THE SELECTION OF IRON TYPE AS FORTIFICANT
IN CHOCOLATE FILLED MILK POWDER
DAVID HARI TJAHJONO
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY (IPB)
BOGOR
STATEMENT ABOUT THESIS AND SOURCE OF INFORMATION
Herewith I declare that thesis “The Selection of Iron Type as Fortificant in Chocolate Filled Milk Powder” is my work with direction from commission of advisor and never been submitted before in any forms by any means to any university. The source of information which come from or cited from book or journal which published or not published from other author has been mentioned in the text and in References on the last part of this thesis.
Jakarta, February 2010
ABSTRACT
DAVID HARI TJAHJONO.
The Selection of Iron Type as Fortificant in Chocolate Filled Milk Powder. Under direction of NURHENI SRI PALUPI and NURI ANDARWULAN.Many infants, children, and women of childbearing age, particularly in the poorer countries of the developing world, are iron deficient. About half of these iron-deficient individuals develop iron deficiency anemia (IDA), the most advanced form of the disease, which has several major negative impacts on health and contributes substantially to the risk of early death and disability (Hurrell 2004).
The objective of this research is to find the appropriate iron type at certain level of fortification in prototype chocolate filled milk powder that is reconstituted at different temperature, based on mainly sensory evaluation, as well as chemical evaluation and keeping quality evaluation.
Types of iron used in this research were Iron sulphate (FeSO4 H2O), Iron fumarate (C4H2FeO4), and Iron pyrophosphate (Fe4 (P2O7)3. 8H2O). These irons cover its properties from low and high bioavailability as well as low and high solubility. The level of iron fortification was set on 10% and 20% of daily value (DV), which corresponds to respectively 2.6 mg and 5.2 mg/serving. The temperature of water for reconstitution was proposed to adapt the consumer consumption behavior, 15oC for cold consumption, 45oC for warm consumption and 80oC for hot consumption.
The selection of appropriate iron type was based on mainly sensory evaluation, chemical analysis and keeping quality evaluation. Only the appropriate iron type would undergo keeping quality evaluation with objective to see the stability of the prototype undergone NRT storage condition for at least 6 months periods of time, where it was represent the normal product age sold in the store.
Based on above research methodology, the acceptable iron for fortification in chocolate filled milk powder at level both 10% and 20% DV, reconstituted at 15 – 80°C, and has stability at estimated 6 months, are Iron Fumarate and Iron Pyrophosphate.
iii
SUMMARY
DAVID HARI TJAHJONO.
The Selection of Iron Type as Fortificant in Chocolate Filled Milk Powder. Under direction of NURHENI SRI PALUPI and NURI ANDARWULAN.Food fortification with iron has been recommended as one of the preferred approaches for preventing and eradicating iron deficiency. Iron supplementation has been mainly targeted at high-risk groups such as pregnant women and young children. Food fortification by mass fortification of staples such as wheat and maize flour or condiments such as salt, soy sauce offers a more cost-effective approach, but this may be not effective for specific age group, where daily iron intake might not be appropriate and iron dietary source from others food might be different (Mehansho 2006). Many infants, children, and women of childbearing age, particularly in the poorer countries of the developing world, are iron deficient. About half of these iron-deficient individuals develop iron deficiency anemia (IDA), the most advanced form of the disease, which has several major negative impacts on health and contributes substantially to the risk of early death and disability (Hurrell 2004). Haem Iron that has good bioavability is mostly found in meat which is classified as expensive foods. Since chocolate filled milk powder which has vegetable fat is designed as affordable milk powder, this iron fortification could be appropriate addressing the iron deficiency amongst poor socio-economic.
The objective of this research is to find the appropriate iron type at certain level of fortification in prototype chocolate filled milk powder that is reconstituted at different temperature, based on mainly organoleptic evaluation, as well as chemical evaluation and keeping quality evaluation.
The success of iron fortification is dependent on delivering a meaningful level of bioavailable iron without affecting the taste and appearance of the finally consumed product. Iron fortification may cause 1) metallic aftertaste 2) unacceptable flavor as a result of the oxidation-mediated rancidity of fats 3) undesirable color changes resulting from interactions with anthocyanins, flavonoids, and tannins and 4) degradation of vitamins (Mehansho 2006).
Types of iron that use in this research were Iron Sulphate (FeSO4 H2O), Iron Fumarate (C4H2FeO4), and Iron Pyrophosphate (Fe4 (P2O7)3. 8H2O). These irons cover its properties from low and high bioavailability as well as low and high solubility. The level of iron fortification was set on 10% and 20% of daily value (DV), which corresponds to respectively 2.6 mg and 5.2 mg/serving. The temperature of water for reconstitution was proposed to adapt the consumer consumption behavior, 15oC for cold consumption, 45oC for warm consumption and 80oC for hot consumption.
The selection of appropriate iron type was based on mainly organoleptic test, chemical analysis and keeping quality evaluation. Only the appropriate iron type would undergo keeping quality evaluation. The chemical and bacto analysis would ensure other factor affecting product stability such as AW, TPC was monitored. The objective of keeping quality was to see the stability of the prototype undergone NRT storage condition for at least 6 months periods of time, where it was represent the normal product age sold in the store. A reference of chocolate filled milk powder without iron fortification was provided to verify other factor affecting the keeping quality, i.e. packaging performance, residual oxygen content.
level both 10 and 20% DV, do not give significant impact on fat oxidation and vitamin C degradation at fresh and keeping quality 6 weeks at 37°C which correspond to 6 months at NRT. Iron sulphate is not acceptable for iron fortification in chocolate filled milk powder due to its high solubility which causes greyish colorisation as well as metallic taste and happened 15 minutes after reconstitution
Since iron fortification does not give a significant impact on vitamin C degradation, and to the fact that vitamin C will help iron absorption, it is suggested to always add vitamin C in iron fortified products. It is suggested to take data iron solubility for any future experimental iron fortification (at pH reconstitution) to predict the impact of greyish colorisation and metallic taste in chocolate filled milk powder.
v
© Copyright IPB, year 2010
All rights reserved
Do not cite partially or fully of this thesis without mentioning its source. Citation is
only for the purpose of education, research, thesis, report preparation, criticism writing
or review of issue; and that citation is not giving any bad image for IPB.
THE SELECTION OF IRON TYPE AS FORTIFICANT IN
CHOCOLATE FILLED MILK POWDER
DAVID HARI TJAHJONO
Thesis
As one of prerequisite to obtain Magister Professional degree On Program Study of Food Technology
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY (IPB)
BOGOR
vii
APPROVAL
Research title : The selection of iron type as fortificant in chocolate filled milk powder
Name : David Hari Tjahjono NRP / Mayor : F252070115 / PTP Program Study : Food Technology
Approved by, Commission of advisor
Dr.Ir. Nurheni Sri Palupi, MS Dr.Ir. Nuri Andarwulan M.Si
Lead advisor Advisor
Acknowledged by,
Head of Program Study For Dean of Graduate School IPB Secretary of Magister Program,
Dr.Ir. Lilis Nuraida, M.Sc. Dr.Ir. Naresworo Nugroho, M.Si
FOREWORD
The fear of the Lord is the beginning of knowledge: but fools despise
wisdom and instruction. (Proverbs 1:7, Old Testaments, Holy Bible
–
King
James Version).
Praise the Lord who give grace and help so that author could finalise
thesis with title
“
The Selection of Iron as Fortificant in Chocolate Filled Milk
Powder
”
. This thesis was started in August 2009 with research which uses
internal facility of PT. Nestle Indonesia.
This thesis was finalized with support from many people and
departments, therefore author would like to express thankfulness to:
1. Dr. Ir, Nurheni Sri Palupi, MS. as lead advisor, Dr. Ir. Nuri Andarwulan
MSi as advisor and Dr. Ir. Feri Kusnandar, MSi as external assessor who
give advice and direction for thesis report preparation.
2. Mr. Marlou Basco Constantino and Mr. Nico Amann
as author’s
superiors who give understanding and support during lecture and thesis
report preparation, and management of PT Nestle Indonesia which allow
and grant the author to take post graduate study.
3. Mr. Eldert Heijkoop as Factory Manager of Kejayan Factory
–
PT. Nestle
Indonesia who allows author to use material and factory facility to
prepare and analyse the prototype samples.
4. Mr. Zulliyan Dachlan, David K Mulia, Achmad Khoirun, Wijoyo, Eko
Sasmito, Endah Sriwulan, Faika Dwiyanti, Juwita Astuti and Shenny
Lumingkewas, as author’s coleaque who help in coordinating the
preparation and analysis of prototype samples.
5. Ms. Fatur Tika, as asistant coordinator of program study Magister
Profesional Food Technology who always help the administrative job for
meeting, seminar and examination.
6.
Author’s family
, Ribkah Christiana, Andre and parents who always give
support and pray in order to finish this study.
Author hope this thesis would benefit for all who concern about food
fortification.
ix
BIOGRAPHY
The author was born in Mojokerto on September 21
st, 1969 as the eldest
children from parents of Soegihartono, B.Sc and Indah Arijatiningsih. In 1987
the author was graduated from Senior High School (SMA 8 Malang) and in the
same year enrolled Sepuluh Nopember Institute of Technology Surabaya (ITS).
Author finished the study in 1993 from the Faculty of Technology Industry
–
ITS
with major Chemical Engineering and got the bachelor degree.
At the moment, author works for PT Nestle Indonesia as AVP (Assistant
Vice President) Product Deployment in Manufacturing Services Department.
The career of author was started from 1993
–
1996 in PT Trias Sentosa as
Quality Incoming Supervisor. Starting 1996
–
now, the author works for PT
Nestle Indonesia and had several function or position Production Supervisor
SCM
–
Waru factory, Product Technologist
–
Waru Factory, Head of production
for Agglomeration Department
–
Waru Factory, Application Group Specialist
–
Kejayan factory, Product Deployment - Manufacturing Service Department till
now.
TABLE OF CONTENT
Page
LIST OF TABLE ………. xii
LIST OF FIGURE ………. xiii
LIST OF ATTACHMENT ………. xiv
I. INTRODUCTION A. BACKGROUND ……… 1
B. OBJECTIVE ……….. 2
C. BENEFIT ………... 2
D. SCOPE ……….. 2
xi
1. Timing 2. Location
B. MATERIALS AND TOOLS ……….. 1. Material and tools for sample preparation
2. Material and tools for analysis
17
C. RESEARCH METHODOLOGY ………..
1. Methodology 2. Working procedure
a. Bulk preparation of chocolate filled milk powder b. Filling/packing procedure
19
D. METHOD OF ANALYSIS ………
1. Accelerated keeping quality procedure 2. Sensory evaluation procedure
3. Chemical analysis procedure 4. Microbiology test procedure
23
IV. RESULT AND DISCUSSION
A. PROTOTYPE OF CHOCOLATE FILLED MILK POWDER ……… 33 B. SELECTION OF IRON TYPE BASED ON CHEMICAL, MICROBIOLOGY
AND SENSORY EVALUATION ………..
1.Chemical, microbiology and sensory evaluation of powder based product
2. Sensory evaluation of reconstituted product
3. Greyish colorisation and metallic taste in reconstituted milk when using iron Sulphate
34
C. STABILITY EVALUATION OF IRON FORTIFIED CHOCOLATE FILLED
MILK POWDER ……….
1.Chemical, microbiology and sensory evaluation of powder based product
2. Sensory evaluation of reconstituted product
37
V. CONCLUSION AND SUGGESTION ……… 41
REFERENCES ………. 42
LIST OF TABLE
Page
1. Nutritional fact per serving of chocolate filled milk powder ………. 4
2. Changes of sugar content in shell-free cocoa bean during fermentation ……. 6 3. Starch content of cocoa beans representing regions of production ………….. 7 4. Starch content in various commercial products ……… 7 5. Distribution of nitrogen compounds in dry cocoa beans ………. 9 6. Phytochemical and polyphenolics of cocoa bean and cocoa products ……… 10 7. Visual detection thresholds (VDT) for selected anthocyanins ……….. 12 8. Relative biological value and solubility of various irons ………. 14 9. Fe content in mixture of cfmp fortified with iron sulphate 10%DV (FMP IS10)
for homogeneity test ………. 33 10. Result of chemical, microbiology and sensory evaluation for all samples …... 34 11. Result of sensory evaluation for all samples ………. 35 12. Result of AW analysis for prototype of chocolate filled milk powder during
storage ……… 37
13. Result of vitamin C analysis (mg/100g) for prototype of chocolate filled milk powder during storage ……….. 37 14. Result of total plate count analysis (cfu/g) for prototype of chocolate filled
milk powder storage ……….. 38 15. Sensory score of prototype of chocolate filled milk powder during storage …. 38 16. pH value (20°C) of prototype of chocolate filled milk powder during storage .. 38 17. Sensory score of prototype of chocolate filled milk powder during storage at
xiii
LIST OF FIGURE
Page
1. Research plan flow chart ……….. 21
2. Processing step of preparation prototype filled milk powder ……….. 24
3. Water activity meter ……….. 27
4. Apparatus for Moisture Karl Fischer determination ………. 28
5. AAS aparatus for Iron determination ……….. 30
6. Titrimetri aparatus for Vitamin C determination ……… 31
7. Powder color for all samples ……… 35
8. Color of reconstituted control Iron (Ferrous) Sulphate 10%DV (FMPIS10) and 20%DV (FMPIS20) ……… 36
9. Appearance of reconstituted control (FMP ref), Fe Fumarate 10%DV (FMPIF10) and 20%DV (FMPIF20) ……… 40
LIST OF ATTACHMENT
Page
1. Keeping Quality of Nestle chocolate milk powder at 37oC and NRT ………….. 44
I. INTRODUCTION
A. Background
Many infants, children, and women of childbearing age, particularly in the poorer countries of the developing world, are iron deficient. About half of these iron-deficient individuals develop iron deficiency anemia (IDA), the most advanced form of the disease, which has several major negative impacts on health and contributes substantially to the risk of early death and disability (Hurrell 2004).
There are five major negative health consequences of IDA. Firstly in the pregnant woman, IDA leads to sub-optimal pregnancy outcome, including lower birth weight, increased morbidity in mothers and neonates, increase infant mortality, and a greater risk of the infant developing iron deficiency after four months of age Secondly, during infancy, IDA leads to delayed mental and motor development with effects on behavior and cognitive performance when the child reach school age. The effects of early IDA on brain development may not be reversible by subsequent treatment, and failure to reach educational goals may affect earning capacity later in life. In children, IDA can also lead to increase frequency and duration of upper respiratory infection and to increased risk for goiter due to diminished utilization of iodinefor thyroid hormone production. Finally, physical work capacity is impaired for all individuals as IDA negatively affects aerobic capacity related to intense physical activity and reduces endurance capacity, voluntary activity, and work productivity. This results in a lower income for the individual, the family, and the country (Hurrell 2004).
Iron deficiency is therefore a major health problem in the developing world and recently WHO ranked it as seventh out of the ten major global preventable risks for disease, disability, and death that together account for 40% of the 56 million deaths that occur world-wide each year, and for one third of the global loss of healthy life years (Hurrell 2004).
For young children iron fortification in chocolate milk product could be a good vehicle to boost iron supplementation, since chocolate flavor is the most favorite flavor milk drink for kids. Furthermore, this product could also increase the intake of vitamins and other minerals such as zinc and calcium which is also important for children.
Haem Iron that has good bioavability is mostly found in meat which is classified as expensive foods. Since chocolate filled milk powder which has vegetable fat is designed as affordable milk powder, this iron fortification could be appropriate addressing the iron deficiency amongst poor socio-economic.
The success of iron fortification is dependent on delivering a meaningful level of bioavailable iron without affecting the taste and appearance of the finally consumed product. Iron fortification may cause 1) metallic aftertaste 2) unacceptable flavor as a result of the oxidation-mediated rancidity of fats 3) undesirable color changes resulting from interactions with anthocyanins, flavonoids, and tannins and 4) degradation of vitamins. In addition, the bioavailability of iron is dependent not only on the iron source but also the type of food and/or beverage consumed with it (Mehansho 2006).
Currently, there are a number of iron sources available as food fortificants. Choosing the appropriate iron fortificant that deliver a meaningful level of bioavailable iron with organoleptically acceptable of finally consumed product remains a challenge for food scientist and food industry.
B. Objective
The objective of this research was to find the appropriate iron types at certain levels of fortification in prototype chocolate filled milk powder that were reconstituted at different temperature, based on mainly sensory evaluation as well as chemical evaluation and keeping quality evaluation.
C. Benefit
This research may give benefit for food industry on how to choose appropriate iron type as fortificant in chocolate milk product. It may also give benefit as reference for researcher that has similar research topic.
D. Scope
II. LITERATURE REVIEW
A. Chocolate Filled Milk Powder
1. Definition
According to SNI 3752-2009, Chocolate milk powder is milk product in powder form, made from milk powder and cocoa powder with or without addition of sugar, other food ingredient and other permitted food additive [BSN 2009]
According to Codex committee on milk and milk products by CAC FAO/WHO 2000, Filled milk powders are products obtained from milk in which milk fat has been replaced wholly or partly by an equivalent amount of edible vegetable oil, edible vegetable fat or a combination thereof, by the partial removal of water to meet the compositional requirement
Based on each definition above, it could be defined that chocolate filled milk powder (cfmp) is milk product in powder form, made from milk powder where the milk fat is replaced wholly or partly with other fat, and cocoa powder with or without addition of sugar, other food ingredient and other permitted food additive.
According to food category system established by GSFA-Codex as well as by BPOM, this product could be classified as food category 01.5.2. Milk and cream powder analogues.
2. Composition
Chocolate milk powder that is available in the market today is normally composed of milk powder of which consist of fat and milk snf (solid non fat), sugar, cocoa, vitamin, mineral and flavour. An affordable chocolate milk powder which should compose of cheap ingredient, yet delivering adequate nutrition, could be made from above composition which fat is using cheap vegetable fat such as palm olein.
A proposal of chocolate filled milk powder which composed of sugar, filled milk powder (using full or partial palm olein), cocoa powder, calcium carbonate, vitamin premix and iron, could be considered.
A nutritional fact of above product per serving (which refer to Nestle Ideal Choco) could be expected as shown in Table 1 below (serving size: 27g).
3. Raw Materials
which fat is composed of majority not milk fat, Cocoa powder, a 10-12% fat alkalized cocoa powder, Calcium Carbonate micronised, Vitamin premix, a free flowing homogeneous mix of vitamins prepared in carrier, Iron fortificant, i.e. Iron Sulphate (FeSO4 H2O), Iron (Ferrous) Fumarate (C4H2FeO4), Iron Pyrophosphate (Fe4(P2O7)3.8H2O)
Table 1 Nutritional fact per serving of chocolate filled milk powder Nutrient Per 27 gr of product % DV (AKG2000)
Total Fat 3.5 g 6
Protein 4 g 7
Sugar 12 g -
Vitamin A 146 mg RE 25
Vitamin C 13.5 mg 25
Calcium 200 mg 30
Iron 3.2 mg 10
Source : Label declaration of Nestle Ideal Choco (2009)
All raw materials listed above are intended to be used in products made by dry mixing without any further heat processing, and normally packed in strippable packaging.
4. Processing
Basically chocolate filled milk powder is produced by means of dry mixing. Mixing (or blending) is a unit operation in which a uniform mixture is obtained from two or more components, by dispersing one within the other(s). During a mixing operation, differences these properties (i.e. particle size, shape, density, surface and flow characteristic) also cause unmixing (or separation) of the components parts. In some mixtures, uniformity is achieved after a given period and then unmixing begins. It is therefore important in such cases to time the mixing accurately, which in turn is related to type of mixer, the operating conditions and the components of foods (Fellows 2000). A homogeneity test could be performed to know the optimal mixing time.
5. Physico-Chemical properties
5
water (10-15oC), warm water (40-45oC) or hot water (75-80oC). Chocolate filled milk powder is normally reconstituted to prepare 150 – 200 mL chocolate milk ready to drink.
6. Organoleptic properties
Refer to technical instructions GI-31.107-1, September 2007, established by Nestle with title The “In/Out” test method for sensory quality control, the organoleptic properties of chocolate filled milk powder should be as follows: typical taste of chocolate milk powder, brown to white color and typical aroma of chocolate milk powder.
B. Cocoa
1. Cocoa Type and Manufacturing
Cocoa beans are seeds found in woody plants (shrubs or small tree) belonging to the genus Theobroma. Only two species of this genus, Criollo, bearing warty fruits containing white or faintly purple seeds, and Forastero, bearing smoother fruits containing seeds of a deeper purple shade, are recognized from a commercial standpoint. Numerous hybrids containing genes of both species have recently been developed (Flament 1989 cited by Shahidi 2004)
2. Cocoa Composition.
Cocoa contains carbohydrate, more so as starch than sugars, but it is more common to consider the contribution from sugars mixed with cocoa during the manufacture of chocolate. Proteins only present in small amount in cocoa. Accordingly, it is for the sensory experience that cocoa is consumed and not for the protein contribution. Nevertheless, since cocoa is often consumed as milk chocolate, the protein contribution will increase with milk protein, and the milk solids improve the taste of a nutritious food (Knight 1999).
a. Carbohydrates Sugars
Cotyledons of fresh cocoa beans contain only 2-4% of free sugar, beside traces of others sugars and sugar alcohols, such as galactose, raffinose, starchyose, melibiose, sorbose, mannitol, inositol, etc. The final content of these sugars varies considerably in fermented beans of various origins, most likely owing to the type and extent of fermentation. Sucrose in well-fermented beans can decrease until near zero, whereas fructose and glucose increase correspondingly (Knight 1999).
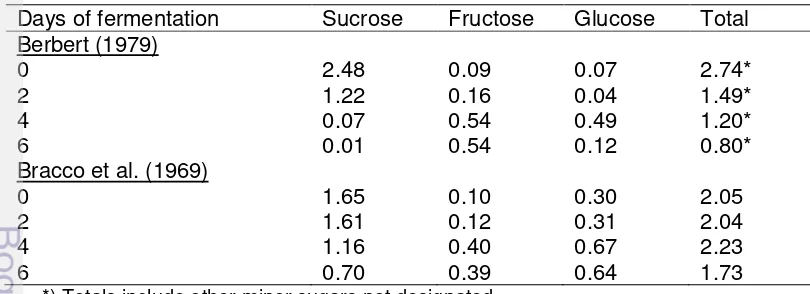
Table 2 Changes of sugar content in shell-free cocoa bean during fermentation.
Days of fermentation Sucrose Fructose Glucose Total Berbert (1979) 0 2 4 6 2.48 1.22 0.07 0.01 0.09 0.16 0.54 0.54 0.07 0.04 0.49 0.12 2.74* 1.49* 1.20* 0.80* Bracco et al. (1969)
0 2 4 6 1.65 1.61 1.16 0.70 0.10 0.12 0.40 0.39 0.30 0.31 0.67 0.64 2.05 2.04 2.23 1.73 *) Totals include other minor sugars not designated
Source : Knight 1999
7
well (Knight 1999). Starch
Schmieder and Keeney reported a mean value of 5.30% starch (4.5 - 7.0%) for 12 lots of cocoa beans (including shell) representing major geographic regions of production (Table 3). Two variables, which might affect the final starch content of cocoa beans, are fruits ripeness at harvesting and fermentation process. Starch in cocoa beans increase progressively from 4.3% to 6.8% between 4.5 and 5.0 months, but then decreased to 6.3% at 5.5 months when pods were harvested (Knight 1999).
Table 3 Starch content of cocoa beans representing regions of production. Cocoa bean source % Starch in whole dry beans including shells Ghana Bahia Samoa Ecuador Venezuela Jamaica Trinidad
7.00 ; 5.80 4.78 ; 4.86 4.77 5.51 ; 5.08 4.66 ; 5.63 4.50 4.77 ; 6.02
Source : Schmieder and Keeney 1980 cited by Knight 1999
Table 4 Starch content in various commercial products
Product % Starch % Starch in cocoa mass (non-fat) Regular cocoa
Dutched cocoa
Regular chocolate liquor Dutched chocolate liquor Dark, Sweet chocolate Milk chocolate (a) Milk chocolate (b)
15.5 15.9 6.9 7.0 3.1 1.1 1.3 18.6 18.9 16.3 16.3 20.4 18.3 18.2 Source : Schmieder and Keeney 1980 cited by Knight 1999
Data for starch analysed in various commercial products are shown in Table 4. When starch content was expressed as starch per unit of cocoa mass, values were quite similar. This indicates that manufacturing processes employed in making these products did not alter the amount of cocoa starch present in cocoa beans (Knight 1999).
Fibre
obtained. Valiente et al. found 17.8% and 16.1% DF in raw and roasted cocoa bean, respectively; using the method according to Prosky et al. Approximately 20% of DF is soluble. Cocoa bean contains also significant amount of polyphenols (5.9%, expressed as tannic acid), but very little has been found in the fibre fractions (0.2% in soluble dietary fibre and 1.6% in insoluble dietary fibre) (Knight 1999).
Geilinger et al. determined the dietary fibre content using the neutral detergent method (15) and found 9.1% in fermented cocoa bean, 12.0% after roasting, and 12.1-15.4% in cocoa mass. This increase of apparent fibre during roasting and processing of the beans was probably due to condensation reaction between protein and polyphenols. These fibre contents are, however, lower than the value reported elsewhere, in part because it does not take into account the soluble fibre fraction (Knight 1999).
b. Protein
Nitrogenous compounds in cocoa beans
Cocoa contains two classes of nitrogenous compounds such as proteins, which contribute up to about 80% of total nitrogen and methylxanthines. Their quantity and relative proportion vary with the bean variety as presented in Table 5 (Knight 1999).
Protein modifications during cocoa processing
From the fresh beans to cocoa powder, cocoa proteins are chemically modified mainly polyphenols, which affect their functional and nutritional properties. This reaction will contribute to reduce the solubility of the cocoa proteins, which decreases from 170 to 65 mg/ fat-free beans during fermentation. The protein quality is therefore expected to decrease similarly. During roasting, the maillard reaction takes place between the amino groups of the small peptides and of the free amino acids liberated during fermentation and the free reducing sugars, some of them also being liberated during fermentation. This maillard reaction is responsible for the development of the cocoa aroma at the expense of the amino acids involved, and to a minor extent, of the nutritional quality (Knight 1999).
c. Lipid
9
Cocoa powder which is commercially available today contains 10-12% fat, 14-16% fat and 20-22% fat. Cocoa powder with 10-12% fat is generally used for beverage application, including chocolate filled milk powder. Cocoa butter is generally used in chocolate confectionary to improve taste and aroma.
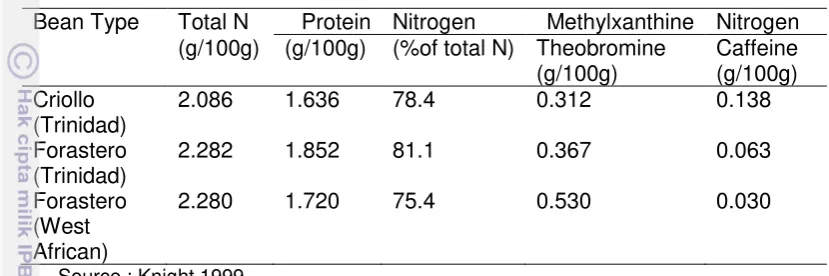
Table 5 Distribution of nitrogen compounds in dry cocoa beans
Bean Type Total N Protein Nitrogen Methylxanthine Nitrogen (g/100g) (g/100g) (%of total N) Theobromine
(g/100g)
Caffeine (g/100g) Criollo
(Trinidad)
2.086 1.636 78.4 0.312 0.138
Forastero (Trinidad)
2.282 1.852 81.1 0.367 0.063
Forastero (West African)
2.280 1.720 75.4 0.530 0.030
Source : Knight 1999
3. Cocoa Polyphenol
Polyphenolic compounds comprise approximately 2% of fresh unfermented cocoa beans (Theobroma cacao) (Porter et al.1991 cited by Shahidi 2004). Epicatechin, catechin, epigallocatechin and epicatechin-based procyanidins have been identified as the predominant phenolics in the flesh of fresh unfermented cocoa seeds (Porter et al.1991;Thompson et al.1972;Villeneuve et al. 1989 cited by Shahidi 2004). The following have been identified as the major flavonoids in fresh cocoa beans: (a) Epicatechin-(4ß8)-catechin (procyanidin B-1), (b) Epicatechin-(4ß8)-epicatechin (procyanidin B-2), (c) [Epicatechin-(4ß8)]2 -epicatechin (procyanidin C-1), (d) Epicatechin-(2ß5,4ß6)--epicatechin, (e) 3T-θ
-ß-D-galactopyranosyl-ent-epicatechin-(2α7,4α8)-epicatechin, (f) 3T-θ -ß-D-arabinopyranosyl-(2α7,4α8)-epicatechin (Porter et al. 1991 cited by Shahidi 2004).
Polyphenolic compounds comprise 12 to 18% of the weight of whole dry bean and are implicated in the formation of the characteristic flavor and color of fermented cocoa (Bracco et al.1969 cited by Knight 1999). Approximately 35% of polyphenol content of unfermented Forastero cocoa beans is (-)-epicatechin (Forsyth, 1955, 1963 cited by Shahidi 2004).
Shahidi 2004).
According to Bonvehi and Coll (1998), the total content of low molecular weight phenolics in cocoa powder, such as phenol, 2-methoxyphenol, 3-methylphenol, 4-methylphenol, 2,3-dimethylphenol, 3-ethylphenol, 4-ethylphenol, 3,4-dimethylphenol and 3,5 3,4-dimethylphenol, should not exceed 9.6mg/kg. Increased levels of these phenolic indicate contamination with smoke and contribute to the development of smoky taste in cocoa powder, Poor drying and storage conditions are responsible for the contamination of cocoa powder with smoke. A sensorially acceptable cocoa powder should contain no more, per kilogram of sample, than 2 mg of phenol, 0.9 mg of 3-methylphenol, 0.55 mg of 2,3-dimethylphenol, 0.9 mg of 3-ethylphenol, and 0.7 mg of 4-ethylphenol (Shahidi 2004).
Commercially, the degree of bean fermentation is determined by cutting 100 beans and recording the color of their cotyledons. Presence of brown color indicates that beans are fully fermented. During fermentation and subsequent drying, flavor precursor responsible for the development of the characteristic flavor of cocoa are formed (Rohan 1963,1964,1967; Rohan and Connell 1964; Rohan and Stewart 1967 cited by Shahidi 2004). These precursors include flavonoids (catechin, epicatechin and gallocatechin), amino acids and sugars; however, unfermented cocoa beans do not contain these aroma precursors (Knapp 1937 cited by Shahidi 2004). Recently, Luna et al.(2002) demonstrated the existence of a positive correlation between polyphenol levels and bitterness and astringency of cocoa liquors and also showed that polyphenol are essential contributors to the overall sensory characteristic of Ecuadorian cocoa liquors (Shahidi 2004).
Table 6 Phytochemical and polyphenolics of cocoa bean and cocoa products
Compound Dry Seeds
(g/100g)
After roasting & conching
(g/100g)
In Milk chocolate (mg/100g) Flavanols Catechins (+)-Catechin (-)-Epicatechin (+)-Gallocatechin (+)-Epigallocatechin Leucocyanidins L1-L4 Polymeric Leucocyanidins Anthocyanins
3-α-L-arabinosyl cyanidins 3-ß-D-galactosidyl cyanidins
3.0 1.60-2.75
0.25-0.45
2.7 2.1–5.4
0.3 0.1 0.03-0.08 0.3-0.5 L1:0.08-0.17 0.01 0.02
Total Phenolics 13.5
11
During the fermentation process, anthocyanins are hydrolysed to anthocyanidins that polymerise along with simple catechins to form complex tannins (Forsyth 1963 cited by Shahidi 2004). Anthocyanins usually disappear rapidly during the fermentation process (Forsyth 1952 cited by Shahidi). After 4 days of fermentation, the content of anthocyanins decreases by up to 7% of initial value. The enzymatic oxidation of (-) epicatechins and leucocyanidins results in the formation of a brown color characteristic of chocolate caused by production of melanin and melanoprotein (Griffiths 1957 cited by Shahidi 2004). Under fermented beans are purple or slaty due to the presence of anthocyanin pigments, whereas fully fermented beans are quite brown. Thus, the content of anthocyanins may be considered a good index for determining the degree of cocoa bean fermentation. Further reduction in anthocyanins content is brought about by the drying process. Depending on the length of fermentation time, drying reduces the content of anthocyanins of beans by 13 to 44%. However, although drying unfermented beans results in loss of 79% of their anthocyanin, they still contain seven times more anthocyanin pigments than those of fermented products. A 97% loss of phenolic results from 4-day fermentation of beans followed by drying (Pettipher 1986 cited by Shahidi 2004).
The fermentation and drying processes alter the content and composition of phenolic compounds. Fermented cocoa beans contain a lower amount of low molecular weight phenolics and an enhanced content of condensed phenolics. The latter compounds are involved in protein-phenol interactions and may contribute to the low digestibility and poor biological value of cocoa proteins (Chatt 1953 cited by Shahidi 2004). Formation of the protein-phenol complex also reduces the bitterness and astringency associated with the presence of polyphenolics and reduces the unpleasant flavors and odors in roasted beans (Griffiths 1957 cited by Shahidi 2004). On the other hand, the low molecular weight polyphenols still present in chocolate may be responsible for its astringent and bitter taste (Shahidi 2004).
4. Anthocyanin
2004). In general, the anthocyanin content in most fruits and vegetables varies between 0.1 and 1% of dry matter content (Swain and Bate-Smith 1962 cited by Kidmose 2002).
Anthocyanins are composed of an aglycone (anthocyanidin), sugar, and sometime phenolic and/or minor organic acids (Kidmose 2002). Most anthocyanins occur as monoglycosides and diglycosides of perlargonidin, cyaniding, peonidin, delphinidin, petunidin, and malvidin. Meanwhile, anthocyanins assume different colors when subjected to pH variation in solutions. In addition, catechins and epicatechins, found in different plants and in high amounts in green tea leaves, are similar in their structures to anthocyanidins; however, they are colorless. The visual detection thresholds for selected anthocyanins are shown in Table 7 (Shahidi 2004).
Table 7 Visual detection thresholds (VDT) for selected anthocyanins.
Anthocyanin VDT (mg/L)
Cyanidin 3-glucoside
Cyanidin 3-xylosyl-galactoside
Cyanidin 3-xylosyl-glucosyl-galactoside
Cyanidin 3-sinapoyl-xylosyl-glucosyl-galactoside Cyanidin 3-feruoyl-xylosyl-glucosyl-galactoside Cyanidin 3-sophoroside-5-glucoside
Cyanidin 3-coumaroyl-sinapoyl-sophoroside-5-glucoside
1.3 0.9 2.4 0.9 0.4 3.6 2.0 Source : Adapted from Stintzing, F.C. et al. 2002, J. Agric. Food Chem., 50:6172-6182
cited by Shahidi 2004
The intensity of color, however depends also on the pH, presence of metal ions, self-association of anthocyanins (Hoshino et al.1980; Mazza and Miniati 1993; Figuerido et al. 1996; Wrolsdat 2000 cited by Shahidi 2004), pigment mixtures and co pigments such as others colorless phenolic compounds (Ohta et al. 1980; Brouillard 1983; Dangles et al. 1993; Mazza and Miniati 1993 cited by Shahidi 2004), as well as processing and storage conditions (temperature,sugar content, presence of ascorbic acid and presence of oxygen, amongst others) (Markakis 1974; Debicki-Pospisil et al.1982; Francis 1989; Dao et al.1998; Delgado-Vargas et al.,2000 cited by Shahidi 2004). Depending on the conditions, ascorbic acid may have positive or detrimental effects on anthocyanin stability (Sarma et al. 1997 cited by Shahidi 2004).
13
base, flavylium cation or oxonium salt, the colorless pseudobase and chalcone. The flavylium cation is weak acid, and a neutral quinonoidal base behaves as a weak acid and a weak base (Brouillard 1983; Mazza and Miniati 1993 cited by Shahidi 2004). Due to the equilibrium between flavylium cation and colorless carbinol structures, most intense red coloration of anthocyanins occurs in the pH range of 1 to 3 (Shahidi 2004).
In slightly acidic solutions (pH 4 to 6.5), anthocyanins undergo color fading. In this range of pH, anthocyanins rapidly transform to red or blue quinonoidal bases as a result of rapid proton loss of any hydroxyl groups at C-4`,C-5`or C-7`. Following this, nucleophilic addition of water to anthocyanins at C-2` or C-4` results in the formation of colorless carbinol structures and this equilibrates to the open, colorless chalcone forms. The reaction involves transferring the proton as well as breaking or forming the C-O bond (Bridle 1967; Brouillard 1982,1988; Mazza and Miniati 1993; Brouillard and Dangles 1994;; Dao et al. 1998; Timberlake and Wrolsdat 2000 cited by Shahidi 2004). Anthocyanins also show a minimum coloration at their isoelectricpoint (Markakis 1960 cited by Shahidi 2004). Shifting the pH of anthocyanins to higher values may even bring about complete loss of color. Thus, alkaline conditions, in particular at high temperature, should be avoided in the processing of anthocyanin-containing foods (Brouillard 1982 cited by Shahidi 2004).
Stability of anthocyanins during processing depends on the composition of food (anthocyanins, enzymatic systems, etc.) as well as temperature, pH, level of sugar, light, organic chemicals (ascorbic acid, tartaric acid, etc) and contaminations with metal ions (Markakis 1982; Macheix et al. 1989; Francis 1989; Wong 1989; Wrolstad et al. 1990; Gil et al. 1997; de Ancos et al. 2000; Wrolstad, 2000; Zabetakis et al. 2000 cited by Shahidi 2004).
C. Iron as fortificant
1. Iron type
Two iron forms that are commonly used in food fortification are ferrous (Fe2+) and ferric (Fe3+). Because both of these species contain unfilled d orbitals, they readily form complexes with electron-rich components yielding species that influence taste and bioavailability. Also, iron has the ability to undergo oxidation-reduction (redox) reactions that cause many of unwanted outcomes related to taste, appearance, and bioavability (Mehansho 2006).
2. Iron bioavailability and solubility
Currently, there are numbers of iron sources available as food fortificants. Based on bioavailability, these iron fortificants are classified into 2 groups. The highly bioavailable iron sources (e.g., ferrous sulphate and ferrous fumarate), which are soluble in neutral and/or acidic aqueous environments but may cause organoleptic changes such as poor product acceptability and shortened product shelf life, and those with poor bioavailability (e.g., iron pyrophosphate and reduced iron), which are less soluble in water but are more compatible with the foods used as vehicle (Mehansho 2006).
Table 8 Relative biological value and solubility of various irons Iron compound Relative Biological Value
(RBV)
Solubility (g/100ml water)
Ferrous Sulphate 100 * 25.6 **
Ferrous Fumarate 95 * 0.63 **
Ferric Pyrophosphate 45 * Insoluble
Source : *) Lynch and Hurrell 1990 **) Wikipedia 2009
From Table 8, eventough ferrous sulphate has excellent bioavailability but this iron has soluble in aqueous solution (water) and may affect the taste and appearance of the finally consumed product. An iron characteristic with acceptable bioavailability but less soluble (in milk solution), that is ideal for particular fortification, seems to be offered by ferrous fumarate. Nevertheless Ferric pyrophosphate which has the least solubility is worth to be evaluated in respect to the effect of taste and appearance of the finally consumed product.
3. Iron price indication
15
research is Iron (Ferrous) Sulphate, with price index 100 ; Iron (Ferrous) Fumarate, with price index 167 ; Iron (Ferric) Pyrophosphate, with price index 133.
From above price index comparison, it shows that Iron sulphate is the most economical iron fortificant. However the iron dosage in chocolate filled milk powder should not more than 0.1% (in dry matter), the cost in use of iron in final product, amongst above iron type, might not be significant different.
D. Ingredient interaction between iron and some ingredients in chocolate filled
milk powder
1. Interaction Iron with Fat
Fat in filled chocolate milk powder is majority come from palm olein, so it may contains 30 – 35% (of total fat) monounsaturated, 8% (of total fat) polyunsaturated fat. This unsaturated will react with oxygen to produce oxidative rancidity either spontaneously on exposure to air (auto oxidation) or in the presence of oxygen, light and a sensitizer (photosensitized oxidation). The transation metal, especially iron and copper, are important prooxidants in foods of both animal and plant origin (Bowers 1992). Therefore the presence of iron will act as catalyst in oxidative rancidity.
If iron was added dry during milk powder processing, the impact of iron as catalyst in this fat deterioration during processing was expected much lower. Milk powder has water activity (AW) maximum 0.2, where any reaction was expected at low speed, therefore it is also expected that the impact of iron fortification in fat deterioration is low during shelf life.
The impact of iron fortification in fat deterioration, so called rancidity, could be evaluated through sensory evaluation.
2. Interaction Iron with vitamin C
Crystalline ascorbic acid (vitamin C) is relatively stable in dry air but is unstable in the presence of moisture. It is readily oxidized in aqueous solutions, first forming dehydro-L-ascorbic acid which is then further and rapidly oxidized (Ottaway 1993).
Traces of heavy-metal ions act as catalysts to the degradation of ascorbic acid. Studies on the stability of pharmaceutical solutions of ascorbic acid showed that the order of the effectiveness of the metallic ions was Cu2+>Fe2+>Zn2+ (De Ritter 1982 cited by Ottaway 1993).
activity (AW) maximum 0.2, this impact is also expected lower during shelf life and that impact could be verified by means of vitamin c analysis.
3. Interaction Iron with Anthocyanin in Cocoa
Refer to Table 6, the anthocyanin in cocoa after roasting and conching is 0.01 g/100g. If chocolate filled milk powder contain 4.5% cocoa powder (dry matter), per 100g chocolate filled milk powder should contain 4.5% x 0.01g/100g anthocyanin (0.45mg anthocyanin). With serving size 27 g to prepare 180 mL of reconstituted chocolate filled milk, this milk solution should contain 0.12 mg anthocyanin or contain anthocyanin with concentration 0.675 mg/L.
A reconstituted chocolate filled milk powder might have pH in the range of 6.5 – 6.9, where at this range of pH, anthocyanin will undergo color fading. Irons that are soluble in neutral and/or acidic aqueous environments will form metal ion (Fe2+), as a result intensity of color, given by anthocyanin, will be increasing.
Refer to Table 8 above, since ferrous sulphate is the most soluble iron amongst others, so it could be predicted that this type of iron will give the most intensity increment of color given by anthocyanin.
III. MATERIAL AND METHOD
A. Timing and Location
1. Timing
Research was started in August - October 2009. 2. Location
Sample preparation, keeping quality (KQ), sensory evaluation, physical analysis, chemical analysis and microbiology analysis was done in application group and laboratory facility at PT. Nestle Indonesia – Kejayan Factory.
B. Materials and Tools
1. Materials and tools for sample preparation
Raw material:
All raw materials explained below were intended to be used in products made by dry mixing without any further heat processing, therefore it should have microbiology requirement as follows ; aerobic mesophilic microorganism max. 3000/g, salmonella negative in 25g, enterobactericeae negative in 1g.
Raw materials used were: a. Sugar
Beet or cane sugar (sucrose, saccharose) having chemical formula C12H22O11, molecular weight 342.3. It is dry crystalline powder, not milled, and not agglomerated. It has color white to slightly yellow or colorless crystals, and must not be blueish. It also has odourless and taste sweet. It should have at least physical and chemical properties as follows; purity polarisation min 99.7°Z (International Sugar Degrees), loss on drying: 0.06 g/100 g (105C, 3hr), invert Sugar: 0.04 g/100 g, color: 100 ICUMSA and particle size : 200 – 700 micron.
b. Filled milk powder
A milk powder which fat is composed of majority not milk fat. It is dried in a spray drier to achieve target moisture content, it is white to yellow color, has milk odour. It should have at least physical and chemical properties; moisture content: Max. 2.4%, AW (water activity) : Max. 0.2, fat total: 26.0% and protein: 25%.
c. Cocoa Powder
of cocoa and absence of any off-aromas. It should have physical and chemical properties; shell: max 1.75% (in alkali free nib), moisture content: max. 5%, pH range: 7.0 – 7.4, fat content: 10 – 12% and particle size: max 75 micron.
d. Calcium Carbonate micronised.
Chemical formula CaCO3, molecular weight 100.09, it is fine microcrystalline powder (micronised), has color of white or colorless. It should have physical and chemical properties; assay: 98.0 – 100.0% as CaCO3, on dry matter, loss on drying: Max 2% (200C, 4 hour), particle size: Average 3 micron and residues (individual oversize fraction): 2.5%, retained on 10 micron.
e. Vitamin premix
A free flowing homogeneous mix of vitamins prepared in carrier. It should have physical and chemical properties; moisture content: 4.5%, vitamin A (retinol): 1,060,000 IUA/100g, vitamin C (ascorbic acid): 45,100 mg/100g, calcium phosphate (anti caking agent) : 0.5% and maltodextrin (as carrier): up to 100%.
f. Iron (Ferrous) Sulphate
Chemical formula FeSO4 H2O, molecular weight 169.9, CAS Nr. 17375-41-6, it appears grey to white powder. Ferrous sulphate monohydrate has an iron content of 33%. It is sourced from Magnesia GmbH, under article code 78203, batch nr. 290372
g. Iron (Ferrous) Fumarate
It has chemical formula C4H2FeO4, molecular weight 170, it is the iron (II) salt of fumaric acid, occurring as a reddish-orange powder. Pure ferrous fumarate has an iron content of 32.87%. It is sourced from Magnesia GmbH, under article code 77717, batch nr. 771707
h. Iron (Ferric) Pyrophosphate
Chemical formula Fe4(P2O7)3.8H2O, molecular weight 745.2 (anhydrous), CAS nr. 10058-44-3, it is fine powder, amorphous and it has color white to slightly yellowish powder. It has loss on ignition: approx. 30g/100g, Iron: 20 – 22g/100g. It is sourced from BK Giulini GmbH, under product code : 76759.
Tools :
19
required were; white PE Plastic bag, weighing Scale (Mettler Toledo PE3600), stainless steel spoons, pilot scale-batch dry mixer with capacity approx 15 liter. For filling and packing preparation, the tools required were; aluminum foils sachet, vacuum pack sealer, sanitised glove and masker.
2. Materials and tools for sample analysis
For sensory evaluation, the materials required was water at temp 15°C, 45°C, and 80°C and tools required were beaker glass 200mL, graduated cylinder 250mL, thermometer, stainless steel spoon, object glass and tasting cup.
For chemical test, the materials required were reagent for Karl Fischer analysis, distilled water, ultra filtered water (18.2 MΩ), hydrochloric acid solution, approx. 3.7 % (v/v), metaphosphoric acid solution 2 g/100 mL, acetic acid solution 10% (v/v) and 2,6-dichlorophenol-indophenol (DCPIP) solution, about 0.5 mg/mL. And the tools required were Rotronic Hygrolab Instrument, Titrator Karl Fischer, Metrohm, KF Titrino 701, pH-meter Metrohm 691 or equivalent (0,01 pH), Atomic absorption spectrometer with Iron hollow cathode lamp, Beaker glass 100mL, magnetic stirrer, pipette and Metrohm Titrino.
For microbiology test, the materials required were Sugar free agar as media, hydrogen peroxide solution 3% (v/v) as reagent and tools required were Petri-dish, Incubation facility.
C. Research Methodology
1. Methodology
This research was started by preparing bulk prototype of chocolate filled milk powder based on three variables, which were type of iron, fortification level of iron and temperature of water for reconstitution. These variables were important parameter which determine the taste and appearance of the finally consumed product, serving suggestions (cold, warm or hot), as well as the claim of iron fortification which relate to level of iron.
Types of iron that use in this research were Iron Sulphate (FeSO4 H2O), Iron Fumarate (C4H2FeO4), and Iron Pyrophosphate (Fe4(P2O7)3.8H2O). These irons cover low and high bioavailability as well as low and high solubility.
Iron” and “Hi-Iron” respectively. Based on local regulation so called “Acuan Label
Gizi Produk Pangan” published by Direktorat Standardisasi Produk Pangan -
Badan POM RI (2007), for consumer group under 2000 kcal energy daily intake, the daily value for iron is 26 mg, therefore to deliver 10% DV, this product should contain Iron 10% of 26 mg per serving; 2.6 mg iron per serving. So the iron level in the finished product would be 2.6 mg per serving to fulfill 10% DV and 5.2 mg per serving to fulfill 20% DV.
The temperature of water for reconstitution was proposed to adapt the consumer consumption behaviour, 15oC for cold consumption, 45oC for warm consumption and 80oC for hot consumption.
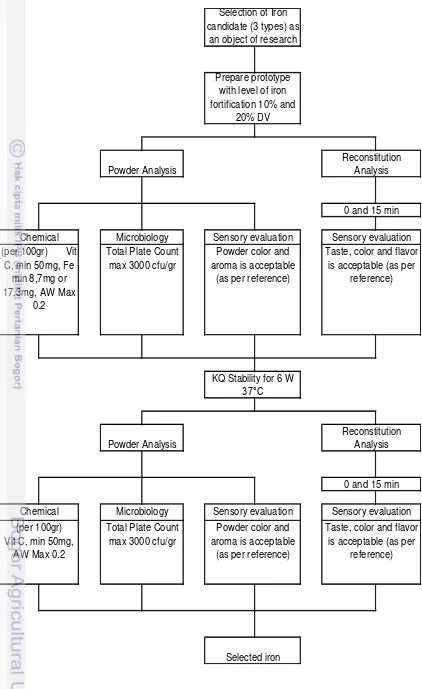
The selection of appropriate iron type at certain level of fortification in protototype chocolate filled milk powder that was reconstituted at different temperature was based on sensory evaluation, chemical analysis and keeping quality evaluation. Only the appropriate iron type would undergo keeping quality evaluation.
The sensory evaluation was designed to test product in both powder and reconstituted form. The sensory evaluation was basically used as main basis to determine appropriate iron type. In powder form, it would be evaluated aroma and color. In reconstituted form, it would be evaluated taste and color at cold, warm and hot water reconstitution, all were observed at 0 and 15 min after reconstitution.
The chemical and microbiological analysis would ensure other factor affecting product stability such as AW, TPC is monitored. The chemical analysis that designed to test the product were as follows : vitamin C content, to observe the impact of vitamin C degradation (if any), which would be used as one of the basis to determine appropriate iron type, water activity and moisture Karl Fischer, to observe the shelf stability, fe content by Atomic Absorption Spectrometry (AAS), to verify the designated iron level, pH, to observe pH of reconstituted chocolate milk at certain water temperature.
The objective of keeping quality was to see the stability of the prototype undergone NRT storage condition for at least 6 months periods of time, where it was represent the normal product age sold in the store.
Chocolate filled milk powder without iron fortification was provided as a reference (control) for sensory evaluation, as well as to verify other factor affecting the keeping quality, i.e. packaging performance, residual oxygen content.
21
(per 100gr) Vit C, min 50mg,
AW Max 0.2
Total Plate Count max 3000 cfu/gr
Powder color and aroma is acceptable
(as per reference)
Selected iron
Sensory evaluation Taste, color and flavor
is acceptable (as per reference) Powder Analysis
Reconstitution Analysis
0 and 15 min Prepare prototype
with level of iron fortification 10% and
20% DV
Chemical
Analysis Taste, color and flavor
is acceptable (as per reference) Sensory evaluation
Reconstitution Analysis
Powder color and aroma is acceptable
(as per reference)
0 and 15 min Selection of Iron
candidate (3 types) as an object of research
Chemical Analysis Microbiology Analysis Sensory evaluation Powder Analysis Microbiology Analysis Sensory evaluation (per 100gr) Vit
C, min 50mg, Fe min 8,7mg or 17,3mg, AW Max
0.2
Total Plate Count max 3000 cfu/gr
[image:36.595.100.522.64.753.2]KQ Stability for 6 W 37°C
The research plan flow chart is shown in Figure 1, which composed of prototype preparation, selection of iron and stability test of iron fortified product.
2. Working procedure
a. Bulk preparation procedure of chocolate filled milk powder
Bulk chocolate filled milk powder which was made fix for all kind of tested iron, composed of filled milk powder, sucrose, cocoa 10-12% fat, CaCO3, vitamin A & C and Iron 0.02-0.08%, to reach min. 2.6mg (10%DV) & min. 5.2mg (20%DV) per serving. Reference bulk chocolate filled milk powder had the same composition as above, but without iron fortification.
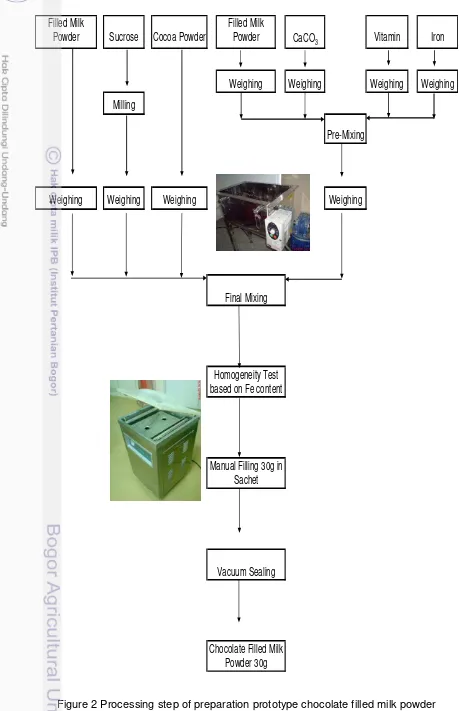
There were six main processing steps to produce prototype of chocolate filled milk powder, which was shown at Figure 2.
Sugar milling
Generally sugar has the highest particle size (200 - 700 micron) than other major ingredient. To improve mixing homogeneity and avoid materials separation, it is necessary to mill sucrose prior to mixing. Sucrose milling was performed in sugar milling available in the factory.
Weighing each ingredient
Each ingredient was weighed to nearest one digit for major materials; filled milk powder, sucrose, cocoa, and to the nearest two digit for minor ingredient; Calcium Carbonate Micronised, Vitamin premix, Iron Sulphate/Fumarate/Pyrophosphate. Preweighed ingredient is labelled with identification of ingredient name, weight, lot nr and when is prepared.
Pre-mixing for minor ingredient
Minor ingredient like Calcium Carbonate Micronised, Vitamin premix, and Iron Sulphate/Fumarate/Pyrophosphate will tend to be hardly homogenous because they are small in percentage (normally < 1%). Pre-mixing would help to improve the homogeneity of these small ingredients when they finally mix with other major ingredient.
Mixing of all ingredient
23
minutes, then discharged it.
Based on operational experience in Kejayan factory, the batch dry mixer has optimal mixing time at 6 - 7 minutes, a homogeneity test must be performed to ensure homogeneous result.
Homogeneity test
To perform homogeneity test, 10 samples must be taken from different point of area in mixing chamber. These samples must undergo chemical analysis (duplo analysis) i.e. Vit C content, then a homogeneity calculation should be made.
Refer to MI.18.348-1, an internal guideline on how to perform dry mixing, made by Nestle, the homogeneity parameter is Coefficient of Variation (CV). If CV is below 1%, the product is considered homogeneous and if the CV is 1 - 2% the product is considered marginally homogeneous, which is also acceptable. The formula is expressed as follows:
CV = Std. Dev. for chemical value (i.e. Fe content) of samples Average for chemical value (ie. Fe content) of samples
A nutritional fact of above product per serving (30g) this could be expected as follows; macronutrient: total fat 4.2 g, protein 4.3 g, Sugar 12.3 g and micronutrient: Vitamin A 162 mg RE, Vitamin C 15.0 mg, Calcium 225 mg and Iron 2.6 or 5.2 mg
b. Filling/Packing procedure
Bulk chocolate filled milk powder must undergo these below test before filing and packing activity was started: AW test (maximum 0.2), iron content (target 8.67 mg/100g or 17.33 mg/100g) and sensory evaluation. The bulk was manually filled into aluminium foil sachet, with net weight 30g, then it sealed under vacuum to minimise residual oxygen content.
D. Method of Analysis
1. Accelerated keeping quality procedure
Through linear regression of KQ data taken from Attachment 1, at temperature NRT and 37°C, it could be established an order 1 equation, as follows :
Filled Milk
Powder Sucrose Cocoa Powder
Filled Milk
Powder CaCO3 Vitamin Iron
Weighing Weighing Weighing Weighing
Milling
Pre-Mixing
Weighing Weighing Weighing Weighing
Chocolate Filled Milk Powder 30g Final Mixing
Homogeneity Test based on Fe content
Manual Filling 30g in Sachet
[image:39.595.64.523.57.768.2]Vacuum Sealing
25
Since the KQ data of taste and appearance was almost the same (see Attachment 1), therefore above equation could be considered valid for both taste and appearance prediction.
From Attachment 3, it showed the correlation between keeping quality at Normal Room Temperature (NRT) and at 37oC, there was a correlation between result of keeping quality 37oC and NRT that was every 1 week at 37oC similar to 1 month at NRT. However, the condition was only valid until 6 weeks at 37°C that was similar to 6 months at NRT.
Refer to technical instructions GI-31.355, May 2006 established by Nestle with title Best Practices for Sensory Evaluation and shelf life assesment, the accelerated keeping quality procedure was developed. The procedure was based on empirical data of keeping quality of similar product produced in PT. Nestle Indonesia, Kejayan factory and considering the average storage time of this similar product sold in the market, which has normally 6 months. It was proposed to perform keeping quality evaluation as follows 1, 3, 6 weeks at 37oC and 1 month at NRT. Both KQ at temperature NRT and 37oC were performed under special room, with temperature control. Each KQ period, the same sensory and chemical test must be performed as it was performed in fresh sample evaluation.
2. Sensory evaluation procedure
Refer to technical instructions GI-31.107-1, September 2007, established by Nestle with title The “In/Out” test method for sensory quality control, a sensory evaluation procedure applicable for this research is made.
General rule to performed sensory evaluation : performed by trained panellist, minimum panellist 6 person, using descriptive In/Out test, reference sample to define in and out must be available, acceptable sensory if “In” above 80%. Reference sample is chocolate filled milk powder composed as describe in “bulk
preparation procedure”, page 27 but without any Iron fortification.
Sample preparation : 30 g samples were weighed or pre-weighed (30g) sachet open and pour in beaker glass, then 150 mL (boiled water) was prepared in beaker glass, with temperature as follows : 15oC for cold reconstitution, 45oC for warm reconstitution and 80oC for hot reconstitution.
Test after reconstitution, pre-weighed 30 g samples was poured in to prepared water (150mL). For color evaluation at fresh and after 15 minutes of reconstitution, the color was evaluated, against reference. And for taste evaluation at fresh and after 15 minutes of reconstitution, the taste was evaluated (milky taste, chocolate taste, metallic taste, rancid taste) against reference. Sensory evaluation evaluation sheet could be seen in attachment 4
Chemical analysis procedure
a. Water activity (AW)
Refer to technical instructions LI-00.015-1, November 2007, established by Nestle with title Water activity using hygrolab from rotronic, an aw test procedure applicable for this research was made.
The measurement procedure: the sample cup is filled with approximately three quarters of their volume with sample. Care should be taken not to overfill the cups, as this may contaminate the sensing element and the sample compartment of the instrument (measuring chamber). On the other hand, too small an amount of sample in a cup means a large headspace which also can affect the results. The cup is placed inside the measuring cell, as rapidly as possible and the measurement is started. The equilibration time with the sample in place must be allowed before recording aW value (Approximately 30 minutes). The temperature value displayed by the instrument is recorded. The awof all samples at 25 ±1 °C is measured, in duplicate and note the readings individually. Then, the recorded aW value must be corrected by using the calibration value. The picture of AW measurement device is shown in Figure 4.
b. Value of pH at fresh and 15 minutes after reconstitution
Refer to technical instructions LI-00.222-2, August 2000, established by Nestle with title General pH and Acidity, a pH test procedure applicable for this research was made.
Sample preparation : the test portion is weighed in a 150 ml beaker. Recently boiled distilled water is added and cooled to 40-50 °C then a 10 % solution (10 g sample + 90 ml water) is prepared. Stir until complete dissolution and cool it to room temperature.
27
Figure 3 Water activity meter
c. Moisture Karl Fischer
Refer to technical instructions LI-08.055-1, March 1999, established by Nestle with title Moisture determination according to Karl Fischer, a moisture KF test procedure for this research was applied. The method is based on the ability of iodine to react with water in the presence of sulfur dioxide (sulphurous anhydride).
Sample is prepared by weighing about 0.5 g sample, to the nearest 0.0001 g into weighing boat, sample is introduced rapidly into the titration vessel and the empty boat is weighed. The difference in mass is the test portion.
Measurement : «mode» is pressed, until «KFT» appears, about 40 ml methanol is introduced by aspiration, «start» is pressed to condition the installation, the product to be analysed is rapidly introduce and the titration vessel is closed. The required data is entered in order to start the titration, the concentration result is displayed in percentage and printed. After each determination, the titration cell is emptied by aspiration and the installation is conditioned with clean solvent and the measurement is repeated. At the end of the second titration, the average of the two determinations is calculated. The apparatus for moisture KF is shown in Figure 4.
Calculation of moisture content, in g/100 g product = v.t / m.10 v = volume of KF reagent used, in millilitres
t = titer of KF reagent, in mg water/ml m = mass of the test portion, in grams 10 = conversion factor of mg/ml into g/100 g
d. Iron (Fe)
procedure applicable for this research was used. The principle of method is acid hydrolysis of food products with nitric acid (for Ca, Na, K and Mg) and hydrochloric acid (for Fe and Zn). Determination of sodium, potassium, calcium, magnesium, iron and zinc by flame atomic absorption spectroscopy.
Figure 4 Apparatus for Moisture Karl Fischer determination
Sample preparation: sample is grinded in a suitable sample mill if necessary and ensure that samples are thoroughly mixed before analysis.
Acid hydrolysis with hydrochloric acid (3.7% v/v) : into a 50 ml PP or glass volumetric flask, 1 g dry product is weighed or 5 – 10 g liquid product, to the nearest 1 mg. And then 5 ml concentrat hydrochloric acid is added carefully, mix well and allow standing for 15 minutes. Then 5 ml pure water is added and swirl gently, and it is heated for 30 minutes in rapidly boiling water bath. Sample is removed from water bath and allowed cooling to room temperature. With pure water, make up to volume and mixed well. Sample is filtered through an ashless filter paper, the first five ml of filtrate is discarded and the remaining filtrate is collected for analysis. This solution should be analysed on the day of preparation.
Calibration procedure : the atomic absorption system is auto-zero with the 0 calibration solution. Using the auto sampler system or manually, each calibration solution is aspirated starting with the lowest concentration. Three readings per solution are taken and the average absorbance of each solution is calculated. Using the instrument software, the calibration line is calculated by plotting the absorbance in the
y axis and the concentration of the element in μg/ml on the x axis. The linearity of the
29
Blank test :a blank test should be carried out in parallel using the same reagents and conditions as described under acid hydrolysis.
Measurement : using the auto sampler of the AAS, each product solution and blank solution is aspirated into the AAS. Three readings are taken per solution and the average of three readings is calculated. The absorbance measured is within the calibration range must be ensured. The concentration of the element to be measured should be near to the middle of the calibration range if possible. The concentration of the element to be determined in the analytical solution must not be higher than the highest calibration point. After a rinse solution (pure water) is aspirated between each analytical solution, the concentration of the element is calculated in each product solution (C) and blank (B) using the calibration curve and the data station of the AAS. The apparatus for Iron determination is shown in figure 5.
The concentration of iron is calculated in the product solution in mg/100 g using the following equation = [(C. D – B) V] / [M.10]
C = concentration of Fe in solution of test portion, in g/ml D = dilution factor, if required
B = concentration Fe in solution of blank, in g/ml V = volume of solution of test portion, in ml M = mass of product analysed, in g
10 = factor to convert g/g to mg/100g