ABSTRACT
LYNN KAAT LAURA KURNIAWAN. Motion Mode Echocardiography of Healthy Male Indonesian Domestic House Cat. Supervised by DENI NOVIANA.
The purpose of this study was to determine the normal echocardiographic heart measurements of healthy male Indonesian Domestic House Cat. Nine male cats weighing between 3.3 – 4.4 kg were anesthetized using zolazepam-tiletamine (8 mg/kgBW). Echocardiographic examination was performed on the right parasternal short axis at the papillary muscles and the aorta level using a 7.5 MHz transducer. Parameters observed were: left ventricular wall thickness (LVW), interventricular septa thickness (IVS), left ventricular internal diameter (LVID), aorta (AO), and left atrium (LA), all during systole (s) and diastole (d). Derivative measurements include left ventricular end-diastolic volume (LVED), end-systolic volume (LVES), stroke volume (SV), cardiac output (CO), ejection fraction (EF), and fractional shortening (FS). Brightness mode shows that the IVS and LVW were hypoechoic, the LA, LVID and aorta lumen were anechoic and the aorta wall was hyperechoic. The average measurements of the Indonesian Domestic House Cat were: LVWs 0.57 ± 0.03 cm, LVWd 0.35 ± 0.06 cm, IVSs 0.56 ± 0.03 cm, IVSd 0.30 ± 0.05 cm, LVIDs 0.78 ± 0.11 cm, LVIDd 1.48 ± 0.12 cm, AOs 0.69 ± 0.06 cm, LA 0.73 ± 0.04 cm. The LA:AO ratio was 1.07 ± 0.1. Derivative measurements: LVED 5.96 1.17 ml, LVES 1.08 0.42 ml, SV 1.08 0.42 ml, CO 865.18 178.66 ml/minute, EF 82.28 4.64 %, FS 48 5 %.
ABSTRAK
LYNN KAAT LAURA KURNIAWAN. Motion mode Ekhokardiografi Kucing Jantan Lokal Indonesia. Dibimbing oleh DENI NOVIANA.
Tujuan dari penelitian ini adalah untuk mengetahui nilai ekhokardiografi normal ukuran jantung dari kucing jantan lokal Indonesia yang sehat. Sembilan ekor kucing jantan dengan bobot badan antara 3.3 – 4.4 kg dianaestesi menggunakan kombinasi zolazepam-tiletamine (8 mg/kgBB). Pengambilan data diambil menggunakan transduser 7.5 MHz pada sumbu pendek jantung (short axis) dengan hewan berbaring pada posisi lateral kanan. Parameter yang diukur antara lain: ketebalan dinding ventrikel kiri (LVW), ketebalan interventrikular septa (IVS), dimensi ventrikel kiri (LVID), dimensi aorta (AO), dan dimensi atrium kiri (LA) pada saat sistole (s) dan diastole (d). Perhitungan turunan antara lain volume ventrikel kiri pada akhir diastole (LVED), pada akhir sistole (LVES), stroke volume (SV), cardiac output (CO), ejection fraction (EF), dan fraksi pemendekan (FS). Brightness mode menunjukkan bahwa IVS dan LVW bersifat hipoekhoik, dimensi LA, LVID dan AO bersifat anekhoik, dan dinding aorta bersifat hiperekhoik. Perhitungan rata-rata pengukuran yang didapat adalah: LVWs 0.57 ± 0.03 cm, LVWd 0.35 ± 0.06 cm, IVSs 0.56 ± 0.03 cm, IVSd 0.30 ± 0.05 cm, LVIDs 0.78 ± 0.11 cm, LVIDd 1.48 ± 0.12 cm, AOs 0.69 ± 0.06 cm, LA 0.73 ± 0.04 cm. Perbandingan LA:AO adalah 1.07 ± 0.1. Perhitungan turunan: LVED 5.96 1.17 ml, LVES 1.08 0.42 ml, SV 1.08 0.42 ml, CO 865.18 178.66 ml/menit, EF 82.28 4.64 %, FS 48 5 %.
20
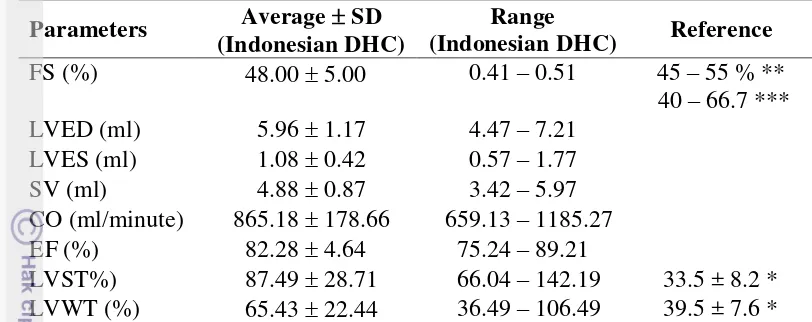
Attachment 1 Heart measurement references Parameter Moise and
Dietz*
Jacobs and
Knight* Fox et al.* Allen**
N 11 30 10 10
AO 0.95 ± 0.15 0.95 ± 0.11 0.94 ± 0.11 0.90 ± 0.07 LA 1.21 ± 0.18 1.23 ± 0.14 1.03 ± 0.14 1.00 ± 0.07 LVWs 0.78 ± 0.10 0.68 ± 0.07 0.55 ± 0.88
LVWd 0.46 ± 0.05 0.33 ± 0.06 0.35 ± 0.05 0.40 ± 0.40 LVIDs 0.69 ± 0.22 0.80 ± 0.14 0.81 ± 0.16 0.86 ± 0.16 LVIDd 1.51 ± 0.21 1.59 ± 0.19 1.40 ± 0.13 1.30 ± 0.12
IVSs 0.76 ± 0.12 0.58 ± 0.06 - -
IVSd 0.50 ± 0.07 0.31 ± 0.04 0.36 ± 0.08 0.40 ± 0.03
HR 182 ± 22 194 ± 23 255 ± 36 175 ± 20
BW 4.3 ± 0.5 4.1 ± 1.1 3.91 ± 1.2 3.64 ± 0.66
LVST 33.5 ± 8.2 - - -
LVWT 39.5 ± 7.6 - - -
FS 55.0 ± 10.2 49.3 ± 5.3 42.7 ± 8.1 0.35 ± 0.25 LA:AO 1.29 ± 0.23 1.30 ± 0.17 1.10 ± 0.18 -
MOTION MODE ECHOCARDIOGRAPHY ON HEALTHY
MALE INDONESIAN DOMESTIC HOUSE CAT
LYNN KAAT LAURA KURNIAWAN
DEPARTMENT OF CLINIC, REPRODUCTION AND PATHOLOGY FACULTY OF VETERINARY MEDICINE
BOGOR AGRICULTURAL UNIVERSITY BOGOR
STATEMENT ABOUT UNDERGRADUATE THESIS AND
INFORMATION SOURCES AND COPY RIGHT*
I hereby state that this undergraduate thesis entitled “Motion Mode Echocardiography on Healthy Indonesian Domestic House Cat” is my own work with the assistance of my supervisor and has not been submitted in any form to other institutes. Sources of information used in this undergraduate thesis quoted from other sources both publicized and unpublicized are written at the end of this thesis.
I hereby present the copy right of my undergraduate thesis to the Bogor Agricultural University.
ABSTRACT
LYNN KAAT LAURA KURNIAWAN. Motion Mode Echocardiography of Healthy Male Indonesian Domestic House Cat. Supervised by DENI NOVIANA.
The purpose of this study was to determine the normal echocardiographic heart measurements of healthy male Indonesian Domestic House Cat. Nine male cats weighing between 3.3 – 4.4 kg were anesthetized using zolazepam-tiletamine (8 mg/kgBW). Echocardiographic examination was performed on the right parasternal short axis at the papillary muscles and the aorta level using a 7.5 MHz transducer. Parameters observed were: left ventricular wall thickness (LVW), interventricular septa thickness (IVS), left ventricular internal diameter (LVID), aorta (AO), and left atrium (LA), all during systole (s) and diastole (d). Derivative measurements include left ventricular end-diastolic volume (LVED), end-systolic volume (LVES), stroke volume (SV), cardiac output (CO), ejection fraction (EF), and fractional shortening (FS). Brightness mode shows that the IVS and LVW were hypoechoic, the LA, LVID and aorta lumen were anechoic and the aorta wall was hyperechoic. The average measurements of the Indonesian Domestic House Cat were: LVWs 0.57 ± 0.03 cm, LVWd 0.35 ± 0.06 cm, IVSs 0.56 ± 0.03 cm, IVSd 0.30 ± 0.05 cm, LVIDs 0.78 ± 0.11 cm, LVIDd 1.48 ± 0.12 cm, AOs 0.69 ± 0.06 cm, LA 0.73 ± 0.04 cm. The LA:AO ratio was 1.07 ± 0.1. Derivative measurements: LVED 5.96 1.17 ml, LVES 1.08 0.42 ml, SV 1.08 0.42 ml, CO 865.18 178.66 ml/minute, EF 82.28 4.64 %, FS 48 5 %.
ABSTRAK
LYNN KAAT LAURA KURNIAWAN. Motion mode Ekhokardiografi Kucing Jantan Lokal Indonesia. Dibimbing oleh DENI NOVIANA.
Tujuan dari penelitian ini adalah untuk mengetahui nilai ekhokardiografi normal ukuran jantung dari kucing jantan lokal Indonesia yang sehat. Sembilan ekor kucing jantan dengan bobot badan antara 3.3 – 4.4 kg dianaestesi menggunakan kombinasi zolazepam-tiletamine (8 mg/kgBB). Pengambilan data diambil menggunakan transduser 7.5 MHz pada sumbu pendek jantung (short axis) dengan hewan berbaring pada posisi lateral kanan. Parameter yang diukur antara lain: ketebalan dinding ventrikel kiri (LVW), ketebalan interventrikular septa (IVS), dimensi ventrikel kiri (LVID), dimensi aorta (AO), dan dimensi atrium kiri (LA) pada saat sistole (s) dan diastole (d). Perhitungan turunan antara lain volume ventrikel kiri pada akhir diastole (LVED), pada akhir sistole (LVES), stroke volume (SV), cardiac output (CO), ejection fraction (EF), dan fraksi pemendekan (FS). Brightness mode menunjukkan bahwa IVS dan LVW bersifat hipoekhoik, dimensi LA, LVID dan AO bersifat anekhoik, dan dinding aorta bersifat hiperekhoik. Perhitungan rata-rata pengukuran yang didapat adalah: LVWs 0.57 ± 0.03 cm, LVWd 0.35 ± 0.06 cm, IVSs 0.56 ± 0.03 cm, IVSd 0.30 ± 0.05 cm, LVIDs 0.78 ± 0.11 cm, LVIDd 1.48 ± 0.12 cm, AOs 0.69 ± 0.06 cm, LA 0.73 ± 0.04 cm. Perbandingan LA:AO adalah 1.07 ± 0.1. Perhitungan turunan: LVED 5.96 1.17 ml, LVES 1.08 0.42 ml, SV 1.08 0.42 ml, CO 865.18 178.66 ml/menit, EF 82.28 4.64 %, FS 48 5 %.
Undergraduate Thesis as a requirement for a degree of Bachelor of Veterinary Medicine
Faculty of Veterinary Medicine
MOTION MODE ECHOCARDIOGRAPHY ON HEALTHY
MALE INDONESIAN DOMESTIC HOUSE CAT
LYNN KAAT LAURA KURNIAWAN
DEPARTMENT OF CLINIC, REPRODUCTION AND PATHOLOGY FACULTY OF VETERINARY MEDICINE
BOGOR AGRICULTURAL UNIVERSITY BOGOR
Title : Motion Mode Echocardiography on Healthy Male Indonesian Domestic House Cat
Name : Lynn Kaat Laura Kurniawan
NIM : B04080055
Approved by
Drh. Deni Noviana, Ph.D Supervisor
Known by
Drh. Agus Setiono, MS, Ph.D, APVet (K) Vice Dean
FOREWORD
Praise and grace to the Lord for His guidance and blessings during the writing of this undergraduate thesis. This thesis entitled “Motion Mode Echocardiography on Indonesian Domestic House Cat” was done from November 2011 until April 2012 in the Clinic, Reproduction and Pathology Department.
The writer would like to thank everyone who made this undergraduate thesis possible:
1. Drh. Deni Noviana, Ph.D as the supervisor for the assistance and guidence in making this thesis
2. Dr. Drh. Trioso Purnawarman, M.Si as the academic advisor for the assistance and support these past three years
3. Dr. Drh. Hj. Gunanti, MS as the head of the Division of Surgery and Radiology for the opportunity to do the research in the Radiology Laboratory 4. Colleagues in the Radiology Laboratory: drh. Mokhamad Fakhrul Ulum,
M.Si, drh. Devi Paramitha, Mr. Katim, Ajeng, Rio, Nengsih, Andi, Pras, Ruri, Cholis, Nisa, Hastin, Ayip, Erly, Medy, Yiyi, Ridwan
5. Father, mother, brother, grandmother, and my whole family in Indonesia, Belgium, China, and the Netherlands for the love, spirit and support
6. My best friends: Puvean, Hana, Arca, Rio, Moniq, Irin, Titus, Cory, Novra for the friendship, laughter, crazyness and everything in between
7. KeMaKI, UKF, HKSA for the second home
The writer hopes that this thesis can be beneficial for the readers, especially veterinary small animal practitioners.
CONTENT
LIST OF PICTURES xiii
LIST OF TABLES xiii
LIST OF ATTACHMENT xiii
INTRODUCTION 1
Background 1
Objective 1
Benefit 1
LITERATURE STUDY 1
Indonesian Domestic House Cat 1
Heart 2
Ultrasonography 3
Heart Ultrasonography (Echocardiography) 4
Right Parasternal Short Axis View 5
Zolazepam-Tiletamine (Zoletil) 6
MATERIALS AND METHOD 7
Time and Place 7
Animals and Materials 7
Method 7
Animals 7
Preparation 8
Echocardiography Technique 8
Data Analysis 8
RESULT AND DISCUSSION 9
Physical Examination 9
Echocardiography 10
Muscular Walls: Left Ventricular Wall and Interventricular Septa 10 Internal Dimensions: Aorta, Left Atrium, Left Ventricle 12
Derivative Measurements 14
Fractional Shortening 15
Left Ventricular Volumes and Ejection Fraction 15 Left Ventricular Stroke Volume and Cardiac Output 16
CONCLUSION 16
REFERENCES 16
ATTACHMENT 20
LIST OF PICTURES
1 The cat heart 3
2 Probe positions and motion mode imaging 4
3 Right parasternal short axis views of the heart 6
4 View of the heart at the papillary muscle level 11
5 View of the heart at the aorta level 13
LIST OF TABLES
1 Normal physiological measurements of a cat 2
2 Comparative heart size of a normal cat 5
3 Equations for the measurement derivates 9
4 Physical examination result of Indonesian Domestic House Cat 9 5 Heart wall thickness of Indonesian Domestic House Cat 12 6 Internal dimension measurement of Indonesian Domestic House Cat 14 7 Derivative cardiac measurements of the Indonesian Domestic House
Cat 15
LIST OF ATTACHMENT
INTRODUCTION
Background
The Indonesian Domestic House Cat (DHC) is a typical Indonesian short hair cat breed from the genus Felis. Cats are prone to various diseases, especially cardiovascular diseases (Scansen 2011b; Mottet et al. 2012). The most common complaint in cats is the evaluation of heart murmur during auscultation. The use of ultrasonographic imaging has had a tremendous impact on the diagnosis of cardiac disease, giving a surrogate measure of the heart size (Chandler et al. 2004; Abbott and MacLean 2006; Coatney 2001). Cardiologists are commonly asked to perform echocardiographic examinations on cats to screen for both acquired and congenital heart diseases (Adin and McCloy 2005; Chetboul et al. 2006).
Echocardiographic values show significant breed variations. Data concerning normal heart-size values have been reported in a number of pedigree cats, however little the normal echocardiogram of the Indonesian DHC has been reported. This is why the purpose of this study was to determine the normal echocardiographic reference values in anesthetized Indonesian DHC. Knowledge of the normal measurements for a specific body weight can help to indicate the degree and direction of the change from normal (Muzzi et al. 2006).
Objective
The objective of this study was to provide the normal values of Motion-mode (M-Motion-mode) echocardiographic cardiac measurements in healthy anesthetized male Indonesian Domestic House Cat.
Benefits
It is hoped that the normal cardiac measurements of the male Indonesian Domestic House Cat can add the physiological database of cat heart measurements to then be used, especially by veterinary practitioners, as a reference when dealing with heart ultrasonography of the Indonesian Domestic House Cat.
LITERATURE STUDY
Indonesian Domestic House Cat (DHC)
2
subject to selective breeding and conform no special standards. They have coats of any length in a range of colors that are usually plain, blotched, striped, or parched (Foss et al. 2008). They usually have green or yellow eyes and a fairly long nose (Edwards 1999).
Domestic House Cats belong to the short hair and non-pedigree group. Male cats are generally larger than females. Table 1 shows the normal physiological data of a cat. A healthy cat has the following physical features: clean and shiny coat, clean eyes and nose, no indications of swollen gums and bad breath, and no physical trauma (Edwards 1999).
Table 1 Normal physiological data of a cat
Adult cat Average adult cat Newborn kitten
Source: Eldredge et al (2008); bpm: beats per minute.
Heart
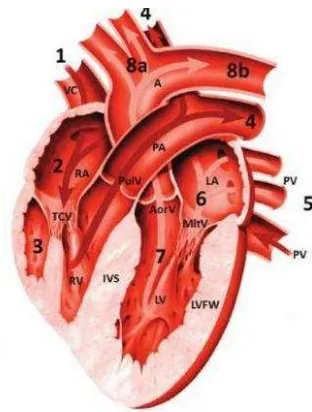
The heart rests in the cavity of the mediastinum near the midline of the thoracic cavity on the diaphragm. About two-thirds of the mass of the heart lies on the left of the body’s midline (Amitrano and Tortora 2007). Anatomically, the heart is a hollow organ with muscular walls and four chambers separated by valves (Norsworthy 2012). The walls of the heart has three layers: the epicardium organized by a single layer of squamous epithelium, the myocardium organized by muscle cells and the endocardium organized by a single layer of epithelium cells (Sebastiani and Fishbeck 2005). Interventricular septum (IVS) is the muscular separation between the left and right ventricle. Papillary muscles are broad, finger-like projections in the cavity of the ventricle. Each papillary muscle has multiple smaller heads giving rise to the chordae tendineae (Factor et al. 2002). The aorta also consists of three layers: the innermost layer, the media and the adventitia (Schmidt 2006).
3
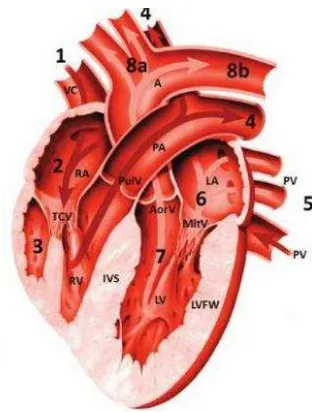
Figure 1 The cat heart; A: aorta, VC: vena cava, RA: right atrium, TCV: tricuspidalis valve, RV: right ventricle, PulV: pulmonary valve, PA: pulmonary artery, PV: pulmonary vein, LA: left atrium, MitV: mitral valve, LV: left ventricle, AorV: aortic valve, LVFW: left ventricular free wall, IVS: interventricular septa; the numbers (1, 2, 3, 4, 5, 6, 7, 8a, 8b) show the sequence of blood flow process; Source: Norsworthy (2012).
Ultrasonography
Ultrasonography is a non-invasive diagnostic tool commonly used by veterinary practitioners (Chandler et al. 2004; Copley et al. 2008; Corwin 2008; Kealy et al. 2011). It uses frequencies greater than 20.000 cycles/second (Hz). The transmission velocity of ultrasound waves in blood and most soft tissues cells is uniform at 1540 m/sec (Coatney 2001). Common ultrasound frequencies used in cats are between 2 and 15 MHz (Chandler et al. 2004; Schober and Todd 2010; Kealy et al. 2011; Noviana et al. 2012). Lower frequencies result in higher penetration but lower resolution (Silkowski 2010). Ultrasound can not be transmitted through vacum and it’s velocity in gas is very low (Noviana et al. 2012).
An ultrasound device consists of a probe and a monitor (Noviana et al. 2012). Ultrasound waves are formed inside the probe by a piezoelectric effect on a medium such as a crystal made of lead zirconate. The probe can act as an emitter (wave producer) and receiver (echo accepter). An electrical impulse causes the crystal to lose its shape, vibrate and release ultrasound waves. The waves transmitted by the transducer are called pulse, while the waves reflected back to the transducer is called echo. The probe is connected to a computer or signal processor which processes the signals and presents it as grey dots. The stronger the echo, the brighter the dots (Kealy et al. 2011; Noviana et al. 2012).
4
echoes/sonolucent present, appearing black on the screen. Hyperechoic is a projection with rich and highly reflective echoes, appearing as varying shades of lighter gray (Silkowski 2010).
There are two kinds of ultrasound imaging: Brightness mode (B-mode) and Motion mode (M-mode). B-mode releases multiple ultrasound pulses at all times and is shown as a two dimensional imaging of the organ (Noviana et al. 2012). M-mode provides a one-dimensional view (depth) into the heart, where the beam transverses as their relative position and moves through time (Ware 2007). M-mode imaging usually provides a clearer resolution of the cardiac borders and a more accurate timing of events within the cycle. Therefore, measurements of the cardiac dimensions and motion are often more accurately assessed from the M-mode tracings (Ware 2007; Boon 2011). The difference between M-M-mode imagings on different probe view angles is illustrated in Figure 2.
Figure 2 Probe positions at the right parasternal long axis view (A) and Motion mode imaging (B); T: transducer, TW: thorax wall, RVW: right ventricular wall, RV: right ventricle, S: septa, LV: left ventricle, AV: aortic valve, AO: aorta, AMV: anterior mitral valve, PMV: posterior mitral valve, LA: left atrium, LVW: left ventricular wall; Source: Ware (2007). RVM: right ventricle muscular, TV: tricuspidal valve, RS: right septa; the numbers (1, 2, 3, 4) indicate the motion mode ultrasound of each level at the right parasternal long axis view.
Heart Ultrasonography
Heart size is important when evaluating cats for cardiac diseases (Chandler et al. 2004; Ghadiri et al. 2008). Diseases such as cardiac neoplasia and lymphoma, idiopathic pericarditis, congestive heart failure, hypertrophic cardiomyopathy, and left ventricular hypertrophy can be diagnosed using ultrasound (Scansen 2001a; Adin and McCloy 2005; Shinohara et al. 2005; Abbot and MacLean 2006; Schober and Todd 2010).
5 is the time when the heart is fully relaxed while systole is the time when the heart is in full contraction (Muzzi et al. 2006). Table 2 shows the comparison sizes of the cat heart.
Table 2 Comparative heart size of a normal cat
Organ Size
Interventricular septal wall (IVS) thickness < 6 mm*
Left ventricular wall (LVW) thickness < 6 mm* or < 1.5 cm** Left atrium (LA) internal diameter <1.6 cm*** or 1.7 x aorta** Right ventricular wall (RVW) thickness ½ LVW**
Right ventricular (RV) internal diameter 1/3 LV Chamber** Fractional shortening (FS) 45 – 55 %****
* Source: Wagner et al. (2010) and Bowles et al. (2010), ** Source: Boon (2011), *** Source: Schober and Todd (2010), **** Source: Norsworthy (2012); LVW: left ventricular wall, LV: left ventricle.
Right Parasternal Short Axis View
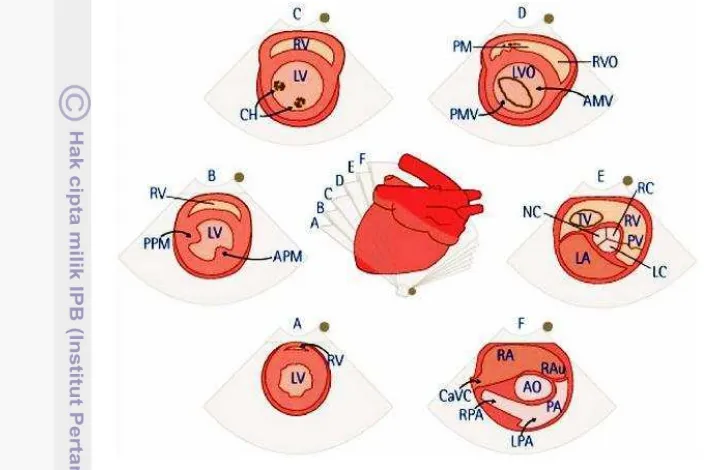
There are at least two rib spaces available for right parasternal views: a cranial location at the fourth intercostal space and a more caudal location at the fifth intercostal space. Examinations are usually done at the right parasternal short axis view at the level of the papillary muscles, measuring the RV and LV (Norsworthy 2012). However, Ware (2007) stated that the LVW, IVS and LVID should be determined at the level of the chorda tendinae. Figure 3 shows different the short-axis views that can be obtained by slightly tilting the probe to another direction. The position of the probe and the position of the animal may affect the size, shape, and relative position of the heart (Ware 2007).
Fractional shortening (FS) is a one-dimensional analogue of ejection fraction that determines the temporal change between LVIDs and LVIDd (Collins et al 2003; Brown and Gaillot 2008). It calculates the percentage of linear reduction in the left ventricular internal dimensions from diastole to systole and is used to evaluate cardiomyopathy and left ventricular performance (Collins et al. 2003, Dehghan et al. 2011). Fractional shortening in normal cats usually ranges from 5–55% and can elevate when excited and decrease when the cat is sedated (Norsworthy 2012).
Left ventricular volume is one of the best way to prognose myocardial infarction (Gottdiener et al. 2004). The left ventricular end-diastolic volume (LVED) and left ventricular end-systolic volume (LVES) are calculated using the equation
and
.
Ejection fraction (EF) is the percentage of volume reduction in the LVID from diastole to systole (Martin 1995) and is calculated by dividing the difference between LVED and LVES with LVED. The American Society of Echocardiography suggested that the EF should be measured from left ventricular volumes rather than estimated by visual inspection (Gottdiener et al 2004).
6
Cardiac output (CO) is the volume of blood pumped by the heart perminute and is the product of the heart rate (HR) and stroke volume (SV) (Collins et al. 2003; Lavdaniti 2008).
Dimensions of LA and the aorta has been used to calculate LA:AO ratio, producing an index for the LA size (Cavalcanti et al. 2009).
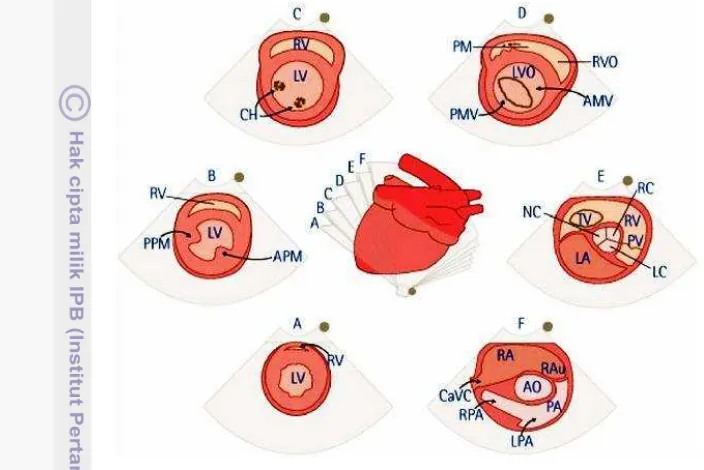
Figure 3 Right parasternal short-axis views of the heart at the level: (A) apex; (B) papillary muscle; (C) chorda tendinae; (D) mitral valve; (E) aortic root/left atrium; (F) pulmonary artery; RV: right ventricle, LV: left ventricle, PM: papillary muscle, CH: chorda tendinae, VS: interventricular septa, PMV: posterior mitral valve, AMV: anterior mitral valve, PV: pulmonary valve, NC: noncoronary cusp, LC: left coronary cusp, RC: right coronary cusp, RAu: right atrial auricle, PA:, LPA: left pulmonary artery. RPA: right pulmonary artery, CaVC: caudal vena cava, PPM: posterior papillary muscle, APM: anterior papillary muscle; Source: Ware (2007).
Zolazepam-Tiletamine (Zoletil)
Zoletil® is a registered trademark of Virbac Laboratories. Itis a non-opoid, non-barbiturate, injectable anesthetic consisting of an equal mixture of tiletamine HCl and zolazepam HCl. It is used intramuscularly to induce and maintain short-term anesthesia. The combination of zolazepam and tiletamine has a short induction time, low dosage, satisfactory safety margins, relatively constant immobilization time, and smooth recovery (Massolo et al. 2003; Kumar et al. 2006).
7 not result in prolonged muscle relaxation (Kumar et al. 2006). The minimal dose of zoletil for a ten minute anesthesia in cats is 4.2 mg/kgBW (Sendler et al. 1994). At the dose of 2.5 mg/kgBW, some cats would be more excited rather than sedated (Forsyth 1995). Sedation of feline patients for diagnostic images is a standard procedure in many veterinarians (Ferasin et al. 2003).
A previous study by Wilson et al. (1993) in adult rats showed that Zoletil® is a cardio stimulatory drug that increases the mean arterial blood pressure and reduces the respiratory depression. A similar research by Hellyer et al. (1989) showed that Zoletil® resulted in a rapid induction of anesthesia and causes an increase in heart rate and cardiac output.
MATERIALS AND METHOD
Time and Place
The study was done from November 2011 until April 2012. Ultrasonography was done at the Division of Surgery and Radiology, Department of the Clinical, Reproduction and Pathology, Faculty of Veterinary and Medicine, Bogor Agricultural University.
Animals and Materials
The animals used for this research were nine (9) healthy male Indonesian Domestic House Cats in the vicinity of the Bogor Agricultural University weighing between 3.3 – 4.4 kg. Cats were quarantined and acclimatized for one week where they were given commercial cat food and water ad libittum. On the first day of quarantine cats were given anthelmintic zipyran plus®. Cats were excluded if they showed any signs of illness and distress.
Ultrasound console used for this research were: Kaixin® ultrasound system, ultrasound scanner (KX5100 Vet), linear probe with frequencies between 5 and 7.5 MHz. Sony video graphic UP-895 MD printer, video camera, shaver, and gavage. The ultrasonography table had a cut out area.
Materials used for this research were ultrasound gel, Zoletil® (50 mg/ml of both zolazepam and tiletamine), atropine sulfat (0.25 mg/ml), commercial cat food, water, syringe (One Med, PT Jaya Mas Medica Industri), and gloves. Zoletil® was prepared by adding 5 ml of sterile water to the vial.
Method
Animals
8
sandbox for defecation and urination. Cats were monitored intensively in the Division of Surgery and Radiology, Department of Clinical, Reproduction and Pathology, Faculty of Veterinary Medicine, Bogor Agricultural University.
Preparation
A physical examination was performed before anesthesia on the cat’s heart rate, respiratory rate, body temperature, and body weight. Cats were given premedication 0.02 mg/kgBW atropine sulfate intramuscularly. After fifteen minutes, cats were anesthetized using 8 mg/kgBW Zoletil® given intramuscularly. The target injection organs were the semitendinosus or semimembranosus muscles. Once the cat was anesthetized, the hair coat at the right lateral axillary area were clipped and shaved clean.
Echocardiography Technique
Echocardiographic examination was performed from underneath with the cat on the right lateral recumbence. The probe was positioned at the fourth or fifth intercostal space. Ultrasonography was performed on the short axis at the papillary muscle and aorta level on both brightness mode (B-mode) and motion mode (M-mode). Ultrasonography was performed by one operator using a 7.5 MHz transducer approximately 15 minutes after the cat was anesthetized. Data using the M-mode were taken until there were three representative data for each measurements. Pictures were taken using a handycam connected to the monitor.
Data Analysis
Data were performed offline using Image-J© obtained from the National Institute of Health. The M-mode right parasternal short axis view at the papillary muscle level was used to measure the left ventricular wall (LVW) thickness during systole and diastole, the left ventricular internal diameter (LVID) during systole and diastole, and the interventricular septa wall (IVS) thickness during systole and diastole. The M-mode right parasternal short axis reading at the aorta level was used to measure the diameter of the aorta (AO) and the left atrium (LA) during diastole. Diastolic measurements were taken at the maximal left ventricular relaxation, while systolic measurements were taken at the maximal left ventricular contraction. The final value used was the average from the three measurements. Data were expressed as average ± standard deviation.
9 Table 3 Equations for the measurement derivates
Parameter Equation
LVIDd: left ventricular internal diameter during diastole, LVIDs: left ventricular internal diameter during systole, HR: heart rate; Source: Boon (2011).
RESULT AND DISCUSSION
Physical Examination
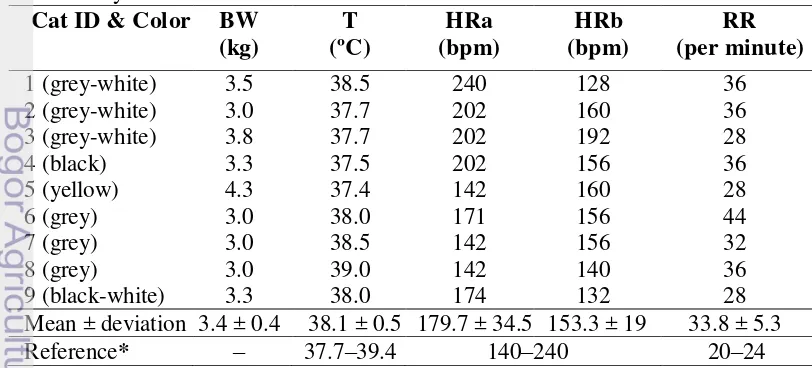
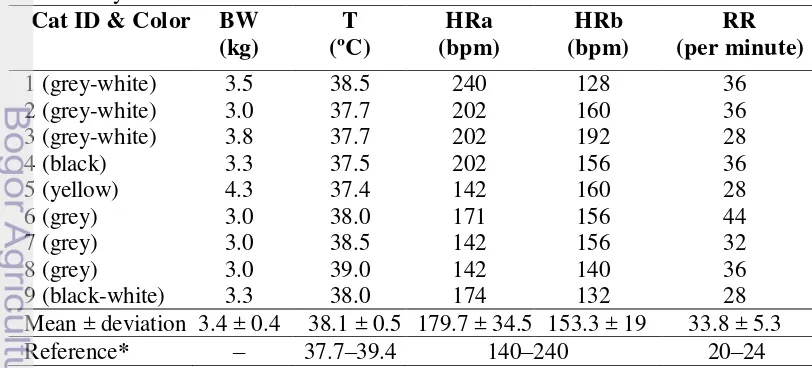
Cats used in this research were nine healthy male Indonesian Domestic House Cats (DHC). Conform to Edwards (1999), the cats were considered healthy when their eyes, coat and ears were clean and there were no indication of swollen gums or physical traumas. Common clinical signs of heart disease in cats such as respiratory distress (tachypnea and dyspnea), per acute paralysis or paresis (usually hind limb or forelimb), anorexia, lethargy, and depression were not observed in the cats used for ultrasonography.
10
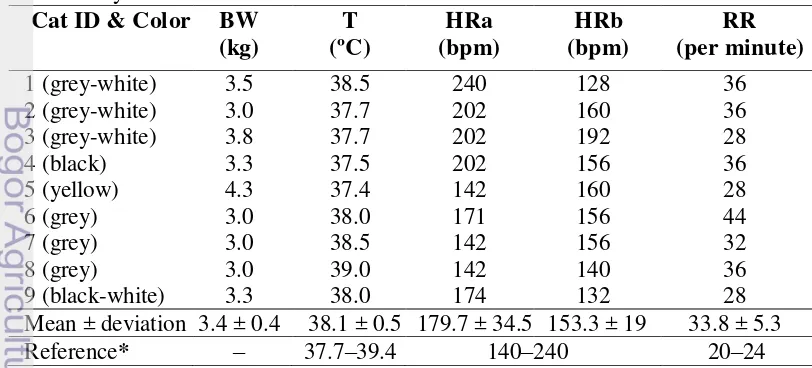
Preliminary physical examination (Table 4) before anesthesia shows that the Indonesian DHC had an average body weight of 3.4 ± 0.4 kg. Their average temperature, 38.1 ± 0.5ºC, was still in the normal range for cats (37.7 – 39.4ºC) (Eldredge et al. 2008). The mean heart rate before anesthesia was 179.7 ± 34.5 bpm and after anesthesia decreased into 153.3 ± 19 bpm. The heart rates of the cats used in this research were still within the normal range for cats (Eldredge et al. 2008). A higher heart rate before anesthesia may be caused the stress during handling. The average respiratory rate was 33.8 ± 5.3 times/minute. This is slightly higher than the normal respiratory range for cats (20 – 24 x/minute) (Eldredge et al. 2008). Anesthesia and stress may cause effects on the heart rate (Lang 2006).
Echocardiography
Echocardiography is a common diagnostic test for the heart, providing an evaluation on the cardiovascular structure, function and hemodynamics. In this research, the probe was positioned on the right parasternal short axis view at the level of the papillary muscle. This is the level at which left ventricular measurements are made (Martin and Corcoran 2006). This research used a 7.5 MHz linear transducer. The sonogram could be displayed despite the linear transducer because cats have relatively thin costae bones penetrable by ultrasound. The measurement results from the Indonesian DHC were compared to measurements by Moise and Dietz, Jacobs and Knight, Fox et al. obtained from Kealy et al. (2011), and measurements by Allen obtained from Brown and Gaillot (2008). The study by Moize and Dietz was done on 11 cats with a body weight of 4.3 ± 0.5 kg and an average heart rate of 182 ± 22 bpm. The study of Jacobs and Knight in Kealy et al. (2011) was done on 30 cats with an average body weight of 4.1 ± 1.1 kg and heart rate of 194 ± 23 bpm. Fox et al. in Kealy et al. (2011) did a study on 10 cats with an average body weight of 3.91 ± 1.2 kg and an average heart rate of 255 ± 36 bpm while Allen did the study on 10 cats with an average body weight of 3.64 ± 0.66 kg and an average heart rate of 175 ± 20 bpm in 1982 (Brown and Gaillot 2008; Kealy et al. 2011). These data were used as a comparison to the Indonesian DHC because the cats had a similar average body weight. Normally, heart measurement values for cats show little variation except for those seen in kitten (Burk and Feeney 2003).
Muscular Walls: Left Ventricular Wall and Interventricular Septa
11 Figure 4 (A) shows that the muscular wall and interventricular septa have the same color projections on the 2D ultrasonography which was hypoechoic, while the papillary muscles were more hyperechoic (Kahn 2004). Pericaridium was seen as a hyperechoic projection surrounding the heart while the lumen of the ventricle was anechoic because it consist of liquid. Figure 4 (B) shows the motion-mode projection of the heart at the papillary muscle level. During systole, the ventricle wall and the interventricular septa moved closer while during diastole, the ventricular walls moved apart. A slight lag between the septum and free wall was because the interventricular septum depolarizes a few milliseconds before the left ventricular free wall (Burk and Feeney 2003). The early contraction of the IVS when compared to the LVW is shown as a and b in Figure 4 (B). During systole, the ventricle wall and the interventricular septa moves closer while during diastole, the ventricular walls move apart.
(A) (B)
Figure 4 View of the heart at the papillary muscle level on the short axis view (A) Brightness Mode view; IVS: interventricular septa, LVID: left ventricular internal diameter, LVW: left ventricular wall, PM: papillary muscle. (B) Motion Mode view; RVIDs: right ventricular internal diameter during systole, IVSs: interventricular septa during systole, LVIDs: left ventricular internal diameter during systole, LVWs: left ventricular wall during systole, RVIDd: right ventricular internal diameter during diastole, IVSd: interventricular septa during diastole, LVIDd: left ventricular internal diameter during diastole, LVWd: left ventricular wall during diastole, arrow: a slight lag between the IVS and LVW.
M-mode echocardiography is commonly used to accurately quantify single dimensions of walls, chambers and valvular structures at a specific location (Collins et al. 2003; Gottdiener et al. 2004; Muzzi et al. 2006). Table 5 shows the heart wall thickness of an Indonesian Domestic House Cat measured from the M-mode echocardiography.
12
(2011) (LVWs = 0.68 ± 0.07 mm), but was quite similar to the results done by Fox et al. in Kealy et al. (2011) (LVWs = 0.55 ± 0.88 cm).
The average IVS during systole and diastole is 0.56 ± 0.03 cm and 0.30 ± 0.05 cm respectively. Wagner et al. (2010) and Bowles et al. (2010) stated that the IVS of the heart of a cat should not be greater than 0.6 cm during systole. Data shows that the IVS obtained from the Indonesian DHC was still within the normal range for a cat. The IVSs thickness of the Indonesian DHC was slightly thinner when compared to the IVSs obtained by Moise and Dietz in Kealy et al. (2011) (IVSs = 0.76 ± 0.12 cm) and Jacobs and Knight in Kealy et al. (2011) (IVSs = 0.58 ± 0.06 cm). Fox et al. in Kealy et al. (2011) did not calculate the IVS during systole but when the data was compared to the IVS during diastole, the Indonesian DHC had a similar IVS thickness. The slight difference in size between cats or breed may be caused by the morphologic adaptations made during training as well as other factors such as breed and body conformation (Muzzi et al. 2006).
The heart’s muscular wall has greater thickness during systole than during diastole. This is because during diastole, the heart was filled with blood causing ventricular hypertrophy. Normal diastolic function allows the heart to fill appropriately at normal filling pressure (Boon 2011).
Table 5 Heart wall thickness of Indonesian Domestic House Cat
Parameter Indonesian ventricular wall thickness during diastole, IVSs: interventricular septa thickness during systole, IVSd: interventricular septa thickness during diastole, BW: body weight.
Internal Dimensions: Aorta, Left Atrium, Left Ventricle
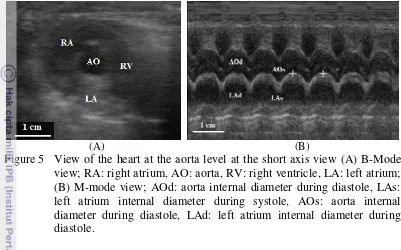
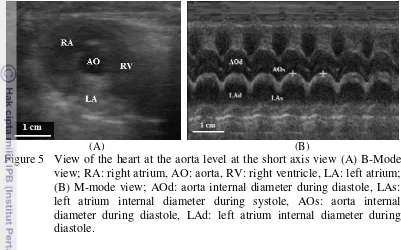
13 (Schmidt 2006). However, in Figure 5 (A) only the hypoechoic layer of the aorta was visible. The lumen of the aorta and the left atrium were anechoic as it contained liquid.
(A) (B)
Figure 5 View of the heart at the aorta level at the short axis view (A) B-Mode view; RA: right atrium, AO: aorta, RV: right ventricle, LA: left atrium; (B) M-mode view; AOd: aorta internal diameter during diastole, LAs: left atrium internal diameter during systole, AOs: aorta internal diameter during diastole, LAd: left atrium internal diameter during diastole.
Table 6 shows the internal dimension measurements of the Indonesian DHC. Results show that the aorta measurements taken during systole and diastole showed no particular difference. This means that during the heart’s muscular contractions, the lumen diameter of the aorta stayed the same. The Indonesian DHC had an AOd of 0.67 ± 0.07 cm. This result was relatively small when compared to the aorta size obtained by Moise and Dietz in Kealy et al. (2011) (AO = 0.95 ± 0.15 cm), Jacobs and Knight in Kealy et al. (2011) (AO = 0.95 ± 0.11 cm), Fox et al. in Kealy et al. (2011) (AO = 0.94 ± 0.11 cm), and Allen in Brown and Gaillot (2008) (AO = 0.90 ± 0.07 cm). The small dimensions of the feline heart provide little room for error and may affect the results (Boon 2011).
14
The Indonesian DHC had an average LVID of 0.78 ± 0.11 cm during systole and 1.48 ± 0.12 cm during diastole. The LVID during systole and diastole in the Indonesian DHC was similar to the LVID results obtained by Moise and Dietz in Kealy et al. (2011) (LVIDs = 0.69 ± 0.22 cm), Jacobs and Knight in Kealy et al. (2011) (LVIDs = 0.80 ± 0.14 cm), Fox et al. in Kealy et al. (2011) (LVIDs = 0.81 ± 0.16 cm), and Allen in Brown and Gaillot (2008) (LVIDs = 0.86 ± 0.16 cm).
Dimensions of the LA and the AO have been used to calculate the LA:AO ratio for objective judgment of LA size (Boller 2010). The ratio of LA:AO will increase with a dilatation in the LA or LV (Nelson and Couto 2009). Data shows that the LA/AO ratio in Indonesian DHC had an average of 1.07 ± 0.13. A LA:AO ratio greater than 1.3 shows a dilatation in the LA (Boller 2010). This shows that the LA of the Indonesian DHC used was not dilated. The LA:AO result was similar to the results of previous researches.
Table 6 Internal dimension measurements of Indonesian Domestic House Cat. Parameters Indonesian systole, AOd: aorta internal diameter during diastole, LAs: left atrial internal diameter during systole, LVIDs: left ventricular internal diameter during systole, LVIDd: left ventricular internal diameter during diastole.
Derivative Measurements
15 Table 7 Derivative cardiac measurements of the Indonesian Domestic House Cat
Parameters Average SD
* Source Moise and Dietz in Kealy et al. (2011) ** Source Norsworthy (2012) *** Source Boller (2010); LVED: left ventricular end-diastolic volume, LVES: left ventricular end-systolic volume, SV: left ventricular stroke volume, CO: left ventricular cardiac output, EF: ejection fraction, LVST: left ventricular septal thickening, LVWT: left ventricular posterior wall thickening, FS: fractional shortening.
Fractional Shortening (FS)
Fractional Shortening in normal cats usually range from 29–55% or 45–55% and can elevate when excited and decrease when sedated (Martin 1995; Norsworthy 2012). The average FS calculated in the Indonesian DHC was 48 0.05% which is still within the average range of a normal cat. This result was quite similar to the result obtained by Moise and Dietz in Kealy et al. (2011) (FS = 55.0 10.2 %), Jacobs and Knight in Kealy et al. (2011) (FS = 49.3 5.3 %), and Fox et al. in Kealy et al. (2011) (FS = 42.7 8.1 %), but was higher than the result obtained by Allen in Brown and Gaillot (2008) (FS = 0.35 0.25 %).
Fractional shortening is affected by preload, afterload and contractility (Martin 1995; Muzzi et al. 2006). It is not significantly affected by age, sex, body surface area and weight but may be affected by heart rate (Boon 2011; Dehghan et al. 2011; Kealy et al. 2011). An increase in preload, a decrease in afterload and hypertrophy of the myocardium may increase the FS while a decrease in preload, increase in afterload, systolic dysfunction, and a reduction in ventricular contractility may decrease the FS (Martin 1995; Ferasin 2009; Boon 2011).
Left Ventricular Volumes and Ejection Fraction (EF)
16
EF, the greater the reduction in systolic function (Tschope and Paulus 2009). The average ejection fraction calculated was 82.28 4.64%.
Left Ventricular Stroke Volume and Cardiac Output
Stroke volume (SV) is determined by the interaction between preload, contractility and afterload. It can be obtained by deducting the LVED with the LVES. The average stroke volume of the Indonesian DHC was 4.88 0.87 ml. Cardiac output (CO) is the volume of blood pumped by the heart/minute and is the product of the HR and SV (Lavdaniti 2008). Actually, echocardiography is not sensitive enough to diagnose CO because many compensatory mechanisms act to maintain normal CO (Muzzi et al. 2006). The average CO in the Indonesian DHC was 865.18 178.66 ml/minute.
CONCLUSION
The left ventricular wall thickness during systole (LVWs), left ventricular wall thickness during diastole (LVWd), intercentricular septa thickness during systole (IVSs), interventricular septa thickness during diastole (IVSd), left ventricular internal diameter during systole (LVIDs), and the left ventricular internal diameter during diastole (LVIDd) of the Indonesian DHC differ slightly from similar research on other cats with the same average weight, while the aorta internal diameter (AO) and the left atrium internal diameter (LA) was slightly smaller.
REFERENCES
Abbott JA, MacLean HN. 2006. Two-dimensional echocardiographic assessment of the feline left atrium. J Vet Intern Med 20 (1):111-9.
Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TSM. 2006. Left atrial size physiologic determinants and clinical applications. JACC 47(12):2357-63.
Adin DB, McCloy K. 2005. Physiologic valve regurgitation in normal cats. J Vet Cardiol 7:9-13.
Amitrano RJ, Tortora GJ. 2007. Laboratory Exercises in Anatomy and Physiology with Cat Dissections. USA: Thomson Brooks.
Boller M. 2010. General Approach and Overview of Cardiac Emergencies. In Drobatz KJ, Costello MF, editor. Feline Emergency & Critical Care Medicine. USA: Blackwell Scientific.
17 Bowles DB, Coleman MG, Harvey CJ. 2010. Cardiogenic arterial thromboembolism causing non-ambulatory tetraparesis in a cat. J Feline Med Sur 12:144-50.
Brown D, Gaillot H. 2008. Heart. In Penninck D, Anjou MA, editor. Atlas of Small Animal Ultrasonography. USA: Blackwell Scientific.
Burk RL, Feeney DA. 2003. Small Animal Radiology and Ultrasonography. USA: Elsevier Science.
Cavalcanti O, Albuquerque G, Muzzi L, Aparecida R. 2009. Evaluation of left atrium in two-dimensional and M-mode echocardiography in Brazilian boxer dogs. REDVET 11(1).
Chandler EA, Gaskell CJ, Gaskell RM. 2004. The Cardiovascular System. In Feline Medicine and Therapeutics. 3rd ed. UK: Blackwell Scientific. P495-526.
Chetboul V, Charles V, Nicolle A, Sampedrano CC, Gouni V, Pouchelon JL, Tissier R. 2006. Retrospective study of 156 atrial septal defects in dogs and cats. J Vet Med A 53:179-84.
Coatney RW. 2001. Ultrasound imaging: principles and applications in rodent research. ILAR J 42(3):233-47.
Collins KA, Korcarz CE, Lang RM. 2003. Use of echocardiography for the phenotypic assessment of genetically altered mice. J Physiol Genomics 13:227-39.
Edwards A. 1999. Cat Breed & Cat Care. USA: Lorenz Books.
Eldredge DM, Carlson DG, Carlson LD, Griffin JM. 2008. Cat Owner’s Home Veterinary Handbook. 3rd ed. Adelman B, editor. New Jersey: Wiley. Factor SM, Lamberti MA, Abadi J. 2002. Handbook of Pathology and
Pathophysiology of Cardiovascular Disease. New York: Kluwer Academic. Ferasin L, Sturgess CP, Cannon MJ, Caney SMA, Gryffydd-Jones TJ, Wotton PR.
2003. Feline idiopathic cardiomyopathy: A restropective study of 106 cats (1994-2001). J Feline Med Sur 5:151-9.
Ferasin L. 2009. Feline myocardial disese: classification, pathophysiology and clinical presentation. J Feline Med Sur 11:3-13.
Fleming K. 2009. the Cat Breeder’s Handbook. 2nd ed. USA: TIBCC. P 45. Forsyth S. 1995. Administration of a low dose tiletamine-zolazepam combination
to cats. N Z Vet J 43(3):101-3.
Foss MA, Stewart N, Swift J. 2008. Cat Anatomy and Physiology. Washington: Washington Univ Pr.
Ghadiri A, Avizeh R, et al. 2008. Radiographic Measurements of Vertebral Heart Size in Healthy Stray Cats. J Feline Med Surg 10(1):61-5.
18
from the American Society of Echocardiography’s Guidelines and standards committee and the task force on echocardiography in clinical trials. J Am Soc Echocardiogr 17(10)1086-119.
Hellyer P, Muir WW, Hubbel JA, Sally J. 1989. Cardiorespiratory effects of the intravenous administration of tiletamine-zolazepam to dogs. J Vet Surg 18(2):160-5
Kahn W. 2004. Veterinary Reproductive Ultrasonography. Germany: Schlutersche Verlagsgesellschaft
Kealy JK, McAllister H, Graham JP. 2011. Diagnostic Radiology and Ultrasonography of the Dog and Cat. 5th ed. Missouri: Elsevier Science. Kumar A, Mann HJ, Remmel RP. 2006. Pharmacokinetics of tiletamine and
zolazepam (Telazol®) in anesthetized pigs. J Vet Pharmacol Therap 29:587-9.
Lafdaniti M. 2008. Invasive and non-invasive methods for cardiac output measurements. J Caring Sciences 1(3):112-7.
Lang J. 2006. Imaging of the heart. In Mannion P, editor. Diagnostic Ultrasound in Small Animal Practice. UK: Blackwell Scientific.
Martin MWS. 1995. Small Animal Echocardiography. In Goddard PJ, editor. Veterinary Ultrasonography. UK: CAB international.
Martin M, Corcoran B. 2006. Cardiorespiratory Diseases of the Dog and Cat. 2nd ed. UK: Blackwell Scientific. indices in normal German shepherd dogs. J Vet Sci 7(2):193-8.
Nelson RW, Couto CG. 2009. Small Animal Internal Medicine. 4th ed. Missouri: Elsevier Science.
Nishimura RA, Tajik AJ. 1997. Evaluation of diastolic filling of left ventricle in health and disease: doppler echoccardiographic is the clinician’s Rosetta Stone. J Am Coll Cardiol 30(1):8-18.
Norsworthy GD. 2012. Cardiology. In Schmeltzer LE, Norsworthy GD, editor. Nursing the Feline Patient. UK: Blackwell Scientific. p155-63.
Noviana D, Aliambar SH, Ulum MF, Siswandi R. 2012. Diagnosis Ultrasonografi pada Hewan Kecil. Noviana D, editor. Bogor: IPB Pr.
Scansen BA. 2001a. Interventional cardiology for the criticalist. J Vet Emerg Crit Care 21(2):123-36.
Scansen BA. 2001b. Feline cardiovascular disease overview of diagnosis, treatment, and management [Internet]. [Downloaded 2012 Mar 17]. On http://www.cincyvma.com/education/archives/ sept27feline.pdf.
Schmidt G. 2006. Differential Diagnosis in Ultrasound Imaging a Teaching Atlas. Germany: Thieme
19 Sebastiani AM, Fishbeck DW. 2005. Mammalian Anatomy the Cat. 2nd ed.
Colorado: Morton.
Sendler K, Lendl C, Henke I, Otto K, Matis U, Mundt S, Erhardt W. 1994. Anesthesia in cats using tiletamine/zolazepam in minimal doses. Tierarztl Prax 22(3):286-90.
Shinohara N, Macgregor JM, Calo A, Rush JE, Penninck DG, Knoll JS. 2005. Presumptive primary cardiac lymphoma in cat causing pericardial effusion. J Vet Cardiol 7(1):65-9.
Silkowski C. 2010. Ultrasound Nomenclature, Image Orientation, and Basic Instrumentation. In Abraham D, Silkowski C, Odwin C, editor. Emergency Medicine Sonography. USA: Jones and Bartlett.
Tschope C, Paulus WJ. 2009. Doppler echocardiography yields dubious estimates of left ventricular diastolic pressures. J Circulation 120:810-20.
Wagner T, Fuentes VL, Payne JR, McDermott N, Brodbelt D. 2010. Comparison of auscultatory and echocardiographic findings in healthy adult cats. J Vet Cardiol 12:171-82.
Ware WA. 2007. Cardiovascular Disease in Small Animal Medicine. London: Manson. p70-82.
William LS, Levy JK, Robertson SA, Cistola AM, Ventroze LA. 2002. Use of the anesthetic combination of tiletamine, zolazepam, ketamine, and xylazine for neutering feral cats. JAVMA 220:10.
20
Attachment 1 Heart measurement references Parameter Moise and
Dietz*
Jacobs and
Knight* Fox et al.* Allen**
N 11 30 10 10
AO 0.95 ± 0.15 0.95 ± 0.11 0.94 ± 0.11 0.90 ± 0.07 LA 1.21 ± 0.18 1.23 ± 0.14 1.03 ± 0.14 1.00 ± 0.07 LVWs 0.78 ± 0.10 0.68 ± 0.07 0.55 ± 0.88
LVWd 0.46 ± 0.05 0.33 ± 0.06 0.35 ± 0.05 0.40 ± 0.40 LVIDs 0.69 ± 0.22 0.80 ± 0.14 0.81 ± 0.16 0.86 ± 0.16 LVIDd 1.51 ± 0.21 1.59 ± 0.19 1.40 ± 0.13 1.30 ± 0.12
IVSs 0.76 ± 0.12 0.58 ± 0.06 - -
IVSd 0.50 ± 0.07 0.31 ± 0.04 0.36 ± 0.08 0.40 ± 0.03
HR 182 ± 22 194 ± 23 255 ± 36 175 ± 20
BW 4.3 ± 0.5 4.1 ± 1.1 3.91 ± 1.2 3.64 ± 0.66
LVST 33.5 ± 8.2 - - -
LVWT 39.5 ± 7.6 - - -
FS 55.0 ± 10.2 49.3 ± 5.3 42.7 ± 8.1 0.35 ± 0.25 LA:AO 1.29 ± 0.23 1.30 ± 0.17 1.10 ± 0.18 -
21
BIOGRAPHY
INTRODUCTION
Background
The Indonesian Domestic House Cat (DHC) is a typical Indonesian short hair cat breed from the genus Felis. Cats are prone to various diseases, especially cardiovascular diseases (Scansen 2011b; Mottet et al. 2012). The most common complaint in cats is the evaluation of heart murmur during auscultation. The use of ultrasonographic imaging has had a tremendous impact on the diagnosis of cardiac disease, giving a surrogate measure of the heart size (Chandler et al. 2004; Abbott and MacLean 2006; Coatney 2001). Cardiologists are commonly asked to perform echocardiographic examinations on cats to screen for both acquired and congenital heart diseases (Adin and McCloy 2005; Chetboul et al. 2006).
Echocardiographic values show significant breed variations. Data concerning normal heart-size values have been reported in a number of pedigree cats, however little the normal echocardiogram of the Indonesian DHC has been reported. This is why the purpose of this study was to determine the normal echocardiographic reference values in anesthetized Indonesian DHC. Knowledge of the normal measurements for a specific body weight can help to indicate the degree and direction of the change from normal (Muzzi et al. 2006).
Objective
The objective of this study was to provide the normal values of Motion-mode (M-Motion-mode) echocardiographic cardiac measurements in healthy anesthetized male Indonesian Domestic House Cat.
Benefits
It is hoped that the normal cardiac measurements of the male Indonesian Domestic House Cat can add the physiological database of cat heart measurements to then be used, especially by veterinary practitioners, as a reference when dealing with heart ultrasonography of the Indonesian Domestic House Cat.
LITERATURE STUDY
Indonesian Domestic House Cat (DHC)
2
subject to selective breeding and conform no special standards. They have coats of any length in a range of colors that are usually plain, blotched, striped, or parched (Foss et al. 2008). They usually have green or yellow eyes and a fairly long nose (Edwards 1999).
Domestic House Cats belong to the short hair and non-pedigree group. Male cats are generally larger than females. Table 1 shows the normal physiological data of a cat. A healthy cat has the following physical features: clean and shiny coat, clean eyes and nose, no indications of swollen gums and bad breath, and no physical trauma (Edwards 1999).
Table 1 Normal physiological data of a cat
Adult cat Average adult cat Newborn kitten
Source: Eldredge et al (2008); bpm: beats per minute.
Heart
The heart rests in the cavity of the mediastinum near the midline of the thoracic cavity on the diaphragm. About two-thirds of the mass of the heart lies on the left of the body’s midline (Amitrano and Tortora 2007). Anatomically, the heart is a hollow organ with muscular walls and four chambers separated by valves (Norsworthy 2012). The walls of the heart has three layers: the epicardium organized by a single layer of squamous epithelium, the myocardium organized by muscle cells and the endocardium organized by a single layer of epithelium cells (Sebastiani and Fishbeck 2005). Interventricular septum (IVS) is the muscular separation between the left and right ventricle. Papillary muscles are broad, finger-like projections in the cavity of the ventricle. Each papillary muscle has multiple smaller heads giving rise to the chordae tendineae (Factor et al. 2002). The aorta also consists of three layers: the innermost layer, the media and the adventitia (Schmidt 2006).
3
Figure 1 The cat heart; A: aorta, VC: vena cava, RA: right atrium, TCV: tricuspidalis valve, RV: right ventricle, PulV: pulmonary valve, PA: pulmonary artery, PV: pulmonary vein, LA: left atrium, MitV: mitral valve, LV: left ventricle, AorV: aortic valve, LVFW: left ventricular free wall, IVS: interventricular septa; the numbers (1, 2, 3, 4, 5, 6, 7, 8a, 8b) show the sequence of blood flow process; Source: Norsworthy (2012).
Ultrasonography
Ultrasonography is a non-invasive diagnostic tool commonly used by veterinary practitioners (Chandler et al. 2004; Copley et al. 2008; Corwin 2008; Kealy et al. 2011). It uses frequencies greater than 20.000 cycles/second (Hz). The transmission velocity of ultrasound waves in blood and most soft tissues cells is uniform at 1540 m/sec (Coatney 2001). Common ultrasound frequencies used in cats are between 2 and 15 MHz (Chandler et al. 2004; Schober and Todd 2010; Kealy et al. 2011; Noviana et al. 2012). Lower frequencies result in higher penetration but lower resolution (Silkowski 2010). Ultrasound can not be transmitted through vacum and it’s velocity in gas is very low (Noviana et al. 2012).
An ultrasound device consists of a probe and a monitor (Noviana et al. 2012). Ultrasound waves are formed inside the probe by a piezoelectric effect on a medium such as a crystal made of lead zirconate. The probe can act as an emitter (wave producer) and receiver (echo accepter). An electrical impulse causes the crystal to lose its shape, vibrate and release ultrasound waves. The waves transmitted by the transducer are called pulse, while the waves reflected back to the transducer is called echo. The probe is connected to a computer or signal processor which processes the signals and presents it as grey dots. The stronger the echo, the brighter the dots (Kealy et al. 2011; Noviana et al. 2012).
4
echoes/sonolucent present, appearing black on the screen. Hyperechoic is a projection with rich and highly reflective echoes, appearing as varying shades of lighter gray (Silkowski 2010).
There are two kinds of ultrasound imaging: Brightness mode (B-mode) and Motion mode (M-mode). B-mode releases multiple ultrasound pulses at all times and is shown as a two dimensional imaging of the organ (Noviana et al. 2012). M-mode provides a one-dimensional view (depth) into the heart, where the beam transverses as their relative position and moves through time (Ware 2007). M-mode imaging usually provides a clearer resolution of the cardiac borders and a more accurate timing of events within the cycle. Therefore, measurements of the cardiac dimensions and motion are often more accurately assessed from the M-mode tracings (Ware 2007; Boon 2011). The difference between M-M-mode imagings on different probe view angles is illustrated in Figure 2.
Figure 2 Probe positions at the right parasternal long axis view (A) and Motion mode imaging (B); T: transducer, TW: thorax wall, RVW: right ventricular wall, RV: right ventricle, S: septa, LV: left ventricle, AV: aortic valve, AO: aorta, AMV: anterior mitral valve, PMV: posterior mitral valve, LA: left atrium, LVW: left ventricular wall; Source: Ware (2007). RVM: right ventricle muscular, TV: tricuspidal valve, RS: right septa; the numbers (1, 2, 3, 4) indicate the motion mode ultrasound of each level at the right parasternal long axis view.
Heart Ultrasonography
Heart size is important when evaluating cats for cardiac diseases (Chandler et al. 2004; Ghadiri et al. 2008). Diseases such as cardiac neoplasia and lymphoma, idiopathic pericarditis, congestive heart failure, hypertrophic cardiomyopathy, and left ventricular hypertrophy can be diagnosed using ultrasound (Scansen 2001a; Adin and McCloy 2005; Shinohara et al. 2005; Abbot and MacLean 2006; Schober and Todd 2010).
5 is the time when the heart is fully relaxed while systole is the time when the heart is in full contraction (Muzzi et al. 2006). Table 2 shows the comparison sizes of the cat heart.
Table 2 Comparative heart size of a normal cat
Organ Size
Interventricular septal wall (IVS) thickness < 6 mm*
Left ventricular wall (LVW) thickness < 6 mm* or < 1.5 cm** Left atrium (LA) internal diameter <1.6 cm*** or 1.7 x aorta** Right ventricular wall (RVW) thickness ½ LVW**
Right ventricular (RV) internal diameter 1/3 LV Chamber** Fractional shortening (FS) 45 – 55 %****
* Source: Wagner et al. (2010) and Bowles et al. (2010), ** Source: Boon (2011), *** Source: Schober and Todd (2010), **** Source: Norsworthy (2012); LVW: left ventricular wall, LV: left ventricle.
Right Parasternal Short Axis View
There are at least two rib spaces available for right parasternal views: a cranial location at the fourth intercostal space and a more caudal location at the fifth intercostal space. Examinations are usually done at the right parasternal short axis view at the level of the papillary muscles, measuring the RV and LV (Norsworthy 2012). However, Ware (2007) stated that the LVW, IVS and LVID should be determined at the level of the chorda tendinae. Figure 3 shows different the short-axis views that can be obtained by slightly tilting the probe to another direction. The position of the probe and the position of the animal may affect the size, shape, and relative position of the heart (Ware 2007).
Fractional shortening (FS) is a one-dimensional analogue of ejection fraction that determines the temporal change between LVIDs and LVIDd (Collins et al 2003; Brown and Gaillot 2008). It calculates the percentage of linear reduction in the left ventricular internal dimensions from diastole to systole and is used to evaluate cardiomyopathy and left ventricular performance (Collins et al. 2003, Dehghan et al. 2011). Fractional shortening in normal cats usually ranges from 5–55% and can elevate when excited and decrease when the cat is sedated (Norsworthy 2012).
Left ventricular volume is one of the best way to prognose myocardial infarction (Gottdiener et al. 2004). The left ventricular end-diastolic volume (LVED) and left ventricular end-systolic volume (LVES) are calculated using the equation
and
.
Ejection fraction (EF) is the percentage of volume reduction in the LVID from diastole to systole (Martin 1995) and is calculated by dividing the difference between LVED and LVES with LVED. The American Society of Echocardiography suggested that the EF should be measured from left ventricular volumes rather than estimated by visual inspection (Gottdiener et al 2004).
6
Cardiac output (CO) is the volume of blood pumped by the heart perminute and is the product of the heart rate (HR) and stroke volume (SV) (Collins et al. 2003; Lavdaniti 2008).
Dimensions of LA and the aorta has been used to calculate LA:AO ratio, producing an index for the LA size (Cavalcanti et al. 2009).
Figure 3 Right parasternal short-axis views of the heart at the level: (A) apex; (B) papillary muscle; (C) chorda tendinae; (D) mitral valve; (E) aortic root/left atrium; (F) pulmonary artery; RV: right ventricle, LV: left ventricle, PM: papillary muscle, CH: chorda tendinae, VS: interventricular septa, PMV: posterior mitral valve, AMV: anterior mitral valve, PV: pulmonary valve, NC: noncoronary cusp, LC: left coronary cusp, RC: right coronary cusp, RAu: right atrial auricle, PA:, LPA: left pulmonary artery. RPA: right pulmonary artery, CaVC: caudal vena cava, PPM: posterior papillary muscle, APM: anterior papillary muscle; Source: Ware (2007).
Zolazepam-Tiletamine (Zoletil)
Zoletil® is a registered trademark of Virbac Laboratories. Itis a non-opoid, non-barbiturate, injectable anesthetic consisting of an equal mixture of tiletamine HCl and zolazepam HCl. It is used intramuscularly to induce and maintain short-term anesthesia. The combination of zolazepam and tiletamine has a short induction time, low dosage, satisfactory safety margins, relatively constant immobilization time, and smooth recovery (Massolo et al. 2003; Kumar et al. 2006).
7 not result in prolonged muscle relaxation (Kumar et al. 2006). The minimal dose of zoletil for a ten minute anesthesia in cats is 4.2 mg/kgBW (Sendler et al. 1994). At the dose of 2.5 mg/kgBW, some cats would be more excited rather than sedated (Forsyth 1995). Sedation of feline patients for diagnostic images is a standard procedure in many veterinarians (Ferasin et al. 2003).
A previous study by Wilson et al. (1993) in adult rats showed that Zoletil® is a cardio stimulatory drug that increases the mean arterial blood pressure and reduces the respiratory depression. A similar research by Hellyer et al. (1989) showed that Zoletil® resulted in a rapid induction of anesthesia and causes an increase in heart rate and cardiac output.
MATERIALS AND METHOD
Time and Place
The study was done from November 2011 until April 2012. Ultrasonography was done at the Division of Surgery and Radiology, Department of the Clinical, Reproduction and Pathology, Faculty of Veterinary and Medicine, Bogor Agricultural University.
Animals and Materials
The animals used for this research were nine (9) healthy male Indonesian Domestic House Cats in the vicinity of the Bogor Agricultural University weighing between 3.3 – 4.4 kg. Cats were quarantined and acclimatized for one week where they were given commercial cat food and water ad libittum. On the first day of quarantine cats were given anthelmintic zipyran plus®. Cats were excluded if they showed any signs of illness and distress.
Ultrasound console used for this research were: Kaixin® ultrasound system, ultrasound scanner (KX5100 Vet), linear probe with frequencies between 5 and 7.5 MHz. Sony video graphic UP-895 MD printer, video camera, shaver, and gavage. The ultrasonography table had a cut out area.
Materials used for this research were ultrasound gel, Zoletil® (50 mg/ml of both zolazepam and tiletamine), atropine sulfat (0.25 mg/ml), commercial cat food, water, syringe (One Med, PT Jaya Mas Medica Industri), and gloves. Zoletil® was prepared by adding 5 ml of sterile water to the vial.
Method
Animals
7 not result in prolonged muscle relaxation (Kumar et al. 2006). The minimal dose of zoletil for a ten minute anesthesia in cats is 4.2 mg/kgBW (Sendler et al. 1994). At the dose of 2.5 mg/kgBW, some cats would be more excited rather than sedated (Forsyth 1995). Sedation of feline patients for diagnostic images is a standard procedure in many veterinarians (Ferasin et al. 2003).
A previous study by Wilson et al. (1993) in adult rats showed that Zoletil® is a cardio stimulatory drug that increases the mean arterial blood pressure and reduces the respiratory depression. A similar research by Hellyer et al. (1989) showed that Zoletil® resulted in a rapid induction of anesthesia and causes an increase in heart rate and cardiac output.
MATERIALS AND METHOD
Time and Place
The study was done from November 2011 until April 2012. Ultrasonography was done at the Division of Surgery and Radiology, Department of the Clinical, Reproduction and Pathology, Faculty of Veterinary and Medicine, Bogor Agricultural University.
Animals and Materials
The animals used for this research were nine (9) healthy male Indonesian Domestic House Cats in the vicinity of the Bogor Agricultural University weighing between 3.3 – 4.4 kg. Cats were quarantined and acclimatized for one week where they were given commercial cat food and water ad libittum. On the first day of quarantine cats were given anthelmintic zipyran plus®. Cats were excluded if they showed any signs of illness and distress.
Ultrasound console used for this research were: Kaixin® ultrasound system, ultrasound scanner (KX5100 Vet), linear probe with frequencies between 5 and 7.5 MHz. Sony video graphic UP-895 MD printer, video camera, shaver, and gavage. The ultrasonography table had a cut out area.
Materials used for this research were ultrasound gel, Zoletil® (50 mg/ml of both zolazepam and tiletamine), atropine sulfat (0.25 mg/ml), commercial cat food, water, syringe (One Med, PT Jaya Mas Medica Industri), and gloves. Zoletil® was prepared by adding 5 ml of sterile water to the vial.
Method
Animals
8
sandbox for defecation and urination. Cats were monitored intensively in the Division of Surgery and Radiology, Department of Clinical, Reproduction and Pathology, Faculty of Veterinary Medicine, Bogor Agricultural University.
Preparation
A physical examination was performed before anesthesia on the cat’s heart rate, respiratory rate, body temperature, and body weight. Cats were given premedication 0.02 mg/kgBW atropine sulfate intramuscularly. After fifteen minutes, cats were anesthetized using 8 mg/kgBW Zoletil® given intramuscularly. The target injection organs were the semitendinosus or semimembranosus muscles. Once the cat was anesthetized, the hair coat at the right lateral axillary area were clipped and shaved clean.
Echocardiography Technique
Echocardiographic examination was performed from underneath with the cat on the right lateral recumbence. The probe was positioned at the fourth or fifth intercostal space. Ultrasonography was performed on the short axis at the papillary muscle and aorta level on both brightness mode (B-mode) and motion mode (M-mode). Ultrasonography was performed by one operator using a 7.5 MHz transducer approximately 15 minutes after the cat was anesthetized. Data using the M-mode were taken until there were three representative data for each measurements. Pictures were taken using a handycam connected to the monitor.
Data Analysis
Data were performed offline using Image-J© obtained from the National Institute of Health. The M-mode right parasternal short axis view at the papillary muscle level was used to measure the left ventricular wall (LVW) thickness during systole and diastole, the left ventricular internal diameter (LVID) during systole and diastole, and the interventricular septa wall (IVS) thickness during systole and diastole. The M-mode right parasternal short axis reading at the aorta level was used to measure the diameter of the aorta (AO) and the left atrium (LA) during diastole. Diastolic measurements were taken at the maximal left ventricular relaxation, while systolic measurements were taken at the maximal left ventricular contraction. The final value used was the average from the three measurements. Data were expressed as average ± standard deviation.