ii
EFFECT OF MYB TRANSCRIPTION FACTOR DURING
NODULE DEVELOPMENT IN Lotus japonicus
SUPRIADI
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
DECLARATION OF THESIS AND INFORMATION SOURCES
OF INFORMATION AND PATENT
I, Supriadi, hereby stated that this thesis entitled Effect of MYB Transcription Factor During Development in Lotus japonicusis true of my own work under the supervisor advisory board and that it has not been submitted before in any form to any university. The content of this thesis has been examined by the advising advisory board and external examiner. Sources of information which is derived or cited either from published or unpublished scientific paper from other writers have mentioned in the script and listed in the references at the end part of this thesis.
I hereby handed the copyright of my thesis to Bogor Agricultural University. Bogor, April 2016 Supriadi
ii
RINGKASAN
SUPRIADI. Efek Faktor Transkripsi MYB Selama Pertumbuhan Nodul pada Lotus japonicus. Dibimbing oleh RAHAYU WIDYASTUTI, DWI ANDREAS SANTOSA, dan MIKA NOMURA.
Dalam beberapa tahun terakhir, beberapa capaian untuk memahami tahap dalam proses perkembangan nodul telah banyak dilakukan dengan pendekatan studi molekuler. Beberapa gen telah diidentifikasi memainkan peran sangat penting baik dalam proses awal nodulasi atau pun nodul organogenesis. Penelitian sebelumnya menemukan bahwa 76 jenis gen teregulasi selama infeksi Mesorhizobium loti ke tanaman Lotus japonicus . Salah satu gen teregulasi adalah faktor transkripsi MYB, molekul yang mengikat pada situs AACG /TG pada DNA. Karena fungsi yang tepat dari faktor transkripsi MYB dalam proses nodulasi masih kurang diketahui, maka fungsi faktor transkripsi MYB selama proses nodulasi penting untuk diteliti. Untuk mengetahui pengaruh faktor transkripsi MYB dalam proses nodulasi Lotus japonicus, kami selanjutnya membuat akar transgenik dari dari Lotus japonicus dengan MYB over ekspresi (MYB OX) dan MYB tersupresi oleh RNAi (MYB RNAi). Kami mengukur jumlah nodul, diameter nodul dan panjang akar sebagai ekspresi fenotip. Untuk mengetahui aktifitas mereduksi Asetilen dari nodul menggunakan analisis kromatografi gas untuk mengetahui aktivitas fiksasi nitrogen. Untuk mengetahui ekspresi gen yang terkait nodulasi, kami mengukur ekspresi gen enod40, gen nin, dan gen sistein protease menggunakan QRT-PCR.
Hasil penelitian menunjukkan bahwa MYB yang dieskpresikan berlebih secara signifikan meningkatkan jumlah nodul dari Lotus japonicus di awal minggu pasca inokulasi dan peningkatan aktivitas fiksasi nitrogen (N2) pada nodul. Pada
penekanan dari MYB secara signifikan menurunkan jumlah nodul dan aktivitas fiksasi N2 serta menginduksi nodul senesens lebih awal. Kami juga menemukan
bahwa MYB tidak memberi efek pada diameter nodul dan panjang akar pada Lotus japonicus.
Data pada QRT-PCR menunjukkan bahwa tingkat ekspresi enod40 memungkingkan MYB mengikat pada enod40. Sebaliknya, tingkat ekspresi nin menunjukkan bahwa MYB mungkin tidak mengendalikan ekspresi gen nin tapi kami memprediksi MYB memiliki fungsi paralel dengan gen nin dalam proses nodulasi. Kami mengusulkan bahwa faktor transkripsi MYB mengikat pada gen enod40 dan diaktifkan selama masa awal nodulasi.
SUMMARY
SUPRIADI. Effect of MYB Transcription factor During Nodule Development in Lotus japonicus. Supervised by RAHAYU WIDYASTUTI, DWI ANDREAS SANTOSA, and MIKA NOMURA
In the past years, some achievments toward understanding the initial stages of nodule developmental process has been done using molecular study. Several genes have been identified that play very important roles whether in the early nodulation process or in nodule organogenesis. Previous research found that 76 genes upregulated during Mesorhizobium loti infection to Lotus japonicus plant. One of upregulated gene is MYB transcription factor, a kind of molecule bind to AACG/TG site in DNA. Due to the precise function of MYB transcription factor in the rhizobial symbiosis is still poorly unknown, we investigated function of MYB transcription factor during nodulation.
To know the effect of MYB transcription factor in nodulation process of Lotus japonicus we further created the transgenic hairy roots of Lotus japonicus with MYB overexpression (MYB OX) and suppression by RNAi (MYB RNAi). We measured the nodule number, nodule diameter and root lenght as phenotype expression. In additional Acetylene reduction activity of nodules has been assayed using gas cromatografh analysis to know nitrogen (N2) fixation activity. To know
the expression of genes related nodulation we measured expression of enod40 gene, nin gene, and Cystein Protease gene using qRT-PCR.
The result revealed that the overexpression of MYB significantly increased the nodule number of Lotus japonicus in early week post innoculation and increased nitrogen (N2) fixation activity of nodule. Particularly the supression of
MYB has significantly decreased the nodule number and nitrogen fixation activity and induce the scenesence nodule earlier. We also found that MYB did not give effect to nodule diameter and root lenght of Lotus japonicus.
The qRT-PCR expression levels of enod40 suggested that MYB might bind to enod40. In contras, the expression levels of nin suggested that MYB might not a regulator of nin but we predicted MYB has a paralel function with nin in nodulation process .We finally proposed that MYB transcription factor binds to enod40 gene and activated during the early time of nodulation.
iv
© Copyright of Bogor Agricultural University, 2015
Protected by the laws
Any unauthorized quotation of all contents or any part thereof is strictly prohibited. Citation is only for educational purpose, research, scientific writing, reports writing, critique and problem analysis; and citations would not give any disadvantage on behalf of Bogor Agricultural University.
EFFECT OF MYB TRANSCRIPTION FACTOR DURING
DEVELOPMENT IN Lotus japonicus
SUPRIADI
A Thesis submitted for the Degree Programs of Master of Science
in Soil and Environtmental Biotechnology
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
vi
viii
FOREWORD
First of all, I would humbly distinguish the Most Gracious Allah SWT, all praises to Allah for the gifts and His blessing in completing this thesis with title Effect of MYB Trancription Factor During Nodule Development in Lotus japonicus. This thesis submitted for the Degree Programs of Master of Science in Master of Science of Soil and Environtmental Biotechnology. I would like to sincerely deliver my greatest gratitude to my advisor: Dr Rahayu Widyastuti, MSc, Prof Dr Ir Dwi Andreas Santosa, MS, Prof Mika Nomura for their advices, expertness, encouragement, and support. My sincere thanks also to Prof Shigeyuki Tajima for all support.
My appreciations were also extended to Japan Student Services Organization (JASSO) Scholarship for granting scholarship during the study and experiment in Kagawa University and also for SUIJI-JDP (Six University Initiative Japan-Indonesia Joint Degree Program) who allowed me to expand my knowledge and experiences in Japan. This master thesis would not have been possible unless the funding of Indonesian Government scholarship (Beasiswa Fresh Graduate).
I also addressed my gratitude to all staff and classmate in Soil and Environtmental Biotechnologi (IPB) and my laboratorymate in Plant Nutrition (Kagawa University) and I would like to take this moment to deeply express my thankful feeling to my father and mother who have been praying, loving and supporting as always. Finally, I hope this thesis can give information about nodulation process in legume, specially the effect of MYB transcription factor during those process.
Bogor, 26 May 2016
x
TABLE OF CONTENT
LIST OF TABLE vi
LIST OF FIGURE vi
INTRODUCTION 1
Objectives 1
LITERATURE REVIEWS 2
Characteristics of Lotus japonicus and Meshorhizobium loti 2
MYB transcription factor and myb 127 2
The formation process of nodule in Lotus japonicus 3
Producing transgenic hairy toot 3
MATERIAL AND METHODS 4 Bacterial strains and medium 4 Plant materials 4 Construction of MYB RNAi and MYBOX in L. japonicus 4 Acetylene reduction assay(ARA) 5 Quantitative real time PCR 5 Counting of bacteroid number 6 Staining of MYB promotor-GUS in L. japonicus 6 Microscope and nodule measurement 6 RESULT AND DISCUSSION 8 DNA amplification and electrophoresis results of PCR 8
Relative expression of transgenic hairy root 9 Phenotype expression of nodulation 9 Nitrogen (N2) fixation activity 12 Bacteroid number 14
GUS staining of MYB promoter-GUS 14 Quantitative real time PCR 15
CONCLUSIONS 17
RECOMENDATIONS 17
REFERENCES 18
LIST OF TABLES
1 Calculation of construct electrophoresis results 8
LIST OF FIGURES
1 Construction of MYB OX, MYB RNAi and controls 5 2 Transformation process of transgenic hairy root 7 3 Relative expression of myb in transgenic hairy roots 8 4 Nodule number per plant of MYB OX and GUS OX 9 5 Nodule number per plant of MYB RNAi and GUS RNAi 9
6 Root lenght of transgenic hairy roots 10
7 Nodule diameter of transgenic hairy roots 11
INTRODUCTION
Excess fertilizer caused pollution. Emissions generated during the application of synthetic fertilizer accounted for 13 % of agriculture greenhouse gas emission in the world (FAO, 2014), it produced from fossil fuel with high economic costs, pollution of the environment and its high energy costs. nitrogen fertilizer which known as the biggest amount of syntetic fertilizer used could be reduced without compromising crop yield using legume plants.
Leguminous plants have capability of establishing nitrogen (N2) fixing
symbiosis with soil bacteria of family Rhizobiaceae. Under conditions of low soil nitrogen, soil bacteria collectively referred as rhizobia infects the roots of legumes and induce the formation of root nodules, while host plants are house and feed the bacterial symbiont. Within the root nodule rhizobia provided reduced nitrogen (N2)
to the plants instead of the host plants provide carbohydrates for exchange mutualism. This critical important symbiosis to fix nitrogen around 200 million tons per year (Ferguson 2010). Understanding the mechanisms of symbiotic nitrogen (N2) fixation between rhizobia and legume expects to decrease the synthetic
nitrogen fertilizer consumption.
During symbiosis process, rhizobia and leguminous plants recognize each other through the exchange of chemical signal. Some advance of researchs said that precise interaction between phytohormones and the other signaling compounds are imperative for nodule organogenesis (Ferguson 2003). Important progress has been achieved in the past years toward understanding the initial stages of this complex developmental process. Several genes have been identified that play a role in the perception of the bacterial Nod factors (NFs), lipo-chito-oligosaccharidic signals essential for triggering the symbiotic genetic program in specific legume hosts, further several of upregulated genes have been identified playing very important roles whether in the early nodulation process or in nodule organogenesis. Some of the genes like nin gene, enod gene were known clearly based on their function but
many genes was still poorly understood. It’s make the complex developmental of
the nodulation process still actively investigated to know about the acts of gene during nodulation.
In previous research, 76 genes upregulated during Mesorhizobium loti infection to Lotus japonicus plant. One of upregulated gene is MYB transcription factor (El Yahyaoui et al. 2004), a molecule binds to AACG/TG site in DNA specifically we found myb127 as the kind of this transciption factor. Due to the precise function of MYB transcription factor in the rhizobia symbiosis is still unknown, this research tried to investigate about function of MYB transcription factor during nodule development in Lotus japonicus.
Objectives
The objectives of this study are to investigate the effect of MYB transcription factor in nodule formation by studying the phenotype expression of nodule, nitrogen (N2) fixation activity and relative expression of gene related
nodulation.
2
Characteristics of Lotus japonicus and Mesorhizobium loti
Mesorhizobium loti MAFF303099 or Rhizobium loti is a Gram negative bacteria that able to set determinant type globular nodule with Lotus japonicus to fix nitrogen from the atmosphere. It’s also known as a bacterial symbiont with several lotus species. The complete sequence of genome of M.loti have been published by Kaneko et al.. (2000). The genome consisted of three circular molecules, a single chromosome with 7,036,071 bp and two plasmids pMLa and pMLb with 351, 911 bp and 208, 315 bp respectively.
Lotus japonicus is a wild legume of family Fabaceae. Members of this family are very diverse more than 20,000 species. Fabaceaea family has significant importance for agricultural and biological as many of others legume species that rich sources of protein and oil and can also fix nitrogen (N2). L.
japonicus has become a model plant for genome studies in legumes, particularly in reference to rhizobia and arbuscular mycorhizal symbiosis. The genome size that known of 470 Mb, the size of plant smaller than other legumes and the abundant of resulted seed for a short life cycle (2 to 3 months) were some reasons why this plants were used to genomic study during these last years (Udvardi et al. 2005). The data of whole genome sequence L. japonicus already been published by Sato et al. (2008).
MYB Transcription Factor and myb127
Nod factor, the signal molecules carried by rhizobia like an oligopolysaccharide can activate a signal pathway that resulted the transcription activation of transcription factors. These regulatory proteins recognize and bind to specific sequences of DNA which located in the promoter of genes, its controlling the activity of gene as the response to environmental. In legumes, several transcription factors have been identified by their capacity to bind to nodulin promoters, but the most significant advances have aroused from genetic approaches in nodule development. The first transcription factor cloned was NODULE INCEPTION (NIN), the other genes such as Astray, Ethylene Response Factor Required for Nodulation 1 (ERN1) and ERN2,5,6 RR1,7 CYCLOPS,8 Nodulation Signaling Pathway 1 (NSP1) and NSP2, Nuclear Factor Y (Y) A1 and NF-YC112 GRAS (NSP1 and NSP2) and NF-Y (NF-YA and NF-YC) (Ripodas et al. 2014).
The MYB proteins family is a group of functionally diverse transcriptional activators found with plants or animal. MYB proteins characterized by DNA-binding domain of more than 50 amino acids. It was known binds to a specific DNA sequence for C/TAACG/TG in most organisms (James et al. 1997) MYB proteins classified into three subfamilies (one, two or three) depending on the number of adjacent which repeats in MYB domain. MYB transcription factors in plants participate in the cell development, plant responses to environmental factors and mediate the action of some hormones (Stracke et al. 2001).
Lotus japonicus root during mycorhizal process, acted as the control for root growth. Kang et al. 2013, also reported MYB subfamily of IPN2 has an important action in nodule organogenesis, in which the suppression of IPN2 by RNAi will significantly decrease the nodule number of L.japonicus.
The myb127 has been upregulated during M.loti infection in L.japonicus. myb127 gene was strongly upregulated after 6 days M.loti inoculation, this expression measured with water and KNO3 as the control. The high expression of
MYB transcription factor is an early indicator of myb127 gene as a gene which affected the nodulation.
The formation process of nodule in Lotus japonicus
The nodule formation process for each plant, generally has the same pathway. The nodules formation has started from the signal that given by plant as a host, flavonoid was known as the compound used to create an early essential signals for nodule establishment. Its known as the early signal for establishing contact and became the first step in infection leading to nodule development (Lateif et al. 2012). The activated signaling of flavonoids by plants then activate the NodD proteins that contributing to stimulate the Nod-gene. Nod factor signal continuously trigger the nodule development after M.loti-rhizobia get to the root and form the root curling, then made the infection thread in conditioning the root to be low oxygen condition. The increase number of rhizobia as bacteroids in plants root then differentiate as the nodule (Madsen et al. 2010).
In Lotus japonicus the activation of Nod factor (NF) will express the early nodulin gene or ENODs, The activation of ENODs is associated with calcium spiking that acts as the signal in linking NF perception to change of gene expression. The early NF signaling pathway is NFR1/NFR5 that encode predicted sugar-binding receptor-like kinases furtherly known as SymRK.
Producing transgenic hairy root
Transgenic hairy roots have been used for more than 30 years. In recent years transgenic hairy has used Agrobacterium-rhizogenes to mediate hairy root production as a biotechnology tool in a variety of plant species to discover novel biological insights. Metabolic enzyme function can be determined by gene overexpression or RNA interference (RNAi) approaches using hairy root transformation or transgenic hairy roots (Ron et al. 2014).
Nowadays, hairy roots transformation used gateway clonning technology. The Gateway clonning system is a molecular biology method that enables researchers to transfer DNA-fragments between plasmids using a proprietary set of recombination sequences efficiently, the "Gateway att" sites, and two proprietary enzyme mixes, called "LR clonase", and "BP clonase". Gateway clonning technique allows transfer of DNA fragments between different clonning vectors while maintaining the reading frame. Using Gateway, one can clone or subclone DNA segments for functional analysis. The system requires the initial insertion of a DNA fragment into a plasmid with two flanking recombination sequences called “attL1”
and “attL2”, to develop a “Gateway entry clone” (Maekawa et al. 2008)
4
M.loti, a Gram-negative nitrogen (N2) fixing bacterium wild type was used
in this research. M. loti lives in soil and establish a symbiosis with legume plants. A tryptone yeast extract (TY) medium was used for cultivation (Beringer 1974). The medium contained 5 g tryptone, 3 yeast extract, 1 g CaCl2.H2O controlled on
pH 6.8. During cultivation M.loti was controled at 28oC.
Plant materials
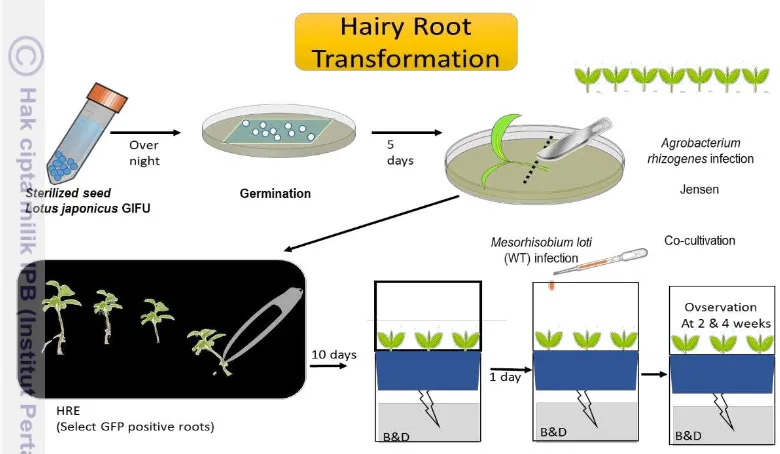
Seeds of L.japonicus B-129 (GIFU) were scarified by sand paper to remove the seed coats. 2 ml of sodium hypochlorite solution and 10 µl of tween-20 fill up 10 ml water was used as sterilization solution of seeds, shacked for 10 minutes and followed by washing with milli-Q water for 3-4 times and final shacked for overnight. One hundred seeds then cultured for each plate for 3 days dark and 2 days light, then infection with Agrobacterium-rhizogenesis LBA1334 which carrying transcription factor of MYB overexpression, MYB suppression, GUS over expression, and GUS suppression, then infected to the shoot which has cut the root and transferred to the Jensen medium for 1 day dark and 4 days light. HRE medium was used as medium to grow the transgenic hairy root for ten days. Transgenic hairy roots were selected under the flourescent microscope, then transferred to the jar pot. Vermiculite was put in the upper compartment of sterile double Magenta Jars, the lower jar was supplied with ½ B&D nitrogen-free nutrient solution (Broughton and Dilworth 1971). Six transgenic hairy root of Lotus japonicus plant were cultured in each jar pot. The Jars were placed in a growth cumber cabinet (EYELA FLI-2000; Tokyo Rikakikai Co., LTD., Japan) controlled at 24oC and exposed to 16 h/light and 8 h/dark. After 1 day plants were inoculated with 10 ml of M.loti, which cultured in TY Medium for 3 days and adjusted to OD550 nm =1.0 (1 x 109 cells ml-1). The phenotype expression of nodule measured at 2 and 4 weeks post inoculation (wpi).
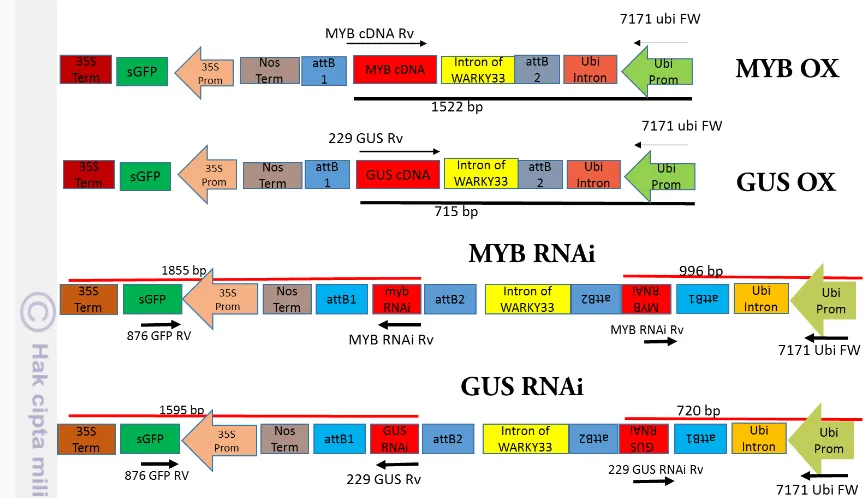
Construction of MYB RNAi and MYBOX in L. japonicus
For making MYB RNAi (chr4.CM0004.2240.r2.d) suppression by RNAi method, a 289 bp fragment (position 544- 832) was amplified from the cDNA using a set of primers containing attB recombination sites for the Gateway vector; FW
primer; 5’
-ACAAGTTTGTACAAAAAGCAGGCTGTTGGGAATGATGCCACTTT-3’; Rv
primer;5’ACCACTTTGTACAAGAAAGCTGGGTACGGTAATTGGTCCAAG CAG-3’ For MYB overexpression (MYB OX), a 900 bp fragment was amplified
using the following primers: FW primer;5’-ACAAGTTTGTACAAAA AGCAGGCTATGGGCAGGAAGTGCTCACA-3’; Rv primer; 5’- ACCACTTTG TACAAGAAAGCTGGGTTTAAGTCACGGTAATTGGTC-3’ (the underline indicates the attB recombination sites). The PCR products were subcloned into pDNOR/Zeo (Invitrogen), and then transferred into a Gateway binary vector, pUB-GWS-GFP for RNAi construction and pUB-GW-GFP for MYB overexpression (MYB OX) (Maekawa et al.. 2008).
Figure1. Constructions of MYB OX, MYB RNAi and controls
Acetylene reduction assay (ARA)
Acetylene reduction assay was determined to know the nitrogen (N2)
fixation activity (Banba et al.. 2001), 2 wpi of nodule and 5 wpi of nodule taken to measure the acetylene reduction activity. Standard vial was prepared by injecting 1 ml of ethylene (C2H4) to 70 ml vial, 0.1 ml of them then taken into 25 ml vial,
then 0.25 ml transferred into 25 ml second vial. 0.1 ml, 0.5 ml and 1 ml gas from 25 ml second vial then injected to the Shimadzu GC-8A gas-chromatography (shimazu, Kyoto, Japan) to make the standardcurve. The flow rate of nitrogen carrier gas was set at 50 ml min-1, range set at 101 , attenuation 64, the injector and
column temperatures at 110 and 70 respectively.
After the standard prepared, 10 nodules for each thairy roots were inserted to each sample vial. Then, 2.6 ml of Acetylene (C2H2) gas injected to the sample
6
Quantitative real time PCR
The Lotus japonicus which carrying the transgenic hairy root of MYB overexpression, MYB suppression, GUS over expression, and GUS suppression were growed in the chamber. Two weeks post inoculation (wpi) and 4 weeks post inoculation were a precise time for RNA extraction. The total RNA has extracted
using the RNeasy plant mini kit by Qiagen, CA, USA. In making cDNA, 0.5 μg of
total RNA mixed with The diluted cDNA stored at -30 oC until it used. A final
concentration of 200 ng cDNA was mixed with 5 μl of specific primer mix. The ubiquitin used as the internal reference for relative quantification (Ubi forward
ATGCAGATCTTCGTCAAGACCTTG, ubi Reverse,
ACCTCCCCTCAGACGAAG), MYB transcripts were amplified using primers 5′ -CTGGGAAAAGGTGACTGGAG-3′ and 5′-CCACTTGTGTTGGGGTTCTT-3′. to check the MYB Expression of each construct and then mixed with Takara SYBR Green premix ExtaqII (Takara, Shiga, Japan) the real time PCR (Thermal Cycler Dice real time SystemII Takara, Japan) the standard cycling conditions were 95 oC for 30 sec, 40 cycles of 95 oC for 5 sec, 60 oC for 30 sec, and dissociation amplification one cycle of 95 oC for 15 sec, 60 oC for 30 sec, and 95 oC for 15 sec.
Counting of bacteroid number
Samples of MYB OX, MYB RNAi, GUS OX and GUS RNAi, nodules at 2 wpi inoculated with Ds Red M. loti, were isolated about 15-20 nodules for each hairy roots construct. The isolated nodules then sterilized using sterilizes solution which contained 10% sodium hypochlorite (concentration of 0.5%), 0.1% tween20, for 3 minutes and followed by water sterilization and shaked for 5 minutes for three times. The sterlized nodules then added with 2 ml sterilized water and grinded with pestle in three mortar for each hairy roots construct ( 1 mortar 5 nodules), 1 ml of suspension bacteroid in the mortar then transferred to eventube and dilute for
10,100, and 1000 dilution. The bacteroid number of 10 μl bacteroids solution that
appear the red color under the microscope then counted in glass slide using microscope and calculated according the formula below :
Bacteroid numbers per nodule =
� � ∗ � � � / / � � �
� �
*10x = 381.18,40 x=6051.27
Staining of MYB Promotor-GUS in L. japonicus
A fragment of 1.0 kb upstream of the start codon of MYB transcription factor was amplified from genomic DNA of L. japonicus using PrimeSTAR HS DNA
polymerase (Takara, Japan) with a specific primers: forward primer, 5’
Microscope and nodule measurement
The fluorescent microscope has used to differentiate the number of transgenic hairy roots before transferred to the pot, the root of transgenic hairy root appeared the green color of roots. Bright-field and fluorescent microscopy were performed with Olympus, the images of transgenic hairy roots were taken using DP Controller (Olympus), NIS Elements (Nikon) or Photoshop (Adobe Systems). The phenotype expression of root nodule such as the number of nodule, nodule diameter, and the root length were observed at 2 wpi and 4 wpi by using Vernier calipers.
Figure 2. Transformation process of transgenic hairy root
8
DNA amplification and electrophoresis results of PCR
The electrophoresis results of PCR product showed that 12 samples of constructed DNA has successfully amplified, it proof by appearing DNA bands under the electrophoresis gel (Figure 3).
Figure 3. PCR electrophoresis results of construct, 1, 2, 7, 8 (MYB RNAi), 3, 4, 9, 10 (GUS RNAi), 5, 11 (MYB OX), 6, 12 (GUS OX)
Samples number 1, 2, 3, 4, 5, 6 are cDNA sample while sample number 7, 8, 9, 10, 11, 12 are sample colony that taken from Agrobacterium transfered the expression clone. The calculation of DNA length using excel program showed the expecteded size and PCR product as result on Table 1. The length of DNA expected size and PCR product mostly same indicated that construction process of each construct has succesfully done.
9 876 GUS
Relative expression of transgenic hairy root
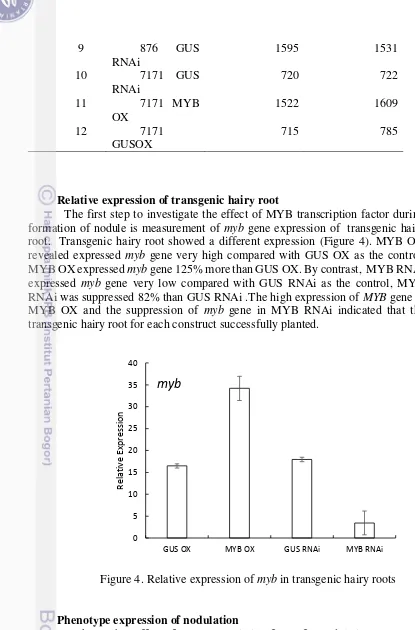
The first step to investigate the effect of MYB transcription factor during formation of nodule is measurement of myb gene expression of transgenic hairy root. Transgenic hairy root showed a different expression (Figure 4). MYB OX revealed expressed myb gene very high compared with GUS OX as the control, MYB OX expressed myb gene 125% more than GUS OX. By contrast, MYB RNAi expressed myb gene very low compared with GUS RNAi as the control, MYB RNAi was suppressed 82% than GUS RNAi .The high expression of MYB gene in MYB OX and the suppression of myb gene in MYB RNAi indicated that the transgenic hairy root for each construct successfully planted.
Figure 4. Relative expression of myb in transgenic hairy roots
Phenotype expression of nodulation
To know the effect of MYB transciption factor for nodulation responses. We investigated the total nodule number, the nodule diamater and main root length used calipers as the measurement. The nodule number for each transgenic hairy root constructs performed the difference nodule number. Figure 5 showed the nodule number average for each transgenic hairy roots of more than 35 plants for each transgenic hairy root construct.
GUS OX MYB OX GUS RNAi MYB RNAi
10
Figure 5. Nodule number per plant of MYB OX and GUS OX The data of nodule number showed MYB Overexpression (MYB OX) produced more numerous nodules (16.4 nodules per plant, n=44) than GUS Overexpression (GUS OX) nodules (11.8 nodules per plant, n=46) as the control of 2 weeks post inoculation (wpi)(Figure 5). The different of nodule number of both construct showed statistically significant. The nodule number of 4 wpi showed both of constructs (MYB OX and GUS OX) produced nodule number with no significant different, 19.1 and 20.4 respectively. The significance of these data that confirmed
by using Student’s t test analysis for (P < 0.01) for 2 wpi but the differences were no significant at 4 wpi. The different of nodule number that showed MYB OX has higher nodule number might indicated the symbiotic interaction of MYB OX formed earlier after M.loti inoculation but it no longer at 4 wpi.
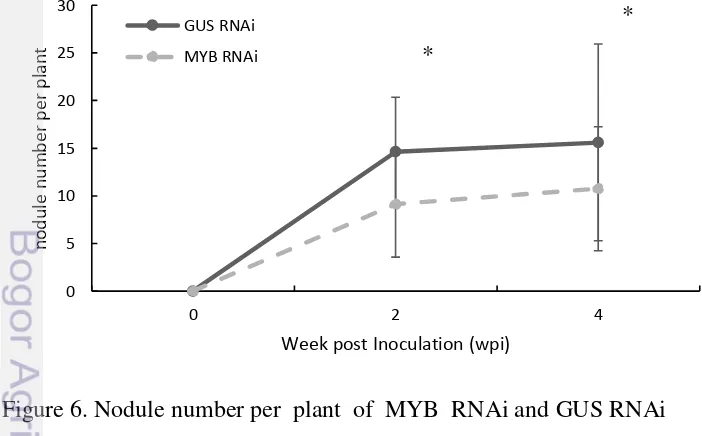
The number of nodules of MYB suppression (MYB RNAi) produced lower number of nodules (9.1 nodules per plant, n=38) than GUS Suppression (GUS RNAi) nodules (14.6 nodules per plant, n=40) of 2 wpi (Figure 6). The different of nodule number of both construct showed statistically significant. The nodule number of 4 wpi showed MYB RNAi and GUS RNAi produced nodule number with signifant different, 15.6 and 10.7 respectively. The significance of these data that confirmed by using Student’s t test analysis for (P < 0.01) for 2 wpi and (P<0,05) at 4 wpi. The different of nodule number that showed MYB RNAi has lower nodule number demonstrated the suppression of MYB transcription factor affected to reduce the nodule number of L. Japonicus.
These data suggested that MYB RNAi has negatively effected the nodule formation. Its also provide evidence that MYB transcription factor is an important for rhizobia infection and nodule formation in L. japonicus, in which the suppression of this gene expression would significantly reduce the numerous of nodule. The expression of MYB might be a limiting step required for the proper development and organization of the nodule tissues like enod40 gene (Charon et al. 1999), so that the expression of MYB transrcription factor directly effected the number of nodule.
In order to know the phenotype expression of root length of transgenic hairy root we also collected the data of 2 wpi and 4 wpi root lenght (Figure 7). MYB OX and GUS OX produced root lenght 6.79 cm and 6.29 cm at 2 wpi, as well 8.51 and 8.21 cm at 4 wpi respectively. Similarly, MYB RNAi and GUS RNAi produced root lenght 6.47 and 6.70 cm at 2 wpi, as well 7.07 and 7.54 cm at 4 wpi recpectively. There is significant different of the root length found (P>0.05). These data (figure 6) indicated that MYB transcription factor did not affected the root lenght, no significant different found in measuring more than 35 plants for each constructs. As the conclusion, we stated that the MYB transcription factor did not give effect to root length of Lotus japonicus.
12
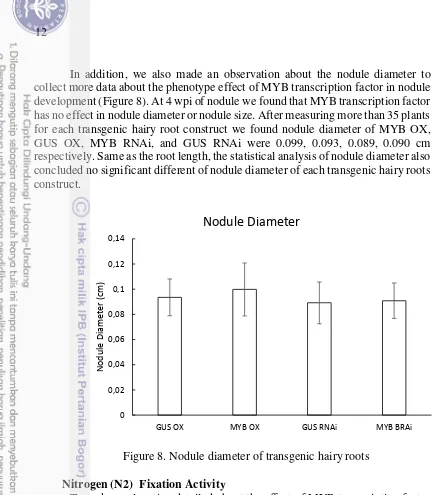
In addition, we also made an observation about the nodule diameter to collect more data about the phenotype effect of MYB transcription factor in nodule development (Figure 8). At 4 wpi of nodule we found that MYB transcription factor has no effect in nodule diameter or nodule size. After measuring more than 35 plants for each transgenic hairy root construct we found nodule diameter of MYB OX, GUS OX, MYB RNAi, and GUS RNAi were 0.099, 0.093, 0.089, 0.090 cm respectively. Same as the root length, the statistical analysis of nodule diameter also concluded no significant different of nodule diameter of each transgenic hairy roots construct.
Figure 8. Nodule diameter of transgenic hairy roots
Nitrogen (N2) Fixation Activity
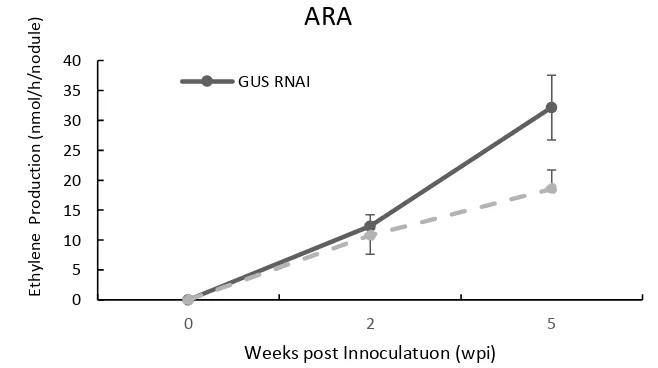
To make explanation detailed about the effect of MYB transcription factor during nodulation we also measured the acetylene reduction assay during nodule formation at 2 wpi and 5 wpi (Figure 9). The positive transgenic nodules of each construct has assayed using acetylene (C2H2) reduction assay (ARA) after 0.5 hours
incubation. ARA can measure the nitrogen fixing activity indirectly. Because nitrogenase does not convert nitrogen to ammonia when excess acetylene is included. Therefore, acetylene can convert to ethylene by nitrogenase. Acetylene reduction Assay (Figure 8) showed that the nitrogen fixation capacity of MYB OX was significantly higher than GUS OX with a different of 14.17 nmol/h/nodule and 11.44 nmol/h/nodule at 2 wpi. The nitrogen fixation assay of both constructs were increased significantly at 5 wpi that nitrogen fixation activity of MYB OX and GUS OX reached 28.68 nmol/h/nodule and 19.12 nmol/h/nodule respectively.
0
GUS OX MYB OX GUS RNAi MYB BRAi
Figure 9. Acetylene reduction assay in OX hairy roots
Different with overexpression hairy roots of MYB OX that has a significant different of activity at 4 wpi , MYB RNAi performed a similiar nitrogen (N2)
fixation assay data with GUS RNAi at 2 wpi (Figure 10). Activity of 2 wpi MYB RNAi reduced acetylene of 10.75 nmol/h/nodule and GUS RNAi reduced achetylene of 12.29 nmol/h/nodule respectively. Interestingly, at 5 wpi the acetylene reduction assay of MYB RNAi was lower than GUS RNAi that are 18.56 nmol/h/nodule and 32.14 nmol/h/nodule respectively.
These result showed that the over expression of MYB has a significant impact on nitrogen (N2) fixation per nodule at 5 wpi, interestingly the number of
nodule in previous data (Figure 5) showed the MYB OX at 4 wpi did not increase the nodule number. By Contrast, the ARA of RNAi constructs at 5 wpi suggested that the nitrogen (N2) fixation of MYB RNAi was lower than GUSRNAi. These
results also showed that the MYB deficiency or MYB RNAi affected not only in decreasing the nodule number but also the nitrogen (N2) fixation activity of nodule
after 5 wpi.
14
The MYB transcription factor effects during nitrogen (N2) fixation activity
probably because of some reasons that already found by some previous studies. Banba et al. (2001) reported the nodules formed with R. etli CE3 cannot utilize photosynthate as an energy source for nitrogen (N2) fixation thereby decreased the
fixation activity of nitrogen (N2) and exhibit senescence. On the other hand, Ott et
al. (2005) also reported that suppression of plant leghemoglobin gene by RNA interference in L. japonicus resulted higher oxygen concentrations inside nodules, lower bacterial nif and fix gene expression, created the absence of nitrogenase activity. However, the future study is needed to know the precious mechanism of MYB transcription factor in effecting nitrogen (N2) fixation activity of nodules.
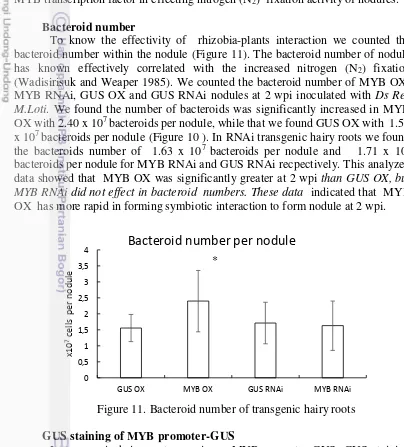
Bacteroid number
To know the effectivity of rhizobia-plants interaction we counted the bacteroid number within the nodule (Figure 11). The bacteroid number of nodule has known effectively correlated with the increased nitrogen (N2) fixation
(Wadisirisuk and Weaper 1985). We counted the bacteroid number of MYB OX, MYB RNAi, GUS OX and GUS RNAi nodules at 2 wpi inoculated with Ds Red M.Loti. We found the number of bacteroids was significantly increased in MYB OX with 2.40 x 107 bacteroids per nodule, while that we found GUS OX with 1.56 x 107 bacteroids per nodule (Figure 10 ). In RNAi transgenic hairy roots we found the bacteroids number of 1.63 x 107 bacteroids per nodule and 1.71 x 107
bacteroids per nodule for MYB RNAi and GUS RNAi recpectively. This analyzed data showed that MYB OX was significantly greater at 2 wpi than GUS OX, but MYB RNAi did not effect in bacteroid numbers. These data indicated that MYB OX has more rapid in forming symbiotic interaction to form nodule at 2 wpi.
Figure 11. Bacteroid number of transgenic hairy roots
GUS staining of MYB promoter-GUS
In transgenic hairy roots carrying a MYB promoter-GUS, GUS staining (blue color) of roots marks confirming the transient expression of GUS (Figure 12). The GUS staining showed the MYB promoter was active within the root and 12 days post inoculation (dpi) nodule. The GUS Expression within the nodule expressed in nodule primordia site (Kang et al. 2013). Further the control experiments with Plambia 134 vector did not give blue coloration.
0
GUS OX MYB OX GUS RNAi MYB RNAi
Figure 12. Blue staining in MYB promotor nodule and control
Quantitative real time PCR
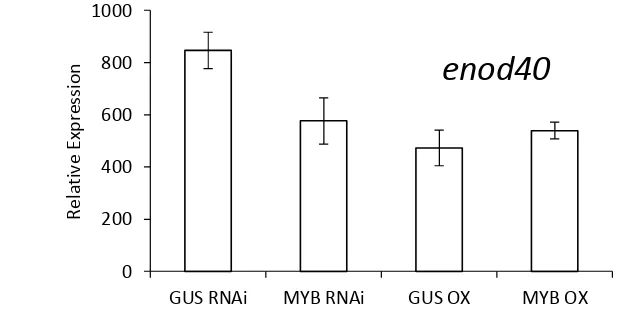
In order to check the correlation of MYB transcription factor compared with the expression of genes in nodule we investigated the expression analysis of enod40 gene and nin gene using qRT-PCR (Figure 13). The expression levels corresponding to enod40 were higher in MYB Supressing nodules than in the GUS OX as control. By parallel the expression of enod40 lower in MYB RNAi. These results suggest that MYB may binds to enod40. By Contrast, the expression levels of early nodule development marker genes, nin in MYB hairyroots (Figure 14). revealed that nin decreased in MYB OX and increased in MYB RNAi compared to their control GUS OX and GUS RNAi. These results suggest that MYB may not a regulator of nin but we predicted MYB has a parallel function with nin in nodulation process .
The expression pattern of MYB was similiar with enod40. The overexpression of enod40 accelerated nodule formation and the suppression of enod40 suppressed nodule formation (Charon et al. 1999). On the other hand The GUS staining of MYB promoter-GUS activity (Figure 12) and enod40 stated by Martirani et al. (1999) was also similiar in which it expressed in the vascular of nodule. These results indicate that MYB might regulate the enod40expression.
Figure 13. Relative expression of enod40 gene of transgenic hairy roots
0
GUS RNAi MYB RNAi GUS OX MYB OX
16
Figure 14. Relative expression of nin gene of transgenic hairy roots To ensure the nitrogen (N2) fixation correlated with nodule senescence we
measured the expression of cyspro gene, the gene marker for nodule senescence in MYB hairy roots (Figure 15). The cyspro expression of MYB RNAi was significantly high compared with GUS RNAi, contrastly the MYB OX expressed low Cyspro gene. These data totally supported the nitrogen (N2) fixation result, in
which the MYB RNAi significantly reduced the nitrogen (N2) fixation activity at
5 wpi nodule. These results indicate that downregulation of MYB expression by RNAi suppression has an effect to form senescence nodule earlier, and negative effect to nirtogen (N2) fixation and nodulation.
Banba et al. (2001) reported that nodules that formed with inoculation CE3/Rhizobium etli that inoculated appeared to be of the early-senescence type because greater amounts of disintegrated membrane structures of nodules. Early senescence formed by disintegration of symbiosome membranes, deterioratio and aggregation of bacteroids, and degradation of the cytoplasmic structures of the infected cells. Although our experiments did not reveal the mechanism of senescence formed in L. japonicus nodule by MYB RNAi but we so far concluded the role of MYB RNAi in forming senescence nodule. Moreover, the study about the mechanism of senescence of MYB RNAi is an interesting issue to be solved in the future research.
GUS RNAi MYB RNAi GUS OX MYB OX
Figure 15. Relative expression of cyspro gene of transgenic hairy roots
CONCLUSIONS
In this present study, we concluded that :
a. the Overexpression of MYB increased the nodule number of nodule during early nodulation time but no longer in mature nodule.
b. MYB overexpression significantly increased the nitrogen (N2) fixation
activity and plant-rhizobia symbiosys.
c. The suppression of MYB (MYB RNAi) decreased the number of nodule and indirectly decreased the nitrogen (N2) fixation activity and symbiosis
process of plant-rhizobia.
d. MYB transcription factor expressed in nodule and root.
e. As an early genetically study we proposed that MYB transcription factor might bind to enod40 gene but not as transcription regulator of nin gene. f. The lack of MYB gene formed early senecence in nodulation.
RECOMENDATIONS
It is needed to analysis the effect of MYB genetic expression during nodulation more deeply. The histological study of MYB nodule also very important to be investigated more detailed.
0
GUS RNAi MYB RNAi GUS OX MYB OX
18
REFERENCES
Banba M,Siddique MAB, Kouchi H, Izui K, Hata S. (2001). Lotus japonicus forms early senescent root nodules with Rhizobium etli.MPMI 173–180
Beringer, J. E. 1974. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol 84, 188–198.
Broughton WJ and Dilworth MJ. (1971). Control of leghaemoglobin synthesis in snake beans. Biocehimistry Journal. Biochem. J. (1971) 125,1075-1080 Charon C, Sousa K, Crespi M, Kondorosia A. Alteration of ENOD40 expression
modifies Medicago truncatula root nodule development induced by Sinorhizobium meliloti. Plant Cell 1999;11;1953-1965. DOI 10.1105/tpc.11.10.1953
El Yahyaoui F, Kuster H, Ben Amor B, Hohnjec N, Puhler A, Becker A, Gouzy J, VernieT , Gough C, Niebel A, Godiard L, Gamas P. (2004). Expression profiling in Medicago truncatula ientifies more than 750 genes differentially expressed during nodulation, including many potential regulators of the symbiotic program. Plant Physiol. 136 (2), 3159-3176
www.plantphysiol.org/cgi/doi/10.1104/pp.104.043612
FAO. Agriculture, forestry and other land use emissions by sources and removals by sinks. Working Paper Series ESS/14-02 (2014)
Fang Y and Hirsch AM. (1998). Studying early nodulin gene enod40 expression and induction by nodulation factor and cytokinin in transgenic alfalfa.. Plant Physiol. 116: 53–68
Ferguson BJ and Mathesius U. (2003). Signaling interactions during nodule development. Plant Growth Reg 22:47–72 doi:10.1007/s00344-003-0032-9
James A, Rosinski, William RA. (1997). Molecular evolution of the myb family of transcription factors: evidence for polyphyletic origin. J Mol Evol 46:74– 83
Kaneko T, Nakamura Y, Sato S, Asamizu E, Kato T, Sasamoto S, Watanabe A, Idesawa K, Ishikawa A, Kawashima K, Kimura T, Kishida Y, Kiyokawa C, Kohara M, Matsumoto M, Matsuno A, Mochizuki Y, Nakayama S, Nakazaki N, Shimpo S, Sugimoto M, Takeuchi C, Yamada M, Tabata S. (2000). Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. Dna Research 7, 331-338
Kang H , Chu1 X, Wang C, Xiao A, Zhu H, Yuan S, Yang Z, Ke D, Xiao S, Hong Z, Zhang Z. (2013). A MYB coiled-coil transcription factor interacts with NSP2 and isinvolved in nodulation in Lotus japonicus. New Phytologist. 201: 837–849 doi: 10.1111/nph.12593
Lateif KA, Bogusz D, Hocher V. (2012). The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, Rhizobia and Frankia bacteria. Plant Signaling & Behavior. 7:6, 636-641. http://dx.doi.org/10.4161/psb.20039
Mai HT, Nomura M, Takegawa K, Asamizu E, Sato S, Kato T, Tabata S, Tajima S .(2006). Identification of a Sed5-like SNARE gene LjSYP32-1 that contributes to nodule tissue formation of Lotus japonicus. Plant Cell Physiol 47: 829-838
Maekawa T, Kusakabe M, Shimoda Y, Sato S, Tabata S, Murooka Y, Hayashi M .(2008).Polyubiquitin promoter-based binary vectors for overexpression and gene silencing in Lotus japonicus. Mol Plant-Microbe Interact 21: 375-382
Marsh T. Rakocevic A, Mitra RM, Brocard L, Sun J, Eschstruth A, Long SR, Schultze M, Ratet P, Oldroyd GED. (2007). Medicago truncatula NIN is essential for rhizobia-independent nodule organogenesis induced by autoactive calcium/calmodulin-dependent protein kinase. Plant Physiol, Vol. 144, pp. 324–335
Martirani L, Stiller J, Mirabella R, Alfano F, Lamberti A, Radutoiu SE, Iaccarino M, Gresshoff PM, Chiurazzi M. (1999). T-DNA tagging of nodulation- and root-related genes in Lotus japonicus: Expression patterns and potential for promoter trapping and insertional mutagenesis. Mol Plant-Microbe Interact 12: 275-284
Ott, T, van Dongen JT, Günther C, Krusell L, Desbrosses G,Vigeolas H, Bock V, Czechowski T, Geigenberger P, and Udvardi MK. (2005). Symbiotic leghemoglobins are crucial for nitrogen fixation in legume root nodules but not for general plant growth and development. Curr. Biol. 15: 531– 535.
Peoples, M.B., et al.. (2009). The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 48:1–17.
Ron M, Kajala K, Pauluzzi G, Wang D, Reynoso MA, Zumstein K, Garcha J, Winte S, Masson H, Inagaki S, Federici F, Sinha N, Deal R B, Bailey-Serres J, Brady SM. (2014). Hairy root transformation using Agrobacterium rhizogenes as a tool for exploring cell type-specific gene expression and function using tomato as a model. Plant Physiol.Oct;166(2):455-69, . doi: http://dx.doi.org/10.1104/pp.114.239392
Rípodas C. Clúa1 J, Battaglia M, Baudin M, Niebel A, Zanetti ME, Blanco F. (2014).Transcriptional regulators of legume-Rhizobia symbiosis Nuclear Factors Ys and GRAS are two for tango. Plant Signaling & Behavior (7): 1-5; 9:e28847; PMID: 24736593; http://dx.doi.org/10.4161/psb.28847
Sato S, Nakamura Y, Kaneko T, , Asamizu E, , Kato T, Nakao M, Sasamoto S, Watanabe A, Ono A, kumiko Kawashima K, Fujishiro T, Katoh M, Kohara M, Kishida Y, Minami C, Nakayama S, Nakazaki N, Shimizu Y, Shinpo S, TakahashiC, Wada T, Yamada M, Ohmido N, Hayashi M, Fukui K, Baba T, Nakamichi T, Mori H, and Tabata S. (2008). Genome structure of the legume, Lotus japonicus. DNA RESEARCH 15, 227–239, doi:10.1093/dnares/dsn008
20
Van Wyk SG., Plessis MD, Cullis CA, Kunert KJ, Vorster BJ. (2014). Cysteine protease and cystatin expression and activity during soybean nodule development and senescence. Plant Biology, 14:294
http://www.biomedcentral.com/1471-2229/14/294
VolpeV. Aglio ED, Giovannetti M, Ruberti C, Costa A, GenreA, Guether M, Bonfante P. (2013). An AM-induced, MYB -family gene of Lotus japonicus(LjMAMI) affects root growth in an AM-independent manner. The Plant Journal 73, 442–455 doi: 10.1111/tpj.12045
APPENDIX
Appendix 1. Phenotype expression of transgenic hairy roots under GFP microscope. The photographs were taken in the GFP mode (A, C, E, G) and in the light field (B, D, F, H). Scale bars represent 2 mm, A and B are MYB OX in 2 wpi, C and D are GUS OX in 2 wpi, E and F are GUS RNAi in 2 wpi, G and H are GUS RNAi in 2 wpi.
E
F
G
H A
B
C
22
40 14 11 overexpression constructs (MYB OX and GUS OX)
Variable 1
Hypothesized Mean Difference 0
df 85
t Critical one-tail 1.6629785
P(T<=t) two-tail supression constructs (MYB OX and GUS OX)
Variable 1 Variable 2
Hypothesized Mean Difference 0
24
29 14 8 12 19 overexpression constructs (MYB OX and GUS OX)
Variable 1 Variable 2
Hypothesized Mean Difference 0
26
Appendix 7. t-Test: two-sample assuming unequal variances of 4 wpi supression constructs (MYB RNAi and GUS RNAi)
Variable 1 Variable 2
Hypothesized Mean Difference 0
df 57
19 4.81 8.011 9.463 6.082
AVE
30
34 0.085 0.103 0.097 0.078
35 0.067 0.117 0.066 0.095
36 0.108 0.128 0.101
37 0.093 0.085 0.090
38 0.089 0.085
39 0.095 0.069
40 0.087 0.108
41 0.103
42 0.091
AVE 0.093 0.100 0.089 0.091
STDEV 0.015 0.021 0.017 0.014
Appendix 11. Table of ethylene production of 2 wpi transgenic hairy roots Plant C2H2(nmol/hr/nodule) STDEV
GUS
RNAi 12.2951 1.903
MYB
RNAi 10.7541 1.039
GUSOX 11.4354 1.078
MYB OX 14.1659 3.392
Appendix 12. Table of ethylene production of 5 wpi transgenic hairy roots Plant C2H2(nmol/hr/nodule) STDEV
GUSRNAi 32.149 5.431694882
MYB
RNAi 18.566 3.142
GUSOX 19.3124 3.374
BIOGRAPHY
Author was born in Bilamporoa. Bontotanga. A village in Indonesia, on May 26t, 1990. He is a single child
of Mr Herman Akmad and Mrs Yasmi. He graduated from highschool SMA Negeri 1 Bulukumba in 2008. He continued his study at Department of Soil Science. Faculty of Agriculture, Hasanuddin University in 2008 and officially got his bachelor degree in 2012. He had enrolled
master degree scholarship ―Beasiswa Fresh Graduate, on
September 2013, by Directorate of Higher Education (DIKTI) Indonesia in Soil and Environtmental Biotechnology at Bogor Agricultural University. In 2015
he accepted in SUIJI-JDP (Six University Initiative Japan-Indonesia Joint Degree Program) at Kagawa University. He spent 1 year in Japan for doing research in plant nutrition laboratory and taking class for several subjects. In 2014 he was selected as secretary of Bogor Science Club, the graduated student organitation at Bogor Agricultutal University and as President of Komunitas Kongkrit in 2016. In 2016 he received 2nd place in National English Competition on Bogor Science Club. In
2015 he joined conference at Japanese Society of Soil Science and Plant Nutrition and presented a research entitled –Effect of MYB Transcription Factor During Nodule Development in Lotus japonicus-. and presented the same article in the SUIJI International Seminar 2015 in Kagawa as IPB student representative. In 2014 he was delegated as IPB student representative on CRISU-CUPT student forum in Konkhaen-Thailand. Author was publishing an article in Plant Biothechnology