstrates anti-oxidant activity. Combined activity of the two fractions produced peptides hydrolysate to lower cholesterol and prevent radical formation, thus is potential candidate for an anti cardiovascular diseases drug.
Recommendation
This study recommends continuing analysis of anti-oxidant using other methods such as ferric reducing power anti-oxidant. Further the bioactivity study can be pursued to analyze peptide as anti-cancer, and anti-hypertension. Formulation of multi functional fish collagen as bioactive peptide hydrolysate drink will give positive contribution in food functional sector.
Acknowledgements
The research was supported by Ministry of Research and Technology, Republic of Indonesia, through funding incentive Sinas Research (Bioactive peptides as anti-hypertension and anti-cholesterol) and Dexa Laboratories of Biomolecular Sciences (DLBS). Thanks to Prof. F. Meinhardt from university of Münster and Dr. Siswa Setyahadi from BPPT for kindly providing the B. statins in inflammation, immunomodulation and atherosclerosis. Current drug target-cardiovascular & hematological disorder. Sharjah (UAE), Bentham Science Publisher 5: 127-134.
Asdornnithee S et al. 1994. Isolation and characterization of a collagenase from
Bacillus licheniformis N22. J Ferment Bioeng 78: 283–287.
Baehaki A, MT Suhartono, Sukarno, D Syah, AB Sitanggang, S Setyahadi and F Meinhardt. 2012. Purification and characterization of collagenase from
Bacillus licheniformis F11.4. African J Microbiol Res 6: 2373-2379.
15 Baie SH and KA Sheikh. 2000. The wound healing properties of Channa
striatus-cetrimide cream- tensile strength measurement. J Ethnopharmacol 71: 93-100. Bergmeyer HU, J Bergmeyer and M GraβI. 1983. Methods of Enzymatic Analysis
Vol. 2. Weinheim (GER): Verlag Chemie.
Borghi C, A Dormi, M Veronesi, V Immordino and E Amerosioni. 2002. Use of Lipid-lowering Drugs and Blood Pressure Control in Patient with Arterial Hypertension. J Clin Hypertens 4: 277-285.
Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.
AnalBiochem 72: 248-254.
Choi NS, JH Choi, BH Kim, YJ Han, JS Kim, SG Lee and JJ Song. 2000. Mixed-substrate (glycerol tributyrate and fibrin) zymography for simultaneous detection of lipolytic and proteolytic enzymes on a single gel. Electrophoresis
30: 2234-2237.
Cronlund AL and JH Woychik. 1987. Solubilization of collagen in restructured beef with collagenase and α-amylase. J Food Sci 52: 857-860.
Chung L, D Dinakarpandian, N Yoshida, GB Laurel-Frields, R Visse and H Nagase. 2004. Collagenase Unwinds Triple Helical Collagen Prior to Peptide bond Hydrolysis. EMBO J 23 : 3020-3030.
Fan X and M Mattey. 1999. Small enzymes with esterase activities from two thermophilic fungi, Emericella nidulans and Taralomyces emersonii.
Biotechnol let 21: 1071-1076.
Gálvez AF. 2012. Abstract 10693: Identification of lunasin as the active component in soy protein responsible for reducing LDL cholesterol and risk of cardiovascular disease. Circulation. 126: A10693.
Gálvez AF and BO de Lumen. 1999. A soybean cDNA encoding a chromatin binding peptide inhibits mitosis of mammalian cells. Nat Biotechnol 17: 495–
500.
Goshev I, A Gousterova, E Vasileva-Tonkova and P Nedkov. 2005. Characterization of the Enzyme Complexes Produced by Two Newly Isolated Thermophylic Actinomycete Strains during Growth on Collagen-rich Materials. Proc Biochem 40:1627-1631.
Harris EDJ and CA Vatar. 1982. Vertebrate collagenases. Methods Enzymol 82:
423-452.
Hoffmann K et al. 2010. Genetic improvement of Bacillus licheniformis strains for efficient deproteinization of shrimp shells and production of high-molecular-mass chitin and chitosan. Appl Environ Microbiol 76:8211-8221.
Indra D, K Ramalingam and M Babu. 2005. Isolation, Purification and Characterization of Collagenase from Hepatopancreas of the Land Snail Achatina fulica. ComparativeBiochemPhys 142:1-7.
Kanth SVR, R Venba, B Madhan, NK Chandrababu and S Sadulla. 2008. Studies on the influence of bacterial collagenase in leather dyeing. Dyes Pigments 76: 338-347.
16
Kim SK, PJ Park, JB Kim and F Shahidi. 2002. Purification and Characterization of a Collagenolytic Protease from the Filefish, Novoden modestrus J Biochem Molecular Biol 335: 165-171.
Kin T, PRV Johnson, AMJ Shapiro and JRT Lakey. 2007. Factors Influencing the Collagenase Digestion Phase of Human Islet isolation. Transplantation 83:
7-12.
Klock G, MB Kowalski, BJ Hering, ME Eiden and A Weidemann, et al, 1996. Fractions from Commercial Collagenase Preparations: Use in Enzymic Isolation of the Islets of Langerhans from Porcine Pancreas. Cell Transplant 5:
543-551.
Korhonen H and A Pihlanto-Leppala. 2003. Food-derived bioactive peptides: opportunities for designing future foods. CurrPharmDes 9: 1297-1308.
Korhonen H and A Pihlanto-Leppala. 2006. Bioactive peptides: Production and functionality. Int Dairy J 16: 945-960.
Kristjansson MM, S Gudmundsdóttir, JW Fox and JB Bjarnason. 1995. Characterization of Collagenolytic Serine Proteinase from the Atlantic Cod (Gadus morhua). ComparativeBiochemPhys 110: 707-717.
KullisaarT, E Songisepp, M Milkesaar, K Zilmer, T Vihalemm and M Zilmer. 2006. Antioxidative probiotic fermented goats milk decreases oxidative stress mediated atherogenicity in human subjects. Brit J Nutr 2: 449-456.
Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685.
Li B, F Chen, X Wang, B Ji and Y Wu. 2007. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem
102: 1135-1143.
Liu L, M Ma, Z Cai, X Yang and W Wang. 2010. Purification and properties of a collagenolytic protease produced by Bacillus cereus MBL13 strain. Food Technol Biotechnol 48:151–160.
Lund T and PE Granum. 1999. The 105-kDa protein component of Bacillus cereus non-haemolytic enterotoxin (Nhe) is a metallo-protease with gelatinolytic and collagenolytic activity. FEMS Microbiol Lett 178: 355–361. Maäkinen KK and PL Maäkinen. 1987. Purification and properties of an
extracelluar collagenolytic protease produced by the human oral bacterium
Bacillus cereus (strain Soc 67). J Biol Chem 262: 12488–12495.
Nagano H and KA To. 1999. Purification of collagenase and specificity of its related enzyme from Bacillus subtilis FS-2. Biosci Biotechnol Biochem 64:
181-183.
Ohyama H and K Hashimoto. 1977. Collagenase of human skin basal cell epithelioma. Biochemistry 82: 175-183.
Okamoto M et al. 2001. A thermostable collagenolytic protease with very large molecular mass produced by thermophlic Bacillus sp. strain MO-1. Appl Microb Biotech 57: 103–108.
17 Petrova DH, SA Shishkov and SS Vlahov. 2006. Novel thermostable serine collagenase from Thermoactinomyces sp. 21E: purification and some properties. Basic Microbiol 46:275-285
Queiroz JA, CT Tomaz and JMS Cabral. 2001. Hydrophobic Interaction Chromatography of Proteins. J Biotechnol 87: 143-159.
Rao CS, T Sathish, P Ravichandra and RS Prakasham. 2008. Characterization of thermo- and detergent stable serine protease from isolated Bacillus circulans
and evaluation of eco-friendly applications. Process Biochem 44: 262– 268. Riu R, L Zhiqiang, and C Min. 2009. Isolation and purification of caseinase and
collagenase from commercial Bacillus subtilis as1.398 enzyme by affinity
chromatography. J Soc Leather Tech Chem 93: 8-11.
Rival SG, CG Boeriu and HJ Wichers. 2001. Caseins and casein hydrolysates. 2. Antioxidative properties and relevance to lipoxygenase inhibition. J Agric FoodChem 49: 295-302.
Roy PC, Bernard and D Patrick. 1996. Purification, Kinetical and Molecular Characterizations of a Serine Collagenolytic Protease from Green Shore Crab (Carcinus maenas) Digestive Gland. Comparative Biochem Physiol 115:
87-95.
Sakurai Y et al. 2009. Purification and caharcterization of a mayor collagenase
from Streptomyces parvulus. Biosci Biotechnol Biochem 73: 21-28.
See SF, PK Hong, KL Ng, WM Wan Aida and AS Babji. 2010. Physicochemical properties of gelatins extracted from skins of different freshwater fish species.
Int Food Res J 17: 809-816.
Senevirathne M and SK Kim. 2012. Development of bioactive peptides from fish proteins and their health promoting ability. AdvFoodNutrRes 65: 235-248. Shah H. 2000. Effects of milk-derived Bioactives: an Overview. British J of characterisation of collagen extracted from the skin of striped catfish (Pangasianodon hypophthalmus). J Food Chem 124: 97-105.
Shmoilov AM, GN Rudenskaya, VA Isev, AV Baydakov and RD Zhantiev. 2006. A Comparative Study of Collagenase Complex and New Homogeneous Collagenase Preparations for Scar Treatment. J Drug Delivery Sci Technol 16: 285-292.
Stricklin GP, EA Bauer, JJ Jeffrey, and AZH Eisen. 1977. Human skin collagenase: isolation of precursor and active forms from both fibroblast and organ cultures. Biochem 16: 1607-1615.
Tyagi SC and JP Cleutjens. 1996. Myocardial Collagenase: Purification and Structural Characterization. Canadian J Cardiol 12: 165-171.
Tran IH and H Nagano. 2002. Isolation and characteristic of Bacillus subtilis
CN2 and its collagenase production. J Food Sci 67: 1184-1187.
Vasilyeva OV, KB Kolygo, YF Leonova, NA Potapenko and TV Ovchinnikova. 2002. Domain structure and ATP-induced conformational changes in
Escherichia coli protease Lon revealed by proteolysis and autolysis. FEBS Lett
18
Waldeck J, G Daum, B Bisping and F Meinhardt. 2006. Isolation and molecular characterization of chitinase-deficient Bacillus licheniformis strains capable of deproteinization of shrimp shell waste to obtain highly viscous chitin. Appl Environ Microbiol 72: 7879-7885.
Wu Q, C Li, H Chen and L Shuliang. 2010. Purification and characterization of a novel collagenase from Bacillus pumilus Col-J. ApplBiochem Biotechnol 160:
19 Appendix1 Reagents for Bradford Assay
1. Bradford Stock Solution
Bradford stock solution is made by mixing 0.35 g coomasie brilliant blue with 100 mL 95 % ethanol and 200 mL 88 % phosphoric acid.
2. Bradford Working Buffer
20
Appendix2 Composition of standard solution for Bradford assay
Volume of BSA solution
(μL) Volume of aquadest (μL) Protein concentration (ppm)
0 200 0
20 180 100
40 160 200
60 140 300
80 120 400
100 100 500
22
Appendix4 Reagents for SDS-PAGE analysis
1. A Solution (30 % w/v acrylamide, 0.8 % w/v bis-acrylamide)
A solution is made by dissolving 14.6 g acrylamide and 0.4 g bis-acrylamide with 50 mL aquabidest. It is stirred until homogen.
2. B Solution (buffer for separating gel, Tris-HCl 2 M, pH 8.8)
B solution is made by mixing 75 mL Tris-HCl pH 8.8 with 4 mL 10 % SDS solution (w/v). Aquabidest is added until the volume reach 100 mL.
3. C Solution (buffer for stacking gel, Tris-HCl 1 M, pH 6.8)
C solution is made by mixing 50 mL Tris-HCl pH 6.8 with 4 mL 10 % SDS solution (w/v). Aquabidest is added until the volume reach 100 mL.
4. Ammonium per sulphate 10 % (w/v)
0.1 g ammonium per sulphate is dissolved with 1 mL aquabidest. 5. Buffer for electrophoresis (pH 8.3)
Buffer for electrophoresis is made by dissolving 1.803 g tris, 8.648 g glysin, and 0.6 g SDS with 600 mL aquadest. Then, HCl 1 M is added to adjust the pH.
6. Buffer for samples
Buffer for samples is made by mixing 0.3 mL Tris-HCl 1 M pH 6.8 with 2.5 mL 50 % glycerol (v/v), 1 mL 10 % SDS (w/v), 0.25 mL β-mercaptoethanol, 0.5 mL 1 % bromphenol blue, and 0.45 mL aquabidest.
7. Staining solution
Staining solution is made by dissolving 0.5 g coomasie brilliant blue R-250 with 225 mL methanol, 50 mL acetic acid glacial, and 225 mL aquabidest. 8. Destaining solution
Destaining solution is made by mixing 225 mL methanol, 50 mL acetic acid glacial, and 225 mL aquabidest.
9. Sodium phosphate (0.1 M) contains 0.5 % SDS (w/v)
23 Appendix5 Composition of separating and stacking gels for SDS-PAGE and
Zymography analysis
Compound Separating Gel (µL) Stacking Gel(µ L)
10% 12% 4 %
A Solution 1670 2000 670
B Solution 1250 1250 -
C Solution - - 1250
Collagen 1000 - -
Aquadest 1100 175 3080
Ammonium per sulphate (APS)
100 100 50
24
AUTHOR BIOGRAPHY
Wenny Silvia Loren Br Sinaga was born on February, 14th 1987 in Jakarta. She is the Eldest daughter of Mr Robinson Sinaga and Mrs Rehulina Br Simbolon. Her parents have only two children, herself and a younger brother. The author started her study in TK METHODIST BERASTAGI, SD METHODIST BERASTAGI, SLTP N 1 BERASTAGI, SMA N 1 BERASTAGI, AND get her bachelor degree in Department of Agroindustrial Technology, Bogor Agricultural University. And now, she is continuing her master degree in Bogor Agricultural University in Food Science Depatement.
The author also joined several committee and student organization during her study. She is a member of AgriaSwara IPB since 2006 and Gita Swara Pasca Sarjana IPB (GSP IPB) since 2011. In GSP organization she takes a responsibility for being Coordinator of Coach Team and Talent and become an Event Organizer for the 1st GSP concert (Semarak Melody Khatulistiwa).
The author also following the PARE Spring Program was held on March 2015. And will presenting her research on 1st University Consortium Graduate Forum in Life Sciences, Food Science and Agriculture (UCGF) 2015(11 – 13 August 2015) in University of Putra Malaysia, Malaysia. She also submitted her manuscript titled “Peptides hydrolysate derived from collagen of snakehead murrel (Channa striata) skin demonstrate anti-cholesterol and anti-oxidant activities” to Journal of Functional Foods.