Studies on Rhizosphere-Bacteria mediated Biotic and Abiotic
stress tolerance in Chickpea (
Cicer arietinum
L.)
Ankita Sarkar, Jai Singh Patel1, Sudheer Yadav1, Birinchi K. Sarma*, Jai Singh Srivastava and Harikesh B. Singh
Received: 01 March 2013 / Revised: 29 Nov 2013 / Accepted: 09 Jan 2014 / Published online: 30 April 2014 This article is published with open access at www.vegetosindia.org
Abstract Rhizospheric bacteria promote plant health and combat with pathogenic microor-ganisms. Available reports indicate the activity of PGPR are in protection of plant under abi-otic stresses. In the present work we have compared the growth promotion and bio-chemical responses of plants influenced bybacteria isolated from the rhizosphere of different plants. Two Pseudomonas strains S1 (P. putida) and Cgr (P. aeruginosa) were isolat-ed from chickpea and congress grass, respec-tively, and their antimicrobial activity was test-ed against Sclerotinia sclerotiorum. Both strains are tested for HCN, IAA and ammonia production. Their surviving ability in salt stress was evaluated and compatibility test was per-formed. We have got some interesting results that plant defense enzymes and phenolic sub-stances were accumulated in higher concen-trations in plants that were treated with the two bacterial strains (Cgr and S1) either indi-vidually or in combination when challenged with biotic (Sclerotinia sclerotiorum) and abi-otic stress (NaCl salt stress) compared to the non-bacterized plants but exposed to biotic as well as abiotic stresses. These results indicates that the Cgr and S1 have potential to be used as biocontrol agents that can help chickpea to combat attack of S. sclerotiorum as well as thrive under salt stress. Moreover, the results also indicated a common pattern of defense response by chickpea against both the biotic and abiotic stress when they are bacterized by the two bacterial strains.
Keywords: Pseudomonas putida, Pseudomo-nas aeruginosa, chickpea, PAL activity, NaCl stress, Sclerotinia sclerotiorum
Introduction
Chickpea (Cicer arietinum L.)
produc-tion is plagued by various diseases of fungal, bacterial, viral and nematode origin and also very sensitive to abiotic stresses like salinity. Most of the susceptible genotypes die in 25 mM NaCl and the resistant genotypes also do not survive even in 100 mM NaCl under hy-droponics condition (Flowers et al. 2010). Scle-rotinia sclerotiorum (Lib.) de Bary is among the most devastating, nonspecific, omnivorous and cosmopolitan plant pathogen. Plants sus-ceptible to this pathogen encompass 64 fami-lies, 225 genera, and 361 species (Purdy 1979). The pathogen causes cottony rot, watery soft rot, stem rot, drop, crown rot, blossom blight and, perhaps most common, white mould (Bolton et al. 1990).
Fluorescent Pseudomonads are used as biocontrol agents in agricultural crops as they have very high adaptive potential (Lugtenberg et al. 2004, Jain et al. 2012). Pseu-domonas fluorescens has been reported to be an effective biocontrol agent against different fungal pathogens by various workers (Meyer
et al. 1992, Singh et al. 2006). Mixtures of fluo-rescent Pseudomonads were used by Pierson and Weller (1994) to suppress “Take All Dis-ease of Wheat” and improve the growth of wheat. Induced systemic resistance (ISR) in plants is a mechanism adopted by fluorescent Pseudomonads for plant disease management (Van Loon et al. 1998; Ramamoorthy et al.
2001). ISR may be effectively used as a strate-gy for biological control. In ISR the existing plant defense is only activated by several incit-ing agents, whereas in direct biological con-trol it relies completely on direct actions of the biocontrol agent like production of antibi-otics, siderophore, HCN as well as competition for nutrients and space. ISR activates multiple defense mechanisms like synthesis and
activa-Department of Mycology and Plant Pathology, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi 221 005 India
1Department of Botany, Faculty of Science, Banaras Hindu University, Varanasi 221005
*Corresponding author E-mail: birinchi_ks@yahoo.com
159 tion of Chitinase, β 1,3- Glucanases,
Peroxidas-es, other PR proteins (Lawton and Lamb., 1987), synthesis and accumulation of phytoa-lexins (Kuć and Rush 1985), formation and deposition of lignin, callose and other Hydrox-yproline rich glycoproteins (Hammerschmidt and Kuć 1979). ISR in plants is triggered by strains of P. fluorescens which belong to plant growth-promoting rhizobacteria (PGPR) (Van Peer et al. 1991, Chen et al. 2000). ISR is acti-vated against several fungal, bacterial and vi-ral diseases (Liu et al., 1995; Maurhofer et al.
1998). Systemic suppression of soil born path-ogen Pythium aphanidermatum in cucumber root was obtained by use of Pseudomonas corrugata strain B and Pseudomonas aureofa-ciens strain 63-28 (Chen et al. 2000). Two strains of Pseudomonas fluorescens Pf1 and FP7 were found to give maximum inhibition of
Rhizoctonia solani and enhanced the vigour of seedlings in vitro conditions (Vidyasekaran and Muthamilan 1999). Mixing of two or more strains of Pseudomonas sp. helped in increas-ing their biocontrol efficacy and PGPR activity (Pierson and Weller. 1994, Singh et al. 1999). An isolate of P. fluorescens Pf1 was used to treat seeds of mungbean and the treated seeds when challenge inoculated with Mac-rophomina phaseolina under glass house con-ditions, increased accumulation of phenylala-nine ammonia lyase (PAL), peroxidase (POx), polyphenol oxidase (PPO), chitinase and β
-1-3-glucanase were observed (Saravanakumar et al. 2007). Lipopeptide was found to be essen-tial for biological control of S. sclerotiorum by
Pseudomonas sp. strain DF41 (Berry et al.
2010). Pseudomonas chlororaphis PA-23, Ba-cillus amyloliquefaciens BS6, Pseudomonas sp. DF41 and Bacillus amyloliquefaciens E16 showed antagonistic activity in vitro against S. sclerotiorum. Double application of PA-23 on canola followed by challenge inoculation with
S. sclerotiorum increased levels of the hydro-lytic enzymes chitinase, β-1,3-glucanase and PR-3 in canola (Fernando et al. 2007).
The first committed step of phenylpro-panoid synthesis is catalyzed by phenylalanine ammonia lyase (PAL) (Jones 1984) and this is the rate limiting enzyme in production of phe-nyl propanoid compounds (Bate et al. 1994). PAL is involved in phytoalexin or phenolics biosynthesis and hence this enzymes has been correlated with defense against pathogens (Binutu and Cordell 2000). Activity of PAL is stimulated by microbial infections and this
leads to synthesis of lignin like wall bound phenolic materials and phenyl propanoid de-rived phytoalexin antibiotics (Jones 1984). Bio-tic and AbioBio-tic stress induces synthesis of phenylpropanoid compounds in plants and these phenyl propanoid compounds do play an important role in plant defense (Dixon and Paiva 1995). An important first line in plant defense against infection is provided by the very rapid synthesis of phenolics and their polymerization in the cell wall. Plant second-ary metabolites especially phenolic com-pounds play a role in mechanisms of plant resistance (Friend 1977). A possible control of
Sclerotium rolfsii infection by enhancing the levels of phenolic compounds in host tissues was suggested by Punja (1985). Many plant phenols possess antimicrobial activities. Phe-nols with antimicrobial activities have the abil-ity to denature proteins and are classified as surface active agents (Sousa 2006). Phenols have the capacity to change the composition of microflora in any environment in which the-se compounds are applied and/or induced in a proper kind and concentration (Puupponen-Pimiä et al. 2001). Production of phenolics with antimicrobial activities gives rise to re-sistance (Luzzatto et al. 2007). Phenols play role mainly in plant protection by contributing to structural integrity, photosynthesis and nu-trient uptake among other functions in vascu-lar plants.
Soil salinity in arid regions is frequent-ly an important limiting factor for cultivating agricultural crops. Nowadays special interest is put on research directed towards develop-ment of inoculants with beneficial bacteria (PGPR) for environmentally friendly agricultur-al management. The main purpose of these biofertilizers is to stimulate the plant’s defen-sive metabolism that will allow crop cultiva-tion in areas with a high pathogen incidence, or on degraded or saline soils or soils subject to water restriction conditions. Reclamation of soils by the use of arbuscular-mycorrhizal fun-gus, and/or PGPR such as Azospirillum, Agro-bacterium, Pseudomonas and several Bacillus
musk et al. 1999). Thus it may be said that plants inoculated with proper biological agent may also show increased adaptability to salt stress and drought stress situations (Timmusk
et al. 1999). Some PGPR elicit Induced System-ic Resistance (ISR) and this ISR elSystem-icited by PGPR suppresses disease resistance in both green house and field conditions (Kloepper et al. 2004). Use of mixtures of PGPR strains were also tried under glass house and field condi-tions to study the alleviation of abiotic stress in plants by these strains. Lettuce was co-inoculated with PGPR (Pseudomonas mendo-cina) and arbuscular mycorrhizal fungi (Glomus intraradicus or Glomus mosseae), an increase in catalase activity under severe drought conditions was observed, thus sug-gesting that these strains may be used as in-oculants to alleviate the oxidative damage elicited by drought (Kohler et al. 2008).
Materials and Methods
T w o b a c t e r i a l c u l t u r e s S 1 (Pseudomonas putida) and Cgr (Pseudomonas aeruginosa) were isolated and used in the ex-periment. The pathogen Sclerotinia scleroti-orum was also isolated from infected chickpea plants and used in the experiment. Chickpea seeds of the cv. ‘Avrodhi’ were used for the
experiments.
Isolation of Pseudomonas
Pseudomonas strain Cgr was isolated from the rhizospheric soil of Congress Grass (Parthenium sp.) growing around the Agricul-tural Research Farm of Banaras Hindu Univer-sity. Pour plate technique was used to isolate bacteria from the suspensions. One ml of sus-pension from each dilution of 10-4, 10-5 and 10 -6 was poured in Petri plates separately and
the plates were then transferred with 20 ml of King’s B (KB) agar (King et al., 1954). The plates were incubated at 28 ± 1 0C for two days for development of colonies. S1 was iso-lated in a similar way from chickpea rhizo-sphere.
When visible colonies appeared, single colonies were carefully picked up by an inocu-lation loop and streaked in another previously poured plate containing King’s B medium for isolation of single cells. These plates were fur-ther incubated in an incubator at 28±1 0C. Colonies developed from single cells were fi-nally picked up and streaked on slants of King’s B Medium for further experimentation. Isolation of Sclerotinia sclerotiorum (Lib.) de Bary
The pathogen was isolated by picking up individual sclerotia from infected chickpea plants. The sclerotia were surface sterilized with 0.1% HgCl2 and sterilized sclerotia were then placed in plates of Potato Dextrose Agar medium (PDA). These plates were incubated at 25±2 0C for a few days till the mycelia grew actively. The cultures were purified by placing mycelia blocks in PDA slants taken from the growing edges of the growing culture.
Characterization of the Pseudomonas strains
Phosphate solubilization
Phosphate solubilisation test of the
Pseudomonas strains was done by using the NBRI-BPB medium (Mehta and Nautiyal 2001).
Cyanogenesis or HCN production
Table 1. Growth of Pseudomonas aeruginosa Cgr and Pseudomonas putida S1 at different NaCl concentra-tions
Salt Concentrations Cfu/ml
S1 Cgr
Control (Broth with-out NaCl)
1.7 × 1010 1.9 × 1012
2% (0.34 M) NaCl 8.6 × 108 4.95 ×1010 3% (0.51 M) NaCl 2.2 × 108 4.2 × 1011 4% (0.68 M) NaCl 4.8 × 107 4.76 ×1011 5% (0.85 M) NaCl 1.2 × 107 4.66 × 109 6% (1.02 M) NaCl 1.5 × 106 6 × 107
7% (1.2 M) NaCl - 2.8 × 102
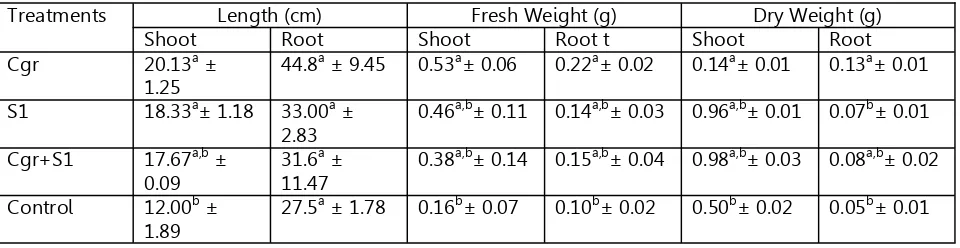
Table 2. Growth parameters of chickpea treated with two Pseudomonas strains S1 and Cgr
Treatments Length (cm) Fresh Weight (g) Dry Weight (g)
Shoot Root Shoot Root t Shoot Root
Cgr 20.13a ±
1.25
44.8a ± 9.45 0.53a± 0.06 0.22a± 0.02 0.14a± 0.01 0.13a± 0.01
S1 18.33a± 1.18 33.00a ± 2.83
0.46a,b± 0.11 0.14a,b± 0.03 0.96a,b± 0.01 0.07b± 0.01
Cgr+S1 17.67a,b ± 0.09
31.6a ± 11.47
0.38a,b± 0.14 0.15a,b± 0.04 0.98a,b± 0.03 0.08a,b± 0.02
Control 12.00b ± 1.89
161
Table 3. PAL activity, total phenol accumulation and polyphenol oxidase activities in chickpea treated with two Pseudomonas strains S1 and Cgr
Treatments† PAL activity (µg TCA g
-1 fresh weight of leaves)
Total Phenol (mg per g fresh weight of leaves)
PPO activity (change in absorbance per mi-nute per micro gram of protein)
24 h 48 h 72 h 24 h 48 h 72 h 24 h 48 h 72 h
Cgr 22.1b,c ± 0.06 50.2f ± 0.12 101.5b,c±0.03 3.2a ± 0.09 2.7g,h ± 0.01 2.5c,d ± 0.04 0.2l ± 0.03 3.9l ± 0.05 4.5h ± 0.01
S1 20.1b,c ± 0.05 39.2g,h ± 0.04 82.6d,e ± 0.04 1.1m ± 0.00 1.5j ± 0.01 1.1j ± 0.15 0.5k ± 0.07 3.7l ± 0.03 8.6h ± 0.04
Cgr+S1 17.7b,c,d±0.04 43.6g ± 0.09 87.4d ± 0.12 2.3f,g ± 0.01 2.9a ± 0.01 1.6h ± 0.02 1.1i,j ± 0.01 2.2n ± 0.02 23.3g ± 0.13
Cgr+P 9.8f,g ± 0.05 39.5g,h ± 0.01 80.8d,e ± 0.04 2.2g ± 0.01 2.9m ± 0.04 2.3e,d ± 0.01 1.1j ± 0.04 3.7l ± 0.01 5.8h ± 0.32
S1+P 15.6c,d,e,f±0.06 34.7k,l ± 0.09 98.0b,c ± 0.15 2.3e,f ± 0.04 2.5l ± 0.01 2.1f ± 0.01 3.9g ± 0.01 4.2l ± 0.01 21.1g ± 0.07
Cgr+S1+P 10.6e,f,g ±0.05 80.2b ± 0.12 96.5c ± 0.08 2.4e,d ± 0.02 2.6k ± 0.01 2.5c,d ± 0.01 9.4c ± 0.09 9.7i ± 0.02 46.6f ± 0.04
Cgr+N50 12.8d,e,f,g±0.07 31.8i,j ± 0.34 69.8g ± 0.06 1.4l ± 0.01 1.5g ± 0.04 1.8h ± 0.03 2.7h± 0.08 3.7l ± 0.01 48.0f ± 0.05
Cgr+N100 15.7c,d,e,f±0.03 79.6b,c ± 0.06 82.3d,e ± 0.13 1.3m ± 0.01 2.1e ± 0.01 2.9b ± 0.02 8.3e ± 0.05 14.6g ± 0.01 61.3e ± 0.01
Cgr+N150 20.8b,c ± 0.05 71.7d ± 0.04 86.6d ± 0.06 1.7j,k ± 0.02 2.4c ± 0.01 3.1a ± 0.01 9.0d ± 0.00 28.0d ± 0.09 77.6b,c ± 0.05
S1+N50 21.1b,c ± 0.06 27.7j,k ± 0.02 101.0b,c±0.09 2.8c ± 0.01 1.6i ±0.01 2.3e ± 0.01 4.0g ± 0.02 11.7h ± 0.03 51.3f ± 0.08
S1+N100 35.2a ± 0.04 55.1e ± 0.09 84.1d,e ± 0.35 2.8c ± 0.01 1.9f ± 0.03 2.5c ± 0.06 8.3e ± 0.23 22.7f ± 0.07 65.3d,e ± 0.04
S1+N150 32.6a ± 0.01 56.9e ± 0.01 103.9b± 0.08 3.1b ± 0.30 1.6i ± 0.02 2.4e,d,c ± 0.06 9.0d ± 0.07 25.0e ± 0.08 72.3c,d ± 0.12
Cgr+S1+N50 8.9f,g ± 0.24 53.0e,f ± 0.07 73.6f,g ± 0.05 1.8h,i ± 0.03 2.0f ± 0.01 1.0j ± 0.01 9.0d ± 0.01 31.3c ± 0.01 84.7b ± 0.30
Cgr+S1+N100 17.43b,c,d
±0.13
75.2c,d ± 0.05 110.4a ± 0.14 2.2f,g ± 0.01 2.3d ± 0.01 2.0f ± 0.01 10.7b ± 0.01 38.0b ± 0.09 85.00b ± 0.40
Cgr+S1+N150 32.2a ± 0.01 86.6a ± 0.12 116.5a ± 0.02 2.7c ± 0.16 2.8b ± 0.01 2.5b ± 0.18 12.6a ± 0.07 44.3a ± 0.10 99.7a ± 0.09
N50 23.9b ± 0.04 39.2g,h ± 0.03 34.7i ±0.06 1.7i,j ± 0.01 1.5j ± 0.05 1.2i ± 0.03 4.0g ± 0.02 5.3k ± 0.09 06.7h ± 0.01
N100 20.2b,c ± 0.06 38.8g,h ± 0.01 46.0h ± 0.17 2.1g ± 0.01 1.7h ± 0.01 1.8g ± 0.06 6.0f ± 0.01 6.7j ± 0.01 08.3h ± 0.02
N150 7.8g ± 0.12 35.8h,i ± 0.02 78.5e,f ± 0.02 1.9h ± 0.02 1.1l ± 0.01 1.8g± 0.03 4.0g ± 0.08 7.0j ± 0.08 7.6h ± 0.03
P 11.8d,e,f,g±0.12 79.1b,c ± 0.05 72.3f,g ± 0.18 1.5d ± 0.01 1.5j ±0.01 1.4h±0.01 1.3i ± 0.04 3.0m ± 0.02 8.0h ± 0.05
Control 11.8d,e,f,g±0.13 21.2l ± 0.12 12.8j ± 0.01 1.1k ± 0.03 1.0g ± 0.02 1.1f± 0.01 0.2l ± 0.01 00.2o ± 0.06 00.3h ± 0.05
†P = Sclerotinia sclerotiorum; N = NaCl
S
tu
d
ie
s o
n
R
h
iz
o
sp
h
er
e
-B
a
ct
er
ia
m
ed
ia
te
d
B
io
tic
a
n
d
A
b
io
tic
s
tr
es
s
to
ler
a
n
ce
in
C
h
ic
kp
ea
Bacterilal isolates were screened for production of HCN by using the protocol pro-posed by Baker and Schipper (1987). King’s B medium amended with Glycine (4.4 g/L) was prepared and sterilized. About 25ml of the prepared medium was poured in the plates sterilized previously. The poured medium was allowed to solidify and then streaking with the bacterial isolate was done. Streaking was done with an inoculating needle in a laminar air flow. Single strains were streaked in each plate. Whatman No. 1 filter papers were cut according to the size of the Petri plate, steri-lized in an autoclave and dipped in freshly prepared solution of 0.5% picric acid in 2% Na2CO3. After soaking the filter papers were attached to lid of Perti plates containing the streaked cultures. Control plates contained the amended medium as well as soaked filter papers attached with the lid of Petri plates without inoculation with the bacterial cultures. The plates were sealed with paraffin and incu-bated in an incubator at 28 ± 1 0C for 3 to 4 days.
Bacterial isolates were tested for pro-duction of Ammonia by using the protocol proposed by Dye (1962). For testing the pro-duction of Indole Acetic Acid bacterial isolate was grown in broth of Luria Bertani for 48 h, there after the culture was centrifuged at 3000 rpm for 30 minutes. Supernatant was collect-ed, 2 ml of supernatant two drops of ortho-phosphoric acid and 4 ml of Salkowski rea-gent (50 ml, 35% per chloric acid and 1 ml of 0.5 M FeCl3 solution) was added. Antagonistic potential of the bacterial strains were tested against the pathogen S. sclerotiorum, a soil borne pathogen, by using the dual culture technique. The two strains were checked for compatibility by spot inoculating them sepa-rately in two plates followed by spraying with the other bacterial strain grown in broth. First, Cgr was spot inoculated in centre followed by spraying with S1 grown in broth , next in se-cond Petri plate S1 was spot inoculated fol-lowed by spraying with Cgr grown in broth.
The PAL assay was carried out accord-ing to the method described by Ross and Se-deroff (1992). Polyphenol oxidase activity was determined following Mayer et al. (2001). To-tal Phenolic Content (TPC) was assayed ac-cording to Sarma et al. (2002).
Identification of strains Cgr and S1
16S rDNA sequence analysis was done
for identification of the strains of fluorescent Pseudomonads.
Results
Identification of bacterial strains
Sequencing of 16S rDNA region con-firmed the identity of S1 as Pseudomonas putida (GenBank Accession: JN020962) and Cgr as Pseudomonas aeruginosa (JN128893). Evaluation of bacterial isolates for salt tolerance
Both the strains Pseudomonas aeru-ginosa Cgr and Pseudomonas putida S1 were able to tolerate high concentrations of salt and showed growth upto 6% (1.02 M) NaCl concentration in King’s B broth. The visible turbidity of the bacterial growth in broths amended with different concentrations of NaCl was decreased with increase in the con-centration of the salt (Plate: K) and (Plate: L). Turbidity in broths inoculated by Cgr and S1 and amended by different concentrations of salt was clearly visible and distinguishable from the control up to 5% (0.85 M). However, at 6% (1.02 M) turbidity was visible but clearly indistinguishable from the control tubes. At 7% (1.2 M) salt concentration no such turbidity was visible in medium, baring just a thin (almost negligible) biofilm in the top most layer. Colony counting of the two strains in salt amended broths showed gradual decline in cfu/ml of broth and the strain S1 failed to grow at 7% NaCl. However, growth of Cgr was recorded at 7% NaCl but significantly less cfu/ml was obtained (Table 1).
Compatibility of the strains
The two strains were found to be fully compatible with one another as indicated by absence of any zone of demarcation between the two strains and the two strains were found to grow comfortably with one another.
Plant growth promotion activities
In presence of the bacterial strains in-dividually or in combination, change observed in most of the growth parameters. Maximum root length, shoot length, root fresh weight, shoot fresh weight, root dry weight and shoot dry weight was obtained in the plants treated with the strain Cgr. The results are summa-rized below:
Root Length: Cgr> S1> Cgr+S1> Control Shoot Length: Cgr> S1> Cgr+S1> Control
163 Statistical analysis using the data from
the CRD experiments subjected to Duncan’s Multiple Range Test (DMRT) shows that growth of root and shoot was highest in Cgr treated plants followed by the combination of both Cgr and S1, S1 alone and control. How-ever in case of shoot growth despite the high-est growth was recorded in Cgr treated plants the next best growth was obtained in S1 treat-ed plants compartreat-ed to their combination. This is in contrast to the results of the root growth characteristics (Table 2).
Biochemical defense responsesPAL activity A higher induction of PAL was ob-served in plants treated with the two strains Cgr and S1 individually as well as in combina-tion (Cgr+S1) compared to control plants without any salt stress. The amount of PAL in-duced was found to increase with time. After 48 h, induction of PAL was maximum in plants treated with both the strains and challenge inoculated with the pathogen S. sclerotiorum
(Cgr+S1+P) compared to plants treated with the strains (Cgr and S1) individually and chal-lenge inoculated with the pathogen (Cgr+P and S1+P). However, increment in level of PAL was observed till 72 h in all the plants that were treated with the two strains, either alone or in combination and this trend was observed irrespective of the fact that the treated plants were exposed to the pathogen or not. An equal amount of PAL activity was observed in Cgr treated as well as in the bacterial combi-nation challenged against the pathogen. How-ever in control plants that were not treated with either of the strains but challenge inocu-lated with the pathogen (C+P), PAL activity was found to be very low when compared to plants that were treated with the strains (alone or in combination and challenged with the pathogen) (Table 3).
Salt stress also induced PAL activity in all the treatments irrespective of the applica-tion of the bacterial strains till 72 h in most of the treatments. However, the level of PAL in-duced in bacterized plants (Cgr, S1 or Cgr+S1) was much more compared to that of the non-treated plants under salt stress and control. PAL activity increased with salt concentrations (50, 100 and 150mM) and time and recorded highest activity at 72 h. The PAL activity was higher in the salt stressed plants treated with both the strains compared to their application in non-stressed plants. However, highest PAL activity was recorded in the salt stressed
plants (100 and 150 mM) that were treated with both the bacterial strains. Between the two strains S1 was more potent than Cgr in inducing PAL activity in the salt stressed chick-pea plants when they were applied alone. Higher PAL activity in the plants under salt stress and treated with both the strains thus shows the synergistic activity of the two bac-terial strains.
Total Phenol Content (TPC)
TPC was increased in all the treatments compared to control plants not subjected to any stress. However, the plants subjected to both the salt and pathogen stress without bacterizing the seeds showed little increase in TPC especially after 24 h. The increased TPC was declined after 24 h. Similarly, the plants treated with the bacterial strains have also showed highest TPC in 24h and declined thereafter. Between the two strains Cgr in-duced more TPC than S1. But when the seed bacterized plants were challenged with the pathogen in all the treatments involved seed bacterization the TPC was highest at 48 h and its content reduced at 72 h. Cgr when applied alone induced TPC maximum than S1 or in combination with S1. However, the sustained effect of the combination was better than their individual effect as its content was highest at 72 h among the three treatments (Cgr, S1 and Cgr+S1) (Table 4). When the plants were ex-posed to salt stress they showed a different trend compared to the plants exposed to bio-tic stress. When the plants were subjected to only salt stress in absence of the bacterial strains highest TPC was observed at 24 h which then declined thereafter. However, the TPC content was higher than the unstressed control plants showing short duration accu-mulation of TPC under salt stress condition. A similar trend was observed in the plants raised from seed bacterization with S1 and subjected to salt stress. But the amount of TPC was sig-nificantly high than the non bacterized plants. The TPC increased with increase in salt con-centration and was highest with the highest salt concentration (150 mM). In contrast, high-est TPC was observed at 48 h in the plants treated with Cgr and subjected to salt stress. The TPC was then declined at 72 h. The results thus showed a role of the bacterial strains in inducing TPC in higher quantity under salt stress conditions (Table 3).
Polyphenol oxidase (PPO) activity
the treatments increased in the pathogen and salt stressed plants treated with the bacterial strains either single or in combination. All the treatments showed a gradual increase in PPO activity and recorded its highest activity at 72 h. The PPO activity in general was relatively higher in salt stressed plants treated with the bacterial strains compared to the pathogen stressed plants treated with the bacterial strains whether singly or in combination. How-ever, the PPO activity was recorded highest in the treatments where the bacterial strains were applied in combination and the plants were subjected to salt stress compared to their single application. The PPO activity in-creased with the increase in salt concentration thereby showing the active role of the mi-crobes under salt stress conditions (Table 3).
Discussion Biotic stress
The bacterial strains used in the pre-sent investigation have shown the potentiality to induce defense responses in chickpea against both S. sclerotiorum and NaCl stress. The plants under both the stress conditions have shown higher defense activities especial-ly when the strains were applied in combina-tion. Assay of defense related enzymes and phenol was done in Pseudomonas fluorescens
1-94 bacterized chickpea plants by Saikia et al.
(2004) after 24 h of challenge inoculation with the pathogen Fusarium oxysporium f.sp. ciceri. The plants were then analysed for phenol, PAL and PR proteins and it was observed that at least 1 day was needed after inoculation for induction of resistance response. In the pre-sent investigation it was observed that a steady and gradual increase in PAL activity was observed up to 3 days. The concentration of PAL was more in plants that were bacter-ized and either inoculated or not inoculated with pathogen, as compared to the control ones. These observations were in agreement with the results of PAL activity in the experi-ment conducted by Meena et al. (2000) where they used Pseudomonas fluorescens for bac-terization of groundnut seeds followed by challenge inoculation with Cercosporidium personatum. The activity of PAL was much more in plants treated with the Pseudomonas
species and then challenge inoculated com-pared to the untreated controls. In the present investigation, in case of Cgr the concentration of PAL was more in plants that were
bacter-ized but not challenge inoculated compared to the plants that were bacterized and then challenge inoculated with the pathogen. A similar phenomenon was observed with S1 as well and the results thus indicate a strong
Pseudomonas-Sclerotinia interaction during this period and therefore the host elicitation of PAL activity was relatively decreased. How-ever, the PAL activity was still higher com-pared to the non-bacterized plants challenged with the pathogen. It shows the role of the bacterial strain in the elicitation of PAL activi-ty. A similar phenomenon was reported by Ramamoorthy et al. (2002) while working with the P. fluorescens strain Pf1 used to treat to-mato plants against the pathogen Fusarium osysporum f.sp. lycopersici. They observed highest activity of PAL on 4th day whereas in the control plants its activity declined after 2nd day. Another similar observation was also re-ported by Nakkeeran et al. (2006). They found that when hot pepper seedlings were treated with Pseudomonas chlororaphsis PA-23 and then challenge inoculated with Pythium apha-nidermatum an increase in activity of PAL was observed reaching maximum in 12 days and declining thereafter whereas in control plants activity of PAL was found to increase up to 4 days and declined thereafter. Moreover, its activity was much lower as compared to that of PA-23 treated plants. In another finding re-ported by Sangeetha et al. (2010) showed that when three beneficial rhizospheric bacteria, viz., non-fluorescent Pseudomonas (NFP6),
Pseudomonas fluorescens (Pf3a) and Bacillus subtilis along with Azospirillum (AS1) and
Azotobacter (AZ1) were used in banana and challenge inoculated with the pathogen La-siodiplodia theobromae and Colletotrichum musae, there was increase in defense related enzymes up to 4 fold compared to that of control. The defense related enzymes had in-creased from 1st day onwards and reached a peak on 5th day after treatment.
165 gen inoculated treatments. In contrast, the
single application of bacterized plants without pathogen challenge showed highest TPC at 24 h which then declined gradually thereafter. Raamamoorthy et al. (2002) while working with Pseudomonas fluorescens Pf1 in tomato plants showed that elicitation of the phe-nylpropanoid metabolites takes place after the first day of challenge inoculation with the pathogen Fusarium oxysporum f. sp. lycoper-sici and their concentration reached peak lev-els on 5th day. These results are in conformity with the results of the present investigation showing an appropriate role of the Pseudo-monas species in triggering the phenylpro-panoid biosynthesis in plants under patho-genic stress. Similar accumulation of the phe-nylpropanoid metabolites over a period of time in the plants under pathogen challenge were also reported by several other workers (Sangeetha et al. 2010, Karthikeyan et al. 2005, Nakkeeran et al. 2006). Singh et al. (2002) and Sarma et al. (2002) also reported higher accu-mulation of TPC in pea against Erysiphe pisi
and chickpea against Sclerotium rolfsii infec-tion, respectively after the plants were bacter-ized by fluorescent Pseudomonas species. PGPR-mediated ISR induction was also report-ed by several workers earlier (Bakker and Schipper 1987, Singh et al. 2003).
In case of Polyphenoloxidase (PPO) activity in the plants treated with the bacterial species and challenged with the pathogen, it was observed that a there was a consistent and steady increase in levels of PPO up to 3 days. The accumulation of PPO was more in plants that were bacterized and challenge in-oculated with the pathogen compared to those that were bacterized but not challenge inoculated. Increase of PPO was also reported in gladiolus plants treated with rhizobacterial strains (S2B C Bacillus atrop haesus, S2BC2+TEPF- SUNGAL, Burlkholderia cepacia). When the bacterized plants were challenged inoculated with the pathogen Fusarium ox-ysporium f. sp. gladioli, it was observed that induction of PPO was more in treated and challenge inoculated plants compared to the control plants (Shanmugam et al. 2011). Simi-lar, findings were also demonstrated by Rama-moorthty et al. (2002a). They reported that pretreatment of tomato and hot pepper seed-lings with P. fluorescens Pf1 and challenge inoculation with the pathogen showed rapid and higher activity of PPO compared to the
non-inoculated ones. In the present investiga-tion plants that were not bacterized but chal-lenge inoculated with the pathogen also showed PPO activity but the activity was much lesser in those cases. Least PPO activity was observed in plants that neither bacterized nor challenge inoculated. Activity of PPO was highest in the plants where the strainal mix-ture was applied and subjected to the patho-gen challenge demonstrating the synergistic activity of the bacterial strains in inducing the defense response. PGPR-mediated increase PPO activity was also reported by Nakkeeran
et al. (2006) in hot pepper and in banana by Sangeetha et al. (2010).
Abiotic stress
Salinity stress caused increase in PAL activity in chickpea plants at varied levels. PAL activity was gradually and constantly in-creased up to 72 h in all the treatments com-prising the bacterial strains and maximum ac-tivity was observed in plants treated with the mixture of the bacterial strains and exposed to high salinity stress. Higher PAL activity in the mixed bacterial inoculation compared to their single application shows the synergistic ability of the bacterial strains in inducing the defense response against the salinity stress. PAL is rec-ognized as an indicator of environmental stresses in plants (MacDonald and D Cunha 2007). According to them Jatropha seedlings when exposed to different salinity levels (0, 50, 100, 150 and 200 mM) an increment in PAL activity with increment in salinity levels was observed. They proposed that the increased PAL activity may be a response to increased cellular damage due to high salinity stress and changes in PAL activity thus reflects a role of the enzyme in helping plants to respond to-wards salt stress. Gholizadeh and Kohnehrouz (2010) while working with two inbreds of maize (A-180 and A-619) reported that when the inbreds were first exposed to salinity stress and thereafter removal of the salt stress caused activation of various defense respons-es including PAL. Analysis of PAL activity sug-gested that phenylpropanoid compounds syn-thesized by Phenylpropanoid pathway may be a component of key importance in salt in-duced maize antioxidative system. One of the early responses of plants to various stresses is the production of Reactive Oxygen Species (ROS) (Alscher et al. 1997, Hernandez et al.
mediate the salt effect on plants cells (Asada 1994; Gossett et al., 1994). Effective removal of ROS in salt tolerant plants is done by using an efficient antioxidative system (Rout and Shaw 2001, Saleh and Plieth 2009). Increase in PAL activity with increase in salt stress in the pre-sent investigation has also showed a similar trend with the trend of total antioxidation pat-tern in maize plants (Gholizadeh and Kohnehrouz 2010).
P G P R s t r a i n s h a v i n g 1 -aminocyclopropane-1-carboxylate (ACC) de-aminase activity were reported to alleviate salt stress in tomato plants and increased plant growth under salt stress conditions (Mayak et al. 2004). Bano and Fatima (2009) used Rhizo-bium and Pseudomonas species to treat two cultivars of maize for alleviation of salt stress. They found that co-inoculation of the bacteri-al strains helped maize plants to adapt in a better way under salinity stress. The rhizobac-terial strains also increased the osmotic po-tential of leaves under stress condition com-pared to that of control. Co-inoculation of
Pseudomonas and Rhizobium was also found to significantly increase the proline content of leaves as compared to untreated control plants under salt stress. Rhizobium was found to perform better than Pseudomonas under unstressed conditions, but under salt stress
Pseudomonas was found to perform better in terms of stimulation of growth and biochemi-cal content of leaves. A similar effect was also seen in the present investigation where the co -inoculation of the bacterial strains (S1 and Cgr) induced more defense responses than their single treatments.
TPC was increased in plants subjected to salt stress irrespective of the plants treated with the bacterial strains. However, TPC con-tent in seed bacterized plants was more com-pared to non-bacterized plants. An interesting observation was noted in the TPC in the pre-sent investigation. The plants when treated with the bacterial strain S1 and exposed to salt stress accumulated highest phenolic con-tent in 24 h compared to the plants treated with Cgr showed highest accumulation in 72 h. However, in contrast to these observations the TPC in co-inoculation of the bacterial strains was highest in 48 h and the amount was less compared to the TPC content in S1 treated plants at 24 h or Cgr treated plants at 72 h. This may be due to difference in the mechanism of elicitation of TPC by the
bacte-rial strains under abiotic stress conditions. Phenols are reported to accumulate in plants under different abiotic stresses (Beckman 2000). Polyphenolic compounds such as phe-nolic acids, flavonoids, and anthocyanins are reported to play an important role in scaveng-ing free radicals produced durscaveng-ing salt stress in plants (Parida et al. 2004, Ksouri et al. 2007, Hichem et al. 2009). The redox properties im-part antioxidant activity to the phenolic com-pounds, and this allows them to act as reduc-ing agents, hydrogen donors and sreduc-inglet oxy-gen quenchers (Beckman 2000). Lettuce plants were treated with VAM 510 or Pseudomonas
and exposed to drought stress, the amount of phenols like ferulic, caffeic, coumaric and cin-namic acids (precursors of lignin) were found to accumulate in higher concentrations in stressed plants pre-treated with VAM 510 or
Pseudomonas (Leinhos and Bergmann 1995). These results are in conformity with the results in the present investigation.
Polyphenol oxidase activity was also increased with increase in salinity level and time. Its activity in plants under salt stress was highest in co-inoculation of the bacterial strains and at 72 h compared to the single ap-plication of the bacterial strains. Higher PPO activity in maize cultivars ‘Hack and Zan’ was also reported by Aghaleh and Nikam (2009). A similar observation was also reported by Niknam et al. (2006) who reported salt stress induced PPO activity in calli and seedlings of
Trigonella aphanoneura and Trigonella foe-num-graecum. The present investigation thus confirms the role of microbial induced PPO activity in chickpea under salt stress condi-tions and the bacterial strains S1 and Cgr elic-its PPO activity more under consortium mode.
References
Abel AJ, Sutherland MW and Guest DI (2003). Pro-duction of reactive oxygen species during non-specific elicitation, non-host resistance and field resistance expression in cultures of tobacco cells. Func Plant Biol 30: 91–99.
Aghaleh M and Niknam V (2009). Effect of Salinity on Some Physiological and Biochemical Parame-ters in Explants of Two Cultivars of Soybean (Glycine max L.). J Phytol 1(2): 86–94.
Alscher RG, Donahue JL and Gramer CL (1997). Re-active oxygen species and antioxidants; relation-ships in green cells. Physiol Plant 100: 224–356.
167 cies in photosynthetic tissue, In: Foyer CH,
Mullineaux PM (Eds) Causes of photo-oxidative stress and amelioration of defense system in plants. CRC, Boca Roton, 77–104.
Bakker AW and Schippers B (1987). Microbial cya-nide production in rhizosphere in relation to pota-to yield reduction and Pseudomonas mediated plant growth promotion. Soil Biol Biochem 19: 451-457.
Bano A and Fatima M (2009). Salt tolerance in Zea mays (L) following inoculation with Rhizobium and Pseudomonas. Biol Fertil Soils 45: 405–413.
Bate NJ, Orr J Ni W, Meromi A, Nadler-Hassar T, Doerner PW, Dixon RA, Lamb CJ and Elkind Y (1994). Quatitative relationship between Phenylala-nine ammonia-lyase levels and phenylpropanoid accumulation in transgenic tobacco identifies a rate-determining step in natural product biosyn-thesis. Proc Natl Acad Sci USA 91: 7608-7612.
Beckman CH (2000). Phenolic storing cells: keys to programmed cell death and periderm formation in wilt disease resistance and in general defense re-sponses in plants. Physiol Mol Plant Pathol 57: 101– 110.
Berry C, Fernando WGD, Loewen PC and de Kievit TR (2010). Lipopeptides are essential for Pseudo-monas sp. DF41 biocontrol of Sclerotinia scleroti-orum.Biol Contr55 :211–218.
Binutu OA and Cordell GA (2000). Gallic acid deriv-ative from Mezoneuron benthamianun leaves. Pharmacol Biol 38 : 284–286.
Bolton H Jr, Elliott LF, Turco RF and Kennedy AC (1990). Rhizosphere colonization of Botrytis ciner-ea in tomato by Pseudomonas aeruginosa 7NSK2: Role of salicylic, acid, pyochelin and pyocyanin. Mol Plant-Microbe Interact 15 : 147-1156.
Chen C, Belanger RR, Benhamou N and Paultiz TC (2000). Defense enzymes induced in cucumber roots by treatment with plant growth promoting rhizobacteria (PGPR). Physiol Mol Plant Pathol 56: 13-23.
Cheong YH, Chang HS, Xun Wang RG, Zhu T and Luan S (2002). Transcriptional profiling reveals nov-el interactions between wounding pathogen, abi-otic stress, and hormonal responses in Arabidopsis. Plant Physiol 129 : 661-677.
Dixon RA and Paiva NL (1995). Stress induced phe-nylpropanoid metabolism. Plant Cell 7: 1085-1097.
Dye DW (1962). The inadequacy of the usual deter-minative tests for identification of Xanthomonas sp. NZT Sci 5 : 393–416.
Fernando WGD, Nakkeeran S, Zhang Y, Savchuk S (2007). Biological control of Sclerotinia scleroti-orum (Lib.) de Bary by Pseudomonas and Bacillus species on canola petals. Crop Prot 26: 100–107.
Flowers TJ, Gaur PM, Laxmipathi Gowda CL, Krish-namurthy L, Samineni S, Siddique KHM, Turner NC, Vadez V, Varshney RK and Colmer TD (2010). Salt sensitivity in chickpea. Plant Cell Environ 33: 490– 509.
Friend J (1977). Phenolic substances and plant dis-ease. Pages 557-588 in: Recent Advances in Phyto-chemistry. Vol 12, Biochemistry of Plant Phenolics. T. Swain, J. B. Hardbone, and C. F. Vansumere, (Eds.) Plennum Press, New York.
Gholizadeh A and Baghban Kohnehrouz (2010). Activation of phenylalanine ammonia lyase as a key component of the antioxidative system of salt-challenged maize leaves. Braz J Plant Physiol 22(4): 217-223.
Gossett DR, Millhollon EP and Lucas MC (1994). Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci 34: 706–714.
Hammerschmidt R and Kuc J (1979). Isolation and identification of Phytotuberin from Nicotiana taba-cum previously infiltrated with an incompatible bacterium. Phytochem18: 874-875.
Hernàndez JA, Ferrer MA, Jiménez A, Ros-Barceló A and Sevilla F (2001). Antioxidant system and O2 and H2O2 production in the apoplast of Pisum sativum L. leaves; its relation with NaCl induced necrotic lesions in minor veins. Plant Physiol 127: 817– 831.
Hichem H, Mounir D and Naceur A (2009). Differ-ential responses of two maize varieties to salt stress: changes on polyphenols composition of foliage and oxidative damages. Indust Crops Prod 30: 144–151.
Jones DH (1984). Phenylalanine ammonia-lyase: regulation of its induction, and its fole in plant de-velopment. Phytochem 23: 1349-1360.
Jain A, Singh S, Sarma BK and Singh HB (2012). Mi-crobial consortium–mediated reprogramming of defence network in pea to enhance tolerance against Sclerotinia sclerotiorum. J Appl Microbiol 112(3): 537-50.
Karthikeyan M, Bhaskarana R, Radhikab K, Mathiya-zhagana S, Jayakumara V, Sandosskumara R and Velazhahan R (2005). Endophytic Pseudomonas fluorescens Endo2 and Endo35 induce resistance in black gram (Vigna mungo L. Hepper) to the pathogen Macrophomina phaseolina., J Plant Inter-actions 1(3): 135 – 143.
King EO, Ward MK and Randey DE (1954). Two sim-ple media for the demonstration of pyocyanin and-fluorescein. J Lab Clin Me 44: 301-307.
Kloepper JW, Ryu Choong-Min, and Zhang Shouan (2004). Induced systemic resistance and promotion of plant growth by Bacillus species. The Nature and Application of Biocontrol Microbes: Bacillus sp. Symposium Phytopath 94 (11) 1259-1266.
Kohler J and Hern A (2008). Plant-growth-promoting rhizobacteria and arbuscular mycorrhi-zal fungi modify alleviation biochemical mecha-nisms in water-stressed plants. Functional. Plant Biol 35 141–151.
Ksouri R, Megdiche W, Debez A, Falleh H, Grignon C and Abdelly C (2007). Salinity effects on polyphe-nol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol Bio-chem 45: 244–249.
Kuć J and Rush JS (1985). Phytoalexins. Arch Bio-chem Biophys 236: 455-472.
Lawton MA and Lamb CJ (1987). Transcriptional activation of plant defense genes by fungal elicitor, wounding and infection. Mol Cell Biol 7:335-341.
Leinhos V and Bergmann H (1995). Effect of amino alcohol application, rhizobacteria and mycorrhiza inoculation on the growth, the content of protein and phenolics and the protein pattern of drought stressed lettuce (Lactuca sativa L. cv. "Amerikanischer Brauner"). Angewandte Botanik 69: 5/6, 153-156.
Liu I, Kloepper JW and Tuzun S (1995a). Induction of systemic resistance in cucumber against Fusari-um wilt by plant growth promoting rhizobacteria. Phytopat 85: 695-698.
Lugtenber BJJ and Bloemberg GV (2004). Life in Rhizosphere; in Pseudomonas : Genomics life style and molecular architecture (1) : JL Ramados (ed) 403-430 New York USA Kleuwer/ Academic, Ple-num Publishers.
Luzzatto T, Golan A, Yishay M, Bilkis I, Ben-Ari, J and Yedidia I (2007). Priming of antimicrobial phe-nolics during induced resistance response towards Pectobacterium arotovorum in the ornamental monocot Calla Lily. J Agric Food Chem 55: 10315– 10322.
MacDonald MJ and D’Cunha GB (2007). A modern view of phenylalanine ammonia-lyase. Biochem. Cell Biol 85: 273–282.
Mayak S, Tirosh T and Glick BR (2004). Plant growth -promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci 166: 525– 530.
Mayak S, Tirosh T and Glick BR (2004). Plant growth -promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42: 565-572.
Mayer AM, Staples RC and Gil-ad NL (2001). Mech-anisms of survival of necrotrophic fungal plant pathogens in hosts expressing the hypersensitive response. Phytochem 58: 33–41.
Meena B, Ramamoorthy V, Marimuthu T and Vela-zhahan R (2000). Pseudomonas fluorescens medi-ated systemic resistance against late leaf spot of groundnut. J Mycol Plant Path 30 (2): 151-158.
Mehta S and Nautiyal CS (2001). An Efficient Meth-od for Qualitative Screening of Phosphate-Solubilizing Bacteria. Curr Microbiol 43: 51–56.
Meyer W, Morawetz R, Borner T and Kubicek CP (1992). The use of DNA Finger printing analysis in the classification of some species of the Trichoder-ma aggregate. Curr Genet 21: 27-30.
Mourhofer M, Reimmann JC, Sacherer SP, Heeb SD and Defago G (1998). Salicylic acid biosynthesis genes expressed in Pseudomonas fluorescens : strain P3 improve the induction of systemic re-sistance in tobacco against tobacco necrosis virus. Phytopath 88 : 678-684.
Nakkeeran S, Kavitha K, Chandrasekhar G, Re-nukadev P and Fernando WGD (2006). Induction of Plant Defense compounds by Pseudomonas chlo-roraphsis PA 23 and Bacillus subtilis BSCBE4 in controlling Damping Off of Hot Pepper caused by Pythium aphanidermatum. Biocontrol Sci Tech 16 (4): 403 – 416.
Niknam V, Razavi N, Ebrahimzadeh H and Shari-fizadeh B (2006). Effect of NaCl on biomass, protein and proline contents, and antioxidant enzymes in seedlings and calli of two Trigonella species. Biol Plant 50: 591-596
Parid AA, Das AB, Sanada Y and Mohanty P (2004). Effects of salinity on biochemical components of the mangrove Aegiceras corniculatum. Aqua Bot 80 : 77–87.
Pierson EA and Weller DM (1994). Use of mixtures of fluorescent pseudomonads to suppress take-all and improve the growth of wheat. Phytopath 84: 940 – 947.
Punja ZK (1985). The biology, ecology and control of Sclerotium rolfsii. Ann Rev Phytopath 23: 97–127
169 Puupponen-Pimiä R, Nohynek L, Meier C,
Kähkönen M, Heinonen M, Hopia A and Oksman-Caldentey KM (2001). Antimicrobial properties of phenolic compounds from berries. J Appl Microbiol 90: 494–507.
Rabie GH and Almadini AM (2005). Role of bio-inoculants in development of salt tolerance of Vicia faba plants under salinity stress. Afr J Biotech 4: 210 – 222.
Ramamoorthy V, Raguchander T and Samiyappan R (2002). Induction of defense-related proteins in tomato roots treated with Pseudomonas fluore-scens Pf1 and Fusarium oxysporum f. sp. lycopersi-ci. Plant Soil 239: 55–68.
Ramamoorthy V, Vishwanathan R, Raghuchander T, Prakasam V and Samiyappan R (2001). Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pests and dis-eases. Crop Prot20: 1-11.
Ross WW and Sederoff RR (1992). Phenylalanine ammonia lyase from Loblolly pine: Purification of the enzyme and isolation of complementary DNA clones. Plant Physiol 98: 380-386.
Rout NP and Shaw BP (2001). Salt tolerance in aquatic macrophytes: possible involvement of anti-oxidative enzymes. Plant Sci 160: 415–423.
Saikia R, Kumar R, Singh T, Srivastava A, Arora KD and LeeM Woong (2004). Induction of Defense re-lated enzymes and Pathogenesis Rere-lated Proteins in Pseudomonas fluorescens Treated Chickpea in Response to infection by Fusarium oxysporium f. sp. ciceri. Mycobiol 32(1): 47 – 53.
Saleh L and Plieth C (2009). Fingerprinting antioxi-dative activities in plants. Plant Meth 5: 2.
Sangeetha G, Thangavelu R, Usha Rani S, Muthuku-mar A and UdayakuMuthuku-mar R (2010). Induction of sys-temic resistance by mixtures of antagonist bacteria for the management of crown rot complex on ba-nana. Acta Physiol Plant 32: 1177–1187.
Saravanakumar D, Harish S, Loganathan M, Vive-kananthan R, Rajendran L, Raguchander T and Samiyappan R (2007). Rhizobacterial bioformula-tion for the effective management of Macrophomi-na root rot in mungbean. Arch Phytopath Plant Protection. 40(5): 323 – 337.
Sarma BK, Singh DP, Mehta S, Singh HB and Singh UP (2002). Plant growth-promoting
rhizobacteria-elicited alterations in phenolic profile of chickpea (Cicer arietinum) infected by Sclerotium rolfsii. J Phytopath 150: 277-282.
Shanmugam V, Kanoujia N, Singh M, Singh S and Prasad R (2011). Biocontrol of vascular wilt and corm rot of gladiolus caused by Fusarium ox-ysporum f. sp. gladioli using plant growth promot-ing rhizobacterial mixture. Crop Prot 30: 807-813.
Singh PP, Shin YC, Park CS and Chung YR (1999). Biological control of Fusarium wilt of cucumber by chitinolytic bacteria. Phytopath89: 92-99.
Singh UP, Sarma BK and Singh DP (2003). Effect of plant growth-promoting rhizobacteria and culture filtrate of Sclerotium rolfsii on phenolic and salicyl-ic acid contents in chsalicyl-ickpea (Cicer arietinum). Curr Microbiol 46: 131-140.
Singh A, Verma R and Shanmugam V (2006). Extra-cellular chitinases of fluorescent pseudomonads antifungal to Fusarium oxysporum f.sp. dianthi causing carnation wilt. Curr Microbiol 52: 310-316.
Singh UP, Sarma BK, Singh DP and Bahadur A (2002). Plant growth-promoting rhizobacteria-mediated induction of phenolics in pea (Pisum sa-tivum) after infection with Erysiphe pisi. Curr Micro-biol 44: 396-400.
Sousa A (2006). Phenolics and antimicrobial activi-ty of traditional stoned table olives ‘alcaparra’. Bioorg Med Chem 14: 8533–8538.
Timmusk S and Wagner EGH (1999) The plant-growthpromoting rhizobacterium Paenibacillus polymyxa induces changes in Arabidopsis thaliana gene expression: A possible connection between biotic and abiotic stress responses. Mol Plant-Microbe Interact 12:951-959.
Van Loon LC, Bakker PAHM and Pieterse CMJ (1998). Systemic resistance induced by rhizosphere bacteria. Ann Rev Phytopath 36: 453–483.
Van Peer R, Niemann GJ and Schippers B (1991). Induced resistance and phytoalexin accumulation in biological control of Fusarium wilt of carnation by Pseudomonas sp. strain WCS 417r. Phytopath 81: 728- 734.
Vidhyasekaran P and Muthamilan M (1999). Evalua-tion of powder formulaEvalua-tion of Pseudomonas fluo-rescens Pf1 for control of rice sheath blight. Bio-control Sci Tech 9: 67-74.
Studies on Rhizosphere-Bacteria mediated Biotic and Abiotic stress tolerance in Chickpea