264
Katili et al. Med J IndonesComparison of
the
EfÏïcacy of Nebulized
and
Intravenous
Salbutamol
in the
Initial
Treatment of Acute Severe
Asthma
Antalia
K-M.
Katili,
Hadiarto Mangunnegoro,
Mohantmad
Farid,
and
Faisal
YunusAbstrak
Penelitian ini dilakulan untuknrenentukan apakah salbutanolyang diberikan uelalui nebuliser jet dengan cara inhalasi intenniten nenberikan respons klinik yang lebih baik dibandingkan dengon penberian intravena pada terapi tahap inisial asna akut berat. Penderita diobati secara randotn pada
uji
klinik buta tunggal dengan5 yg,/kgBB
salbutanol intravena atau inhalasi 0,15 ng/kgBB selana 10nenit.
Peninglatan Arus Puncak Ekspirasi (APE) lebih besar pada kelontpok inhalasi dibandingkan dengan kelonpok intravena. Peningkatanpadakelonpokinhalasidarinilaiawalsanpaiakhirjanpertanadankeduasetelahpengobatanadalahsebagai berikut 19,69!6,84V.,
5l,U
+ 15,56%
dan 56,64+
15,93% nilai prediksi denganp
< 0,01, dibandingkan 18,18 + 6,21%, 41,01 + 12,63% dan 50,83 + 24,36% denganp
< 0,5. Terdapat perbedaan bennakna antara kedua kelonpok padajan
pertama (p < 0,1). Perbedaan bennknajuga terbukti dari perbaikan skor berdasarkan nrcdifikasi dari cara Brtdsh Thoracic Society dan Cochrane. Berat obstruksi awal, lmkni APE t0, tidak meupunl'ai korelasi denganlana
sesak napas sebelwtt datang ke runah sakit, tetapi uenpunyai korelasi sedang dengan besar perubahan APE pada nenit ke 45 pengobatan. Palpitasi, tentor dan sakit dada lebih nenonjol padajant pertana penberian intravena, teruta,ila pada nenit ke I 5 - 30 pengobatan. Satu kasus dari keloupok intravena tidak dapat neneruskan pengobatan karena sakit kepala dan ,nuntah-uunlah, sedangkan infus harus diperlanùat pada satu kasus dengan elcstrasistole.Abstract
This study was undertaken to deteruine if internûttent inhalation of salbutauol delivered by jet nebulizer provided better clinical response than intravenous salbutamol in the initial therapy of acute severe osthtna. In a randonized single blind clinical trial, patients with acute severe asthna were given salbutatnol either intravenously 5 ltglkgBW or by inhalation of 0.15 ug/kgBWfor
I0
ninutes. A grearcr inprovemenl in peak expiratorS,flow rate (PEFR) was observed in the inhalation group cornpared to the inlravetrcus gtoup. The PEFR in the inhalation group v,asinitially
19.69 + 6.84% of the predictive value, 51.04+ 15.56% one
hour, and 56.64+ 15.93%
two hours afler trealilrcnt, v,ith p < 0.01, whereas in the group receiving intravenous salbutanol, the values were 18.18 !6.21%, 41.05 + 12.63,50.83 + 24.36% respectivels,, v,ithp
< 0.05.A
statistically significantdffirence
was found between both groups in thefirst
hourafTerrreailnentv,asinitiated(p<0.01). TherewasalsoasignificantdifferenceintheresultsassessedbyntodifiedBritishThoracic Society and Cochrane scoring nethods. No correlation wasfound benveen the severity of initial airway obstruction with the duration of dyqtneaprior
to adnission, instead, nrcderate corellation was found between the severity of obstructiott and the inprovenent oJ PEFR 45 minutes after initial treaunent. Palpitation, treilrcr, and chest painwere ilore pronounced in intravenous group thefirst hour alter treatnrent, particularly during thefirstl5
- 30 uinutes. Syntptonts ofvoniting and headache were so severe in one patient given intraveous salbutantol that treatnent hacl to be discontinued. In another patient, the rate of infitsion had to be reduced because of the ectopic heartbeat it caused.Keywords : Acute severe asthnta, initial theral4', nebulization, salbutamol
Acute
severe asthmais
alife
threatening situation
that demands adequate andrapid therapeutic intervention.
Simple assessmentof
theseverity of
the attackcoupled
to the
responseto the initial
treatment
will
serve to
identify
early
ahigh risk group of
asthmatic patientsin
which
the usual
emergency
room
therapeutic
modalities
will
often prove
ineffective.l
Theimmedi-Departuenl of Pulnonology, Faculty of Medicine, University of Indonesia, Persahabatan H osltital, Jakarta, Indonesia
ate
relief
of symptoms
and the nragnitudeof
theinitial
improvement
will
determinefurther
therapy and prog-nosis.inhala-favorable regarding side effects.
Several studies haveshowed
that even though the
peak
responsedid
not
differ
significantly,
there
was a
tendency
for
earlier
occurence
of
bronchodilatation after inhalation
the-rapy2 and
a
slower decline
in
effect,3
whereas
in-travenoussalbutamol
was found to increase PaOzz
andbetter
affect
paradoxical
pulse.a
A
multicenter study
organized
by
the Swedish Society
of
ChestMedicine
(SSCM) provided
definitive
evidenceof superiority
of
inhaled salbutamol
in
thetreatment of
acute episode .)The
principal aim
of
this
study was
to determine
whether nebulized
inhalation of
salbutamol
wasmore
effective in
treating severe asthmatic attack,particular-ly
within the
initial
phase,when compared
to an
in-travenous
bolus
infusion
of
the
same
drug.
An
additional
purpose wasto determine
thedifference
in
side effects and whether
additional
medication could
improve
the results.METIIODS
This study
is arandomized single
blind clinical trial
of
short term
responses
to
compare nebulized
to
in-travenous salbutamol. The
trial
wascarried out
at the EmergencyUnit
of
the PersahabatanHospital
in Jakar-ta,from August
l99l to
May
1992.One hundred patients aged
l5
- 45 years wereincluded
in
this study. The number was sufficiently large
toenable
statistically reliable
conclusions
to
be
drawn,All
were admitted to the respiratory
emergencyroom
becauseof
acute severeasthma.
Thesepatients
wereclinically
defined
ashaving
anindex
of
> 4according
to a
modified
British
Thoracic Society (BTS)
and Cochranescoring methods
(table
l).o'/
The peak
Ex-piratory Flow
Rate
(PEFR) value on
admission
was Iess than200
l/min. or
less than 4O7oof
thepredictive
value.' The
patients excluded were those
who
could
not
beevaluated
by
a
peakflow
meter,or
thosewith
a history
of
cardiovascular,
liver,
or
renal
diseases, diabetesmellitus, hiperthyroidism,
pregnancy, ornon-asthmatic
chronic
respiratory
insufficiency.
Patientsreceiving corticosteroids,
oral
or
intravenous
82-agonists less
than
four hours
prior to
admission, or
aminophylline
less
than
six
hours before
admission
were
alsoexcluded.
Thosewith
aPEFR value
of
lesslhan l57o
of
the predictive value
30
minutes
after
initial
treatment
or suffering
severeside effects
were alsoexcluded
from
this
study.Subjects
fulfilling
the
criteria were divided
into
2groups
(figure
l),
group
one(n
=48)
was treatedwith
of salbutamol,
while
group
two (n
=5l)
wasgiven
abolus
infusion
of salbutamol.
The PEFR,
measuredby
a
Mini
Wright peak Flow
Meter (Airmed, Clement Clarke International Ltd.,
London,
England), pulse rate, and
systolic
and
dias-tolic
blood
pressures measuredby a
sphygmomano-meter were variables recorded
prior
to
the
initial
treatment andevery
15minutes thereafter
for
2 hours.The
best of
3
PEFR values
for
each patient
wascalibrated
to normal
Indonesian predicted values
ob-tained
by the
Indonesian Pneumobile
Project.
Eachpatient was
askedto
note the
presenceor
absenceof
side
effects, such as tremor,
palpitation,
nausea, headache,or other symptoms.
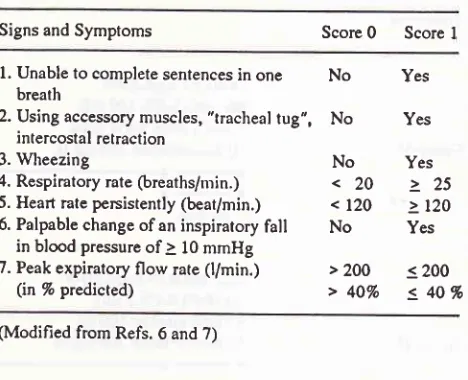
Tabel 1. Assessment of severity
Signs and Symptoms Score
0
Score Il.
Unable to complete sentences inone No
Yes breath2. Using accessory muscles, "tracheal
tug",
No
yes intercostal retraction3.
Wheezing
No
yes4. Respiratory rate
(breaths/min.)
< 20 >
25 5. Heart rate persistently(beat/min.)
<l2O
> l2O 6. Palpable change ofan inspiratoryfall
No
yesin blood pressure of
à l0
mmHg7. Peak expiratory flow rate
(l/min.)
>2OO
< 2OO(in%predicted)
>4OVo
540%
(Modified from Refs. 6 and 7)
The dose
for inhalation
was0.l5
mg/kgBW of
5mg/ml
salbutamol solution diluted
with
2 ml0.97o NaCl
sotu-tion
in
a jet
nebulizer (Pari-Master,
PaulRitzau,
pari-Werk
GmbH).
The compressed airnebulizer
produceda mass median
pa
;r and anintrapulmonary
d
aperiod
of7+4minutes
ulization
was
interrupted during
expiration.
The procedure wascontinued
until
the nebulizer was
dry,
in
order
to reduce wastageof
the nebulisedsolution.e
Subjectsin
group
two
were given
5
pg,/kgBflmin
salbutamol,
diluted
in
100ml of
O.9VoNaCl solution, intravenously
over a period
of l0
minutes.s,l0 Aminophylline
5-6nrg/kgBV/, diluted
in
20 ml
of
5%
glucose solution,
was
given 60
minutes
after
theadministration of
sal-butamol. This
dose was reducedto
half
if
thepatient
havetaken
oral theophylline during
the
last24
hours.All
patients weregiven 200
mgintravenous
hydrocor-tisone andcontinous oxygen 6-8
l/min
by
nasal canule at thestart.l0
The study was completeain
tZO minutes. [image:2.595.319.553.285.475.2]-266
Kaûli et aI.asthmatic symptoms, whether
it
was
improved,
wor-sened or remained unchanged, and
whether
thepatient
needed additional
treatment
with
bronchodilators
or
antibiotics for
respiratory tract infection.
Nonparametric statistics, Chi-square
andMann-Whit-ney analysis
for unpaired data, were
usedto
analyze
the
changesin side effects
and scoreof the asthmatic
attack.
PairedZ
test wasapplied for comparing pulse
rate,
respiratory frequency,
and PEFR(in llmin.
andin
STUDY PROTOCOL
Treatment
Group V
Salb iv 5 FglKgBW (in NaCl0.97o 100 ml) + NaCl inhal 0.97o 2 ml Hydrocortisone 200 mg
iv
Group H
Salb inh 0.15 mg/KgBW (in NaCl 0.9Vo 2 ml) + NaCl inhal
iv 100
ml Hydrocortisone 200 mgiv
Med J lttdones
%
of
the predictive value)
in
patients
of
the
samegroup. Comparison
of
changesin symptoms
between thetwo
groups was made using an analysisof variance
with 3 variables. Measurements at
agiven
time were
compared
by using non paired
Z
test.
Kolmogorov-Smirnov test was used to evaluate similarities
in
theduration
of
dyspnea
prior to
admission
and the
fre-quency
of
attacks between thetwo groups. Correlation
analysis was used
in evaluating two
variablesquantita-tively"
Aminophylline bolus
x-x
x-x
l0
min.Aminophylline bolus
x-x
l0
min.xxxx
xxxx
xxxx
xxxx
xx x x
x x x
x
x x x x
x x x
x Time
Assessment Score Syst. press.
Diast. press.
Side+ffects
Salb = Salbutamol
[image:3.595.61.558.114.711.2]Inhal = Inhalation Syst,pres = Systolic Diast.pres = Diastolic pressurepressure
Except
for
the duration
of
breathlessnessprior to
ad-mission,
no
significant difference
in
characteristics
betweenpatients
in
thetwo
study
groups was
found
(p <
0.05).
The occurence
of
respiratory
tract
infec-tions
was also
proportional
in
both
groups (table
2).Differences
in
theseverity
of
the 7variables assessed
were
apparent
at
minute-15
and continued
until
minute-105 after treatment (table
3).
Patientsreceiv-ing
intravenous
salbutamol were slower
to
respond than thosereceiving
inhalation (figure
2).inability
tocomplete
a sentence disappearedafter
30 minutes
of
treatment,while
in
the
intravenous
group
it
took 60
minutes.There was
a significant difference
in
the
number
of
patients using
the accessory musclesfor
respiration
atminute-60,
L2% vs
2.087o cases(p <
0.05).
No
sig-nificant
changein
the incidence
of
paradoxical
pulsewas found
between
both
groups.
There
was
still
asignificant
difference in
the number of patientswheez-ing
atminute-120 (p
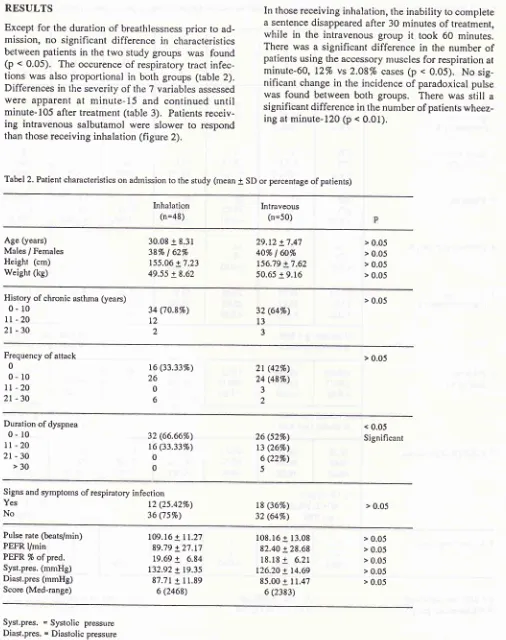
< 0.01).Tabel 2. Patient characteristics on adtnission to the study (mean
I
SD or percentage of patients)Inhalation
(n=48) Intraveous(n=50)
Age (years)
Males / Females
Height (cm) Weight (kg)
30.08 + 8.31 387o
|
627o155.06 + 7.23
49.55 + 8.62
29.t2 + 7.47
40% I 60%
t56.79 + 7.62 50.65 + 9.16
> 0.05
> 0.05
> 0.05 > 0.05
History of chronic asthma (years)
0- l0
ll
- 202l
-30
> 0.05
34 ('t0.8%) L2
)
32 (64Vo)
l3
3
Frequency of attack
0
0- l0
It
-20
2t
-30
> 0.05 t6 (33.3370)
26
0 6
2t
(42Vo) 24 (48%)3
2
Duration of dyspnea
0- l0
lt
-20
2t-30
>3032 (66.6670)
t6 (33.337o)
0
0
26 (527o)
t3 (26Vo) 6 Q2%)
5
< 0.05
Significant
Signs and symptoms of respiratory infection Yes
No 36 t2 (25.427o)(75Vo)
t8 (367o)
32 (64%)
> 0.05
Pulse rate (beats/min) PEFR l/min
PEFR 7o ofpred. Syst.pres. (mmHg) Diast.pres (mmHg)
Score (Med-range)
109.16 + 11.27
89.79 + 27.t7
19.69
+
6.84 132.92 + 19.35 87.71 + 11.896 (2468)
108.16 + 13.08
82.40 + 28.68 18.18
+
6.21 126.20 + t4.6985.00 + I1.47 6 (2383)
> 0.05
> 0.05
> 0.05 > 0.05 > 0.05
Syst.pres. = Systolic pressure
[image:4.595.40.546.79.719.2]-Katili et al.
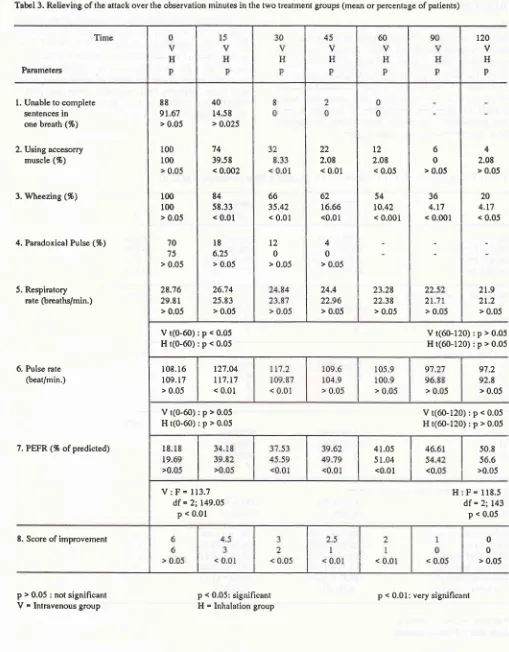
Tabel 3. Relieving of the attack over the observation minutes in the two treatment groups (mean or percentage of patients)
Parameters
l.
Unable to completesentences in
one brcath (%)
2. Using acce.sorry
muscle (%)
3. Wheezing (%)
4. Paradoxical Pulse (%)
5. Respiratory
rate (breaths/min.)
Vt(0-60):p<0.05
Ht(0-60):p<0.05
Med J Indones
4
2.08
> 0.05
20
4.t7 < 0.05
21.9
21.2
> 0.05
Vt(60-120):p>0.05
Ht(60-120):p>0.05
97.2
92.8 > 0.05
Vt(60-120):p<0.05
Ht(60-120):p>0.05
H:F=118.5
df = 2;143p < 0.05
0 0
> 0.05 120
v
H
P
6. Pulse rate
(beat/min.)
7. PEFR (% ofpredicted)
8. Score of improvement
p > 0.05 : not signihcant
V
-
Intravenous groupVt(0-60);p>0.05
Ht(0-60):p>0.05
V:F=113.7
df = 2:149.05 p < 0.01
50.8
56.6
>0.05
p < 0.05: significant H = Inhalation group
66
35.42
< 0.01
t2
0
> 0.05
22
2.08 < 0.01
62 16.66 <0.01
4 0
> 0.05
24.4 22.96
> 0.05 88
9r.67
> 0.05
r00
100 > 0.05
70
75 > 0.05
28.16
29.81 > 0.05
40 14.58 > 0.025
74 39.58 < 0.002
84
58.33
< 0.01
l8
6.25 > 0.0526.74 25.83
> 0.05
L2
2.08
< 0,05
54
r0.42
< 0.001
23.28 22.38
> 0.05
6 0
> 0.05
36
4.r7 < 0.001
108.16 109. 17
> 0.05
r27.04
tt7.r7
< 0.0141.05 51.04
<0.01
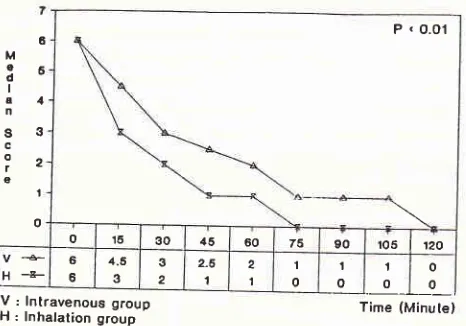
[image:5.595.51.560.81.733.2]6
M
qE o
I
a4
n
s3
c
iz
g
'|
o
o '16 30 il5 60 75 90 r05
|
12OH+ 66 4.5
3 3 2
2-6
1 2
1
'|
0 t o
I o
o 0
V:
lnlravenous groupH:
lnhalation group Time (Minulelfrom
84.4
+28.68
l/min.
(18.18 +
6.27o)to
185.25 + 56.03l/min.
(41.05
+
12.637oof
the
predictive value) in
minute-60, and to 216.3 +
57.08
l/min.
(50.g3
+24.367o
of
the
predictive value)
in
minute-120
after
administration
of
intravenous
salbutamol (p <
O.Ol). In theinhalation
group, the PEFRimproved from
89.79+
2',7.17l/min.
(19.69
+
8.84%)
to
234.79 +
67.85l/min.
(51.04
+
15.56%
of the predictive value)
in
minute-60
andto 260.73
+70.5
l/min.
(56.64
+
I5.g70of
thepredictive value)
in
minute-120 (p
<0.05).
Thegreatest increase
in
both
groups was achieved in
minute-60
(p
< 0.01),
with inhalation
achieving
more [image:6.595.40.273.77.240.2] [image:6.595.290.532.291.470.2] [image:6.595.298.527.500.673.2]significant
increase when compared to thoserecieving
intravenous salbutamol
(p <
0.01).
There
wasalso
asignificant
difference
in
therespiration
rate(p
< O.O5). Figure 2Group
Inproveuent of Score in Inhalatiott and Intravenous
Systenric
Effects
No significant difference
in
blood
pressure was
ob-served between
the
2
groups
(p
>
0.05)
during
theduration of
thestudy.
Although
the meandiastolic
andsystolic
pressures
were
lower
in
minute-60
andminute-120 when
compared
with
minute-O,
the
dif-ference was
not
statistically
significant.
The
dif-ference
in
pulse rates between
two
treatment
groupswere
significant at minutes-15, 30,
and
75,
with
themean pulse rates
higher
for
the intravenousgroup;
127+ 13.2
vs
117.2+ I4.3(p
<0.01),
117.2+
1l.8
vs
109.9+
12
(p <
0.01), and 103,9 +
t7.9 vs
97.5
+
il.6
beats/min.
(p <
0.05), respectively (table
3).
The
in-creaseoccurred
in minute-15,
19 beats comparedto
gbeats/min.,
with
the
difference
becoming
statistically
significant
in minutes-15
and 30. The mean pulse ratesof
both groups returned to
thepre-treatment
valuesin
minute-45.
More complaints
of
tremor (figure 3)
andpalpitation
(figure
4)
were
observed antong
patients
in
the
in-travenous
group.
The peak difference was
seen
in
minute-3O.
One
patient with
complaints
of
vomiting
and headaches 5
minutes after
initial treatnent
had to bewithdrawn from
the
trial.
The
infusion
rate hadto
be
reduced
in
one patient, when ectopic
heartbeatsoccurred
5minutes after
initial
treatment.The Effects on Lung Function
There was a
significant
improvement
in
lung
function
in
both groups
(figure
5).
A
greater increasein
pEFR
was observedin
theinhalation
group,with
asignificant
increase apparent
in
minute-15 (p <
O.O5).
This
in-crease peakedin
nrinute-45 (p
<0.01)
and lasteduntil
V : lntravenous group H : lnhelation group
Figure 3. Distribuliott of patients v,ith
treuor
(%)
zoP
f
o I o f
t I
o n
60
50
10
30
20
10
o
Time (Minute)
Time (Minute) V : lntravenouc group
H : lnhalation group
Figure 4. Distributiott of patients wirh palpitatiott
The
asthnta
index
assessedin
minute-I5
and
105-minute was significantly different
for both
groups,vf
6
N\NvI
27O
Katili et al.with
theinhalation
groupshowing
anearlier decline
in
symptoms
(figure
2).
No relationship
was
found
be-tween the
duration of
breathlessness andthe severity
of
airway obstruction (PEFR)
on
admission
to
thestudy
(r
=
0.09). In
the group
receiving
intravenous
salbutamol, the improvement
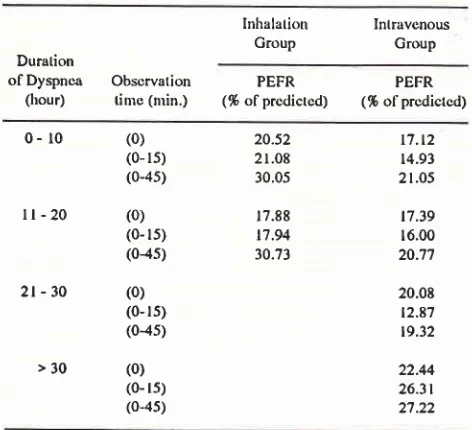
in
the averagePEFR
of
patients experiencing
dyspneafor
more than
30 hoursllm
V :
Intravenous group
H
: lnhalation
group
Figure 5. Conparison ofntean PEFR between the inhalation and intravenous groups
PEFR (l/min.) at minute-45
Med J Indones
prior
toadmission
was greater than thoseof
more than 10hours.
The
initial
severity
of
airway
obstruction
was moderately correlated
with
the
magnitude
of
bronchospasm
relief
atminute-45
(r = 0.544),although
the change was
slightly lower
in
patients
of
both
groups
with
more
severe
initial
obstruction (table
4;figure
6)..:-E:.J
r:.=, I
i.lE;
i::[15
1E:.1
1.::=l
4t
r:l+t
T
+.1
+.a t
-.-il'|---"r
---
.)+.f
+
.)?
Ç
I
+ç
.)t
)
i+1
Time (Minute)
+
a+
r:
0.544p:
< 0.05:l.l:J:
:L:t::t 1:::r::
J5::l
J-?LJXË'H,,n,,".,
Figure 6. The correlation between initial obstruction and the inprovetnent of lung function at uùnute 45.
in
P E F R
300
250
200
150
100
50
o
o 15 so 45 60 75 90 ro5 r20
V
-ê-H
--g- 8e.79281.1 158.t 213.125170r802
loo.lo,r lE5.2 2S1.7ei
r93.8 245.ôg
202.5 214.47
219 251.79
Vol 4, No 4, October - December 1995
Table
4.
Distribution of mean PEFR in % of predictive value based on treatment group and duration ofdyspnea before admissonInhalalion
IntravcnousGroup
GroupDuration
ofDyspnca
Obscrvation(hour)
tine (nrin.)PEFR
PEFR (%
ofprcdictcd)
(% ofprcdictcd)0-
r0ll-20
2t-30
>30
(0)
(0- rs)
(0-45)
(0)
(0- 15)
(0{s)
(0)
(0-rs) (04s)
(0)
(0- ls)
(0-4s)
20.52
2 r.08 30.05
17.88 t7.94
30.73
17.t2
14.93
21.05
t7.39 16.00 20.7'l
20.08
12.87 19.32
22.44
26.3t
27.22
The patients were
followed-up
even
after
completion
of
the
treatment.
Of
the 98
subjects treated
in
this
study,
93 (94.897o) were dischargedwith
BL (82.657o)subjects having
ascore
of 0
and therest
a scoreof
l.
The
PEFR
values were270.81
+ 60.94l/nrin.
(58.22
+ 14.08%)in
theinhalation
group
and 247 .3 + 36l/min.
(52.29
+
lO.497o)
in
the intravenous
group.
The
greatest improvement
in
PEFR was achieved in
minute-60,
the
inhalation group achieved
51.04%
of
the predictive
value and
the
intravenous group
achieved
4l7o
of
the predictive
value.
Thirty
six
(36.737o) patients had
successfully
overcome the acute asthmatic episode,achieving
a PEFR value 50%of
thepredictive
value.DISCUSSION
It
has
been suggested
that
in
patients
with
severebronchial
obstruction,
nlucus
plugging of
theairways
might
prevent nebulized sympathonrinretics from
having an effect on the peripheral airways and
thatintravenous
infusions miqht
therefore be more
effec-tive in
theseconditions.4'm
In
this presentstudy,
how-ever, thebronchodilating
effectsof
inhaledsalbutanrol
was
found
to
be
superior
to
intravenous salbutamol,
irrespective
of
the baselineventilatory function. This
significant
difference was apparent
in
minute-15,
peaked
in
minute-60
and lasteduntil
minute-9O.During
an acuteasthmatic attack,
even aslight
degreeof
inrprovement
needs
a higher
dose
of
inhaled
R2-Initial Treanuent of Acute Sev,ere
Asthna
27I
agonists.
Theairway obstruction
leads todifficulty in
inhaling
andsubsequently
only
asmall
percentageof
the
drug
can bedeposited
in
theairways.
This
condi-tion is further
aggravated
by
the increased
diffusion
barrier
between theairway
mucosa and the B- receptorson the
vesselswalls
and smooth muscle, and
by
thediminished
responsiveness
of
the
B2-receptors:ll-13Several studies have compared the
efficacy of
inhaled
to
intravenous
R2-agonist. The results varied from
slight superiority (R=5) of
parenteral
salbutamol,14'lsto equal effectiveness (R= lO) of both treatments,2'16
or
slight
or
mor-emarked
superiority (R=30)
of
inhaled
salbutamol.ap
The
difference in
results
can bepartty
attributed
to
theinhaled
to
intravenous
doseratio
(R)
used andto
the modeof
administration.
The
Swedish Society
of Chest
Medicine
have found
that
0.15 mg/kgBW is
the threshold dose
for
inhaled
salbutamol, since
repetition
of
this
dose
causedsys-temic si{e-effects and
high
plasma levels
of
sal-butamol.s
The threshold dose
for
intravenous
sal-butamol was found
to
be
5 prg/kgBw. It
producedpeak plasma
concentration levels that
washigher
thanthat noted
after
the second inhaled
dose.
This
peak
plasma concentration
of
intravenous
salbutamol
ap-peared
much
sooner
with
a
more
rapid
decline.
In-creasing
the
dose caused
an
increase
in
systemic
side-effects.
Although
theinhalation
dose wasalmost
30
that
of
the intravenous dose,
Yanson
have found
that the
average
systemic
availability
of
0.15
nrg/KgBW of
salbutamol
wasapproximately egual
to thatof
5pg/kgBW of
intravenous
salbutamol.r/
B2-receptors are present in the
airway
smooth nluscles,nicrovasculature,
glands, andepithelial cells
from
the tracheato
the
terminal bronchioles. Their
density
in-creases
with
decreasingairway size.
Both
thecentral
and the peripheral airways dilate after inhalation
of
B2-agonist
drugs.
The inhaled drug
will
be mainly
centrally deposited.
It
not only
exerts
a
local effect
but
will
also be
absorbedand
distributed
throughout
the
bronchial
tree
via
the
densesubmucosal vascular
network.
l3
Therefore the site
of
deposition
of
the B-agonists^playsa
lessimportant role
it
is below
theglottis. rE'le This
processwill
depend on theinhalation
n'laneuvresperformed.
A
large
amount
of
intrapul-monary deposited
fraction
(20-607o)
and apeak
con-centration
of .357qwas produced
with
deepand slow
inspiration.s
Inhalations
of
large amountsof
solution
aretime
con-suming and
tiring
for
the
patients,20while a
small
amount
or
concentrated solution
will
decrease
the [image:8.595.53.289.118.333.2]272
Katili et al.salbutamol
in this
study
wasdiluted
to 2
ml. Several
studies,
however,
havefound
that there was nocorrela-tion
between the amount
of solution
andthe
increaseof
PEFR
after
inhalation.2o'21
Intermittent
inhalation
through a fingertip controlled nebulizer
used
in
this
study permits
ahighly efficient
and consistent methodof delivery.
The duration
of
dyspnea
prior
to
admission was not
observed to
becorrelated
with lung function
changesin minutes-60
and-
120for
both treatmentgroups.
But
there was a moderate
correlation
(r = 0.54) between theseverity
of
the
initial
obstruction
andPEFR
improve-ment
in
minute-45.
Should
the duration
of
dyspneainfluenced
thePEFR
changes,it will
not
be apparentat the
initial
phaseor
the
rapid
phaseof
the biphasic
response
to
treatment due
to
the resolution
of the
smooth
musclecontraction.l'22 In
addition,22% of
îhe
cases
in
the intravenous group,
with a
duration
of
breathlessness
of
more than
2l
hours,
were
patients
with
an acute episode
of
chronic
asthma
requiring
intense
treatment
andlonger periods
of
observation to
relieve the mucosal
airway inflammation.
Studies
have
showed
that
the
increase
in
pulse
ratewere
19-24beats/min.
in
intravenously
treated groupscompared
with
5-8
beats/min.
in
the
inhalation
g.oup..2'a'5'23
Thi.
study
found
that the
increase wasl9
beats/min.
in
intravenous group,
which
double
thatof
the rate
of
8
beats/min.
for
the inhalation
group.Thos
rise
was
significant
in
minutes-15 and
-30
anddeclined
in minute-45.
Overall,
the average pulse ratewas
significantly higher
in
the intravenously
treatedgroup.
The
slight
decrease
of
nrean
systolic and diastolic
pressures
in
both groups were not found
to
be
sig-nificant.
This finding
was
not similar to
the
double-blind
randomized
study
using terbutalin,
which
showed a more significant
decrease
in
mean blood
pressure
(p
<0.02).2
Theside-effects,
such astremor,
palpitation,
andcephalgia
were moreprominent
in
theintravenously
treatedgroup.
They
can beattributed
tohemodynamic
changes causedby salbutamol
stimula-tion of
theBl
and 82 adrenergicreceptors. Tremor
andpalpitations were marked
in
minutes-15
and -30.Previous studies have
only
included patients
with
aninitial
PEFR
of
33%
to
theinhalation group
and3l7o
the
predictive value to the
intravenous
group.s
Thit
present study have also
included
patientswith
aninitial
PEFR
of
less
than l57o
the
predictive value,
unlessthey could
not be
evaluated
with
a
mini
Wright
ap-Med J Indones
paratus. The
averageinitial
PEFR
valuesin
this study
werc
19.69%for
theinhalation
group and
18.18% thepredictive value
for
theintravenous
group. In
thefirst
hour
of
treatment, nosignificant
increasein
PEFR wasfound
in
28
patients
(56%)
of
the intravenous group
and
31
patients (64.58%)
of
the
inhalation
group,
whereas
in l9
patients(38%) of
theintravenous
andl5
patients
(31.85%)
of
the inhalation group the
PEFR
remained
unchanged.
There was adecline
in the PEFRof
5 patientsprior
toaminophylline infusion.
But
there was asignificant
risein
PEFRafter
abolus infusion
of
aminophylline was
administered.
It
was unclear
whether the
rise
in
PEFR was due
to
aminophylline.
The
improvement
in
lung function
by
administration
of
corticosteroids was
slow
and
not
generally
seenduring the
first
4
hours
of
treatment.
Intravenous
hydrocortisone
restored 82- adrenergic. responsivity
in
3-5
hours
after
administration
(24). Early
use
of
steroids
istherefore indicated (25),
sinceit
canpoten-tiate the
effects
of
B2-agonist
drugs
even
though
noeffect
was
seenwithin
the
first 2
hours
of
treatment.Although the addition
of
aminophylline
showed
noimmediate benefit,
it
can provide additional
thera-peutic benefit
in
the
ftrsl
24 hours
of
patients
with
severe
airway obstruction
treated
with
systemic
cor-ticosteroids.
The
patients improved
enough to be dischargedwith
ascore
of
0
or
I
and aPEFR
of
58.24%
thepredictive
value
for
the
inhalation
group
and
51.587o
of the
predictive
valuefor
theintravenous group.
Theinhala-tion
group
had an increaseof 5l7o
in
PEFRin
minute-60,
while in
theintravenous group
therise
was4l%
of
thepredictive value. Thirty
six (36.737o) patients had passed the acute episodeachieving
a PEFR 507oof
thepredictive value. In
aprevious study
only
357oof the
caseshad
passedthe
acute episode
in
the
first
hour,
even
with
intensive
useof
drugs
of
proven
effective-n"r..'
Th"
rymptoms
disapp.eared when the PEFR was43 7o
of
thepredictive
va Iue"
and al 5 4 7o the predicti vevalue, patients were scored as
clinically
free
of
symptoms.
Th'eresults
of physical
examination
wassatisfactory,
while lung function
was
still
lower
thannormal.
This
was due
to the
tendency
of
the
larger
airways
to
increase
their
resistence
quickly
when
stimulated,
which
can be reversedrapidly.
CONCLUSIONS
Intermittent inhalation
of
nebulized salbutamol
was proven to beeffective in
relieving
bronchospasm. The
inrprovement of
theclinical
symptoms in
severe acutewere
minimal.
Combining aminophylline to
inhaled
B2-agonist can
provide additive therapeutic benefits.
Acknowledgements
The
authorswish
to thank
Dr. Armen
Mochtar,
of
theDepartment
of
Pharmacology
of
theUniversity
of
In-donesia,
for
his valuable
advice
on
pharmacological
and
statistical
aspectsof
this study.
REFERENCES
l.
Fanta CH, Rossing TH, Mc Fadden ER. Emergency treat-ment treattreat-ment of asthma. Relationship among therapeutic combination, severityof
obstruction andthis
courseof
response. Am
I
Med 1982;122416-22.2. Van Renterghem P, Lamont
H,
ElincW.
Intravenous vs nebulized terbutalin in patients with acute severe asthma.A
double blind randomized srudy. Ann Allergy 1987;59:313.
3. Hetzel
MR,
Clark TJH. Comparisonof
intravenous andaerosol salbutamol. Br Med
I
1976; Oct: 919.4: Bloomfield P, Carmichael J, Petrie GR. Comparison of given
intravenously and
by
intermittent spontaneous pressurebreathing
in life
threatening asthma.Br
MedI
1979; L:848-50.
5. Swedish Society of Chest Medicine. High dose inhaled vs intravenous salbutamol combined
with
theophyllinein
severe acute asthma. Eur Respir J 1990; 3: 183-70. 6. Cochrane. Acute severe asthma.
A
colour atlas of asthma.England. Wolfe Medical Publication Ltd 1989: 70. 7.
British
Thoracic Society. Guidelinefor
managmentof
asthma adults. Acute severe asthma. Br Med
J
1990; 301: 797-30t.8. Matthys H, Kohler D. Puhnonary deposition of aerosols by different mechanical devices. Respiration 1985 ;4 8 : 269 -'t 6. 9. Siez D, Chai H. A standard method of intermittent inhaled
'
therapy via a jet nebulizer. Ann Allergylglg;
57:245.10. Cochrane GM. The role of bronchodilators in severe acute asthma in Clark
TIH.
Bronchodilator therapy. The basisof
asthma and chronic obstructive airways disease manage-ment. New Tnaland. Adis press Ltd 2nd ed; l9g4: 167.London :2nd Chapman & Hall 1983; 393.
12. Barnes P. Neural control of.human airways
in
health and disease. State of an. Am Rev Respir Dis 1986; 134:12g9 13. Kerreblyn PK. Beta agonists.In
KalinerMA,
Barnes pI,Pierson eds: Asthrna.
New York. Marcel
Dekker Inc;l99l:523.
14. Cheong
B,
Reynold S, RajanKG.
Comparisonof
in-trâvenouswith
nebulized salbutamolin
the treatmentof
acute severe asthma. Br Med J 1988; 29j:448-50. 15. William S, Seaton A, Parrish R. Cornparison of intravenous
aminophylline and salbutamol in severe asthma. Br Med J 1975; Dec: 685.
16. Lawford P, Jones
IM,
Milledge JS. Comparisonof
in-trâvenous and nebulized salbutamol ininitial
treatrnentof
severe asthma. Br Med J 1918;2:84.
17. Yanson
lvfl.
Plasma levels and effectof
salbutamol after inhaled or i.v adnrinistration in stable asthma. Eur Respir Jl99l;
4: 544-50.18.
Ruffin
RE, MontgomeryJM,
NewhouseMT.
Siteof
B-adrenergik receptorsin
the respiratory tract. Deliveryof
fenoterol administration by two methods. Chest
1978:256-,
80.,19.
Kohler
D,
FleischerW.
Established factsin
inhalation therapy. Arcis Verlag Munchen 1991.20. Haldfield
fW,
Windenbank WJ, Bareman JRM. Is driving gas flow rate clinically important for nebulizer therapy ?. BrJ Dis of Chest 1988; 80:50-4.
2l . Mitchel DM, Solomon MA, Tolfree SEJ, Shorr M, Spiro SG. Effect of particles size of bronchodilator aerosols on lung distribution and pulrnonary function in patients with chronic asthma. Thorax 1987
;
42: 457 -61.22. McFadden ER, Kiser
R,
DeGrootWI.
Acute bronchial asthma Relation between clinical and physiological manifes-tation. The N Engl J Med 1973; 288:221-25.23. Noseda A, Yernault IC. Syrnpathomirnetics in acute severe asthma, inhaled
or
parenteral, nebulizeror
spacer ? Eur RespirI
1989;2 :377-82.24.
Holgate ST. Bronchoconstriction.
In
Clark
TJH. Bronchodilator therapy. T1te basicof
asthma and chronic obstructive airways disease ntanâgment. New Zealand. Adis Press Ltd 1984; l-13.