MORPHOLOGICAL STUDY OF GOBY (GOBIIDAE) FROM
LOMBOK ISLAND
–
INDONESIA
YULIADI ZAMRONI
POST GARDUATE SCHOOL BOGOR AGRICULTURAL UNIVERSITY
STATEMENT OF THESIS
AND INFORMATION SOURCE
Hereby I express that the thesis entitled: Morphological study of goby
(Gobiidae) from Lombok Island – Indonesia is the original result of my research and has never been published. All data and information I provided in the thesis are
based on evidence and available references.
Bogor, March 2011
Yuliadi Zamroni
ABSTRAK
YULIADI ZAMRONI. Studi Morfologi Ikan Gobi (Gobiidae) dari Pulau Lombok - Indonesia. Dibimbing oleh BAMBANG SURYOBROTO dan MOHAMMAD MUKHLIS KAMAL.
Studi morfologi ikan gobi telah dilakukan berdasarkan 22 karakter morfometrik dan 8 karakter meristik. Ikan gobi dikoleksi dari enam lokasi di Pulau Lombok, tiga lokasi di sungai (Jangkuk, Sidutan, dan Belimbing) dan tiga lokasi di hutan mangrove (Selindungan, Teluk Sepi, dan Labuan Treng). Sampel dikoleksi dengan menggunakan electro-fishing (12 V, 10 A) untuk ikan gobi diperairan sungai dan campuran minyak cengkeh dan alkohol 50% (1 : 3 bagian) sebagai anastesi untuk ikan gobi diperairan mangrove. Dari hasil identifikasi, didapatkan 21 spesies ikan gobi. Analisis pengelompokan menunjukkan ikan gobi dapat dikelompokkan menjadi 7 kelompok yang merujuk pada level genus, antara lain Acentrogobius (kelompok 1), Amoya (kelompok 2), Periophthalmus
(kelompok 3), Stiphodon (kelompok 4), Sicyopterus (kelompok 5),
Boleophthalmus (kelompok 6), dan Oxyurichthys (kelompok 7). Dari 22 karakter morfometrik dan 8 karakter meristik, didapatkan 6 karakter morfometrik (panjang moncong sampai sirip ventral, panjang batang ekor, panjang dasar dari sirip dorsal ke-2, panjang dasar dari sirip anal, panjang sirip ventral, dan panjang postorbital) dan 2 karakter meristik (jumlah sisik lateral dan jumlah sisik predorsal) memberikan variasi yang tinggi pada data sehingga karakter ini sangat baik digunakan sebagai karakter taksonomi untuk mengelompokkan ikan gobi.
SUMMARY
YULIADI ZAMRONI. Morphological study of goby (Gobiidae) from Lombok Island – Indonesia. Supervised by BAMBANG SURYOBROTO, MOHAMMAD MUKHLIS KAMAL.
Gobies (family Gobiidae) are bottom-dwelling fishes with flattened head, rounded snout, and bulbous cheeks that having a characteristic of two soft-spined dorsal fins and united ventral fins (disc-like) adapted to bottom-living life style. These modified fins are sucker device which enable the fish to grasp on surfaces and prevent from drifting in fast water current. Gobies are one of fish group who dominate ocean islands because of amphidromous larvae stage, euryhalinity, small size, an excellent climbing ability, a wide range of trophic level, frequent lack of gas bladder, and an associated bottom-living life style.
Taxonomically, Gobiidae is one of the most poorly known family and consists of numerous undescribed species. There are many unanswered question about them, such as how many species there are and their phenetic relationship. Morphological study can be used to clustering and understanding relationship of taxa. Variation in morphological data can be used to characterize the distance between them. The distance makes each taxon uniquely diagnosable
Lombok is an ocean island and present study analyze samples of gobies that were collected from six locations in Lombok. They were three locations at rivers (Jangkuk, Sidutan and Belimbing) and three locations at mangrove forests (Selindungan, Sepi Bay, and Labuan Treng). Electric fishing gear (12 V, 10 A) was employed for about one hour per location in rivers, whereas in mangrove forest sampling was carried out using anaesthetic during low tide in pools and creeks. A mixture of one part clove oil and three parts of 50% alcohol was used as anaesthetic agent.
Morphometric and meristic data have been analyzed using R software version 2.12.1. The morphometric measurements were standardized on Standard Length (SL) to remove size effect. These morphological data were analyzed using Principal Components Analysis (PCA) to remove the correlation between variables.
Clustering of gobies was performed using K-means method to select a prespecified number of cluster centers to minimize the within-class sum of squares from those centers. This analysis was followed with elbow criterion method to estimate the best number of clusters. The criterion used the ratio of within-group to the total variances. The best number of clusters could be estimated when adding a group did not improve the explained variance, and the closeness between clusters were estimated using Minimum Spanning Tree (MST). Based on morphological identification result, 214 specimens of gobies from Lombok could be distinguished into 21 species in 10 genera and 4 subfamilies. In this study, 12 species were included in analysis and 9 species were excluded because consisting of only few samples. However, after clustering 12 species, 9 species which were excluded in analysis were plotted in principal component space to find their relation with other clusters.
cluster), Periophthalmus (3rd cluster), Stiphodon (4th cluster), Sicyopterus (5th cluster), Boleophthalmus (6th cluster), and Oxyurichthys (7th cluster). These specimens had six morphometric characters (snout to ventral fins length, caudal peduncle length, 2nd dorsal fin base length, anal fin base length, ventral fin length, and postorbital length) and two meristic characters (lateral scale rows and predorsal scale rows) gave higher variation and good as taxonomic characters to separate the taxa.
Copyright©2011 by Bogor Agricultural University
Copyright are protected by law,1. It is prohibited to cite all or part of this thesis/ dissertation without referring to and mentioning the source.
a. Citation only permitted for sake of education, research, scientific problem.
b. Citation doesn’t inflict the name and honor of Bogor Agricultural University.
MORPHOLOGICAL STUDY OF GOBY (GOBIIDAE) FROM
LOMBOK ISLAND
–
INDONESIA
YULIADI ZAMRONI
Thesis
As partial fulfillment of the requirements for Master Degree in Animal Biosciences
POST GARDUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
Title : Morphological Study of Goby (Gobiidae) from Lombok
Island- Indonesia
Name : Yuliadi Zamroni
Registration number : G352080011
Certified by:
Advisory committee
Dr. Bambang Suryobroto Dr. Ir. Mohammad Mukhlis Kamal, M.Sc
Chairman Member
Acknowledged by
Coordinator
Major of Animal Biosciences Dean of Postgraduate School
Dr. Bambang Suryobroto Dr. Ir. Dahrul Syah, M.Sc.Agr
O man! What has deluded you in respect of your Noble Lord?
He Who created you and formed you and proportioned you and assembled you
in whatever way He willed.
(Al-Qur’an, Surat al-Infitar: 6-8)
THIS THESIS IS DEDICATED TO
FOREWORD
On the blessing of God I am able to finish my thesis. This paper is made to fulfill the requirement for master degree at Bogor Agricultural University. The
title of my paper is “Morphological Study of Goby (Gobiidae) from Lombok
Island- Indonesia”.
I would like to thank my advisor committee Dr. Bambang Suryobroto and Dr. Mohammad Mukhlis Kamal. I am indebted to Dikti Depdiknas for the Postgraduate Scholarship (BPPS); Indonesian Institute of Science and Conservation International Indonesia for the sponsored my training in Indonesian Marine Taxonomy; and Raffles Museum for allowing me to study of gobies.
Special thank to Dr. Zeehan Jaafar (Nasional University of Singapore, Singapore) and Dr. Helen Larson (Museum and Art Gallery of The Northern Territory, Australia) for introduce to me how to identified of gobies, collecting methods, and all of gobies information. I express my gratitude to Novita Tri Artiningrum (my wife), Annisa Hishnul Izza (my daughter), Andy Darmawan (my friend), and Mrs Tini and Ani (laboratory assistant) for their support.
May this paper is usefull for all who study on gobies taxonomy.
Bogor, March 2011
CURRICULUM VITAE
The author was born in Mataram on July 10th, 1981 as the seventh son of
Masyhur Hamnur (†) and Saidah. The author got married with Novita Tri
Artiningrum and have one daughter (Annisa Hishnul Izza).
CONTENTS
Page
LIST OF TABLE ... xiii
LIST OF FIGURE ... xiv
LIST OF APPENDIX... xvii
INTRODUCTION ... 1
LITERATURE STUDY Taxonomic Remarks of Gobies ... 3
Lombok Gobies ... 3
Morphological Analysis ... 4
MATERIALS AND METHODS Study Site ... 5
Sample Collection ... 5
Gobies Identification ... 6
Morphometric data ... 7
Meristic data ... 9
Data Analysis ... 10
CLUSTER ANALYSIS Clustering of Gobies Based on Morphometric Characters ... 11
Clustering of Gobies Based on Meristic Characters ... 14
IDENTIFICATION ... 17
DISCUSSION ... 42
CONCLUSION ... 45
LITERATURES CITED... 46
LIST OF TABLES
Page
1 Twenty one species in this study ... 7
2 List of morphometric characters of gobies used in this study ... 8
3 List of meristic characters of gobies used in this study ... 10
4 Percentage of variance explainable with adding number of clusters ... 11
5 Eigen value, proportion of variance, and cumulative of variance. ... 13
6 Eigen vector of each character on first two PCs. ... 14
7 Percentage of variance explainable with adding number of clusters. ... 14
8 Eigen value, proportion of variance, and cumulative of variance. ... 16
LIST OF FIGURES
Page
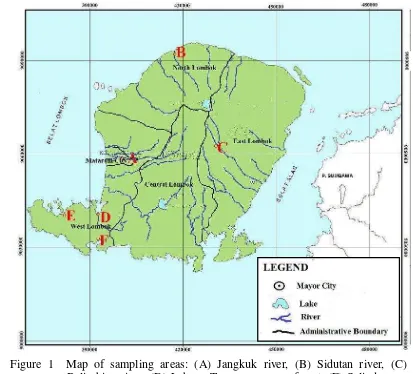
1 Map of sampling areas: (A) Jangkuk river, (B) Sidutan river, (C) Belimbing river, (D) Labuan Treng mangrove forest, (E) Selindungan mangrove forest,
and (F) Sepi Bay mangrove forest. ... 6
2 Morphometric characters of gobies. ... 7
3 Meristic characters (A) scale counts: LSR (red), TSR (yellow) and PSR (blue). (B) Fin-ray count... 9
4 Plotting of all speciments in two first PC indicate centroid of clusters and their minimum spanning tree ... 12
5 Plotting of all speciments in two first PC indicate centroid of clusters and their minimum spanning tree ... 15
6 Ventral view of pelvic fins (a) Without frenum; (b) Frenum without fleshy lobes; (c) Frenum with fleshy lobes ... 17
7 (a) Ventral fins with frenum and fleshy lobes; (b) eyes lateral and without eyelid ... 18
8 Head pores type (a) A pair and (b) A single of anterior interorbital pore(s)... 18
9 Dermal crest on top of head. ... 19
10 Tongue distinctly bilobed. ... 19
11 Acentrogobius audax, 43.33 mm in SL, Selindungan, Sekotong distric-western Lombok. ... 20
12 Acentrogobius janthinopterus, 49.87 mm in SL, Sepi Bay, Sekotong distric-western Lombok. ... 20
13 Acentrogobius viridipunctatus, 66.14 mm in SL, Selindungan, Sekotong distric-western Lombok. ... 21
14 Amoya gracilis, 42.35 mm in SL, Labuan Treng, Labuan distric-western Lombok. ... 22
15 Amoya moloana, 44.83 mm in SL, Selindungan, Sekotong distric-western Lombok. ... 23
17 Cristatogobius rubripectoralis, 49.80 mm in SL, Selindungan, Sekotong distric- western Lombok. ... 24
18 Glossogobius celebius, 47.00 mm in SL, Sidutan River, Kayangan distric- northern Lombok. ... 25
19 Dorsal view of head ... 26
20 Oxyurichthys tentacularis, 66.96 mm in SL, Labuan Treng, Labuan distric-western Lombok. ... 27
21 Oxyurichthys ophthalmonema, 41.51 mm in SL, Selindungan, Sekotong distric-western Lombok. ... 28
22 Pseudogobius javanicus, 27.75 mm in SL, Selindungan, Sekotong distric-western Lombok. ... 29
23 Ventral view of head showing (a) black branchiostegal membranes and (b) pale brachiostegal membrans... 30
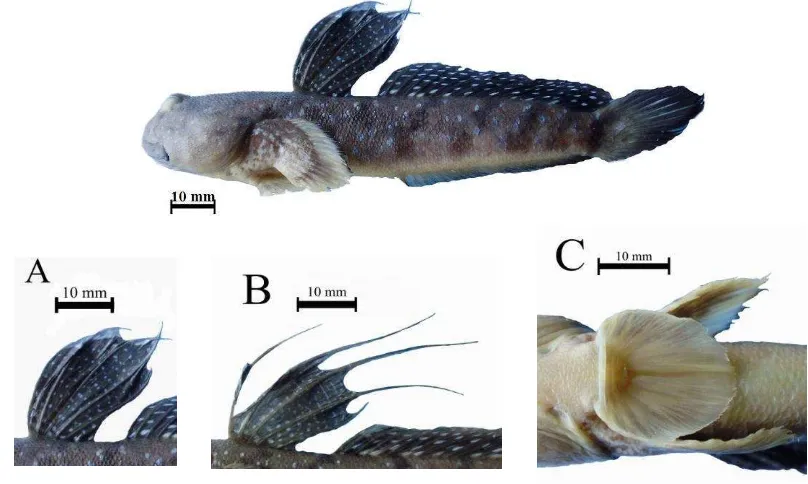
24 Boleophthalmus boddarti, 89.94 mm in SL, Labuan Treng, Labuan district-western Lombok. (A) Male 1st dorsal fin, (B) Female 1st dorsal fin, and (C) Ventral fins with frenum. ... 31
25 Periophthalmus argentilineatus, 37.85 mm in SL, Sepi Bay, Sekotong distric-western Lombok. (A) First and second dorsal fins and (B) Ventral fin without frenum. ... 32
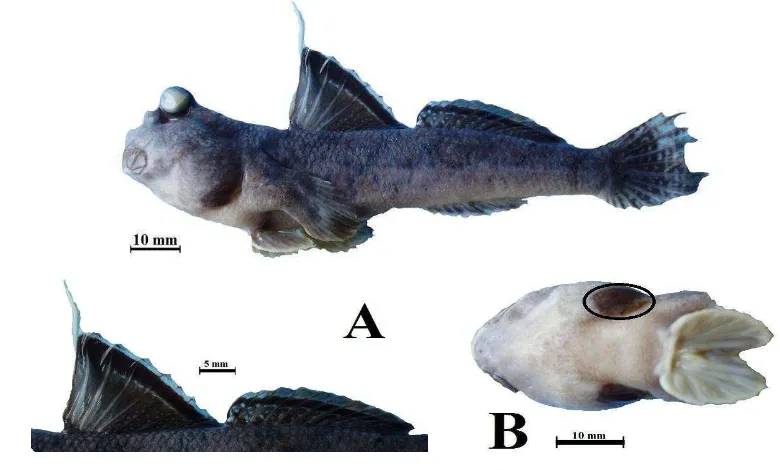
26 Periophthalmus gracilis, 33.45 mm in SL, Sepi Bay, Sekotong distric-western Lombok. (A) First and second dorsal fins and (B) Ventral fin without frenum. ... 33
27 Periophthalmus malaccensis, 69.28 mm in SL, Sepi Bay, Sekotong distric-western Lombok. (A) First and second dorsal fin, (B) Ventral view of head showing pale branchiostegal membranes and ventral fin with frenum. ... 34
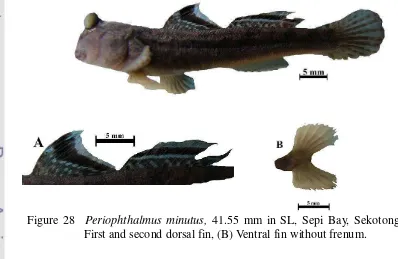
28 Periophthalmus minutus, 41.55 mm in SL, Sepi Bay, Sekotong. (A) First and second dorsal fin, (B) Ventral fin without frenum. ... 35
29 Periophthalmus novemradiatus, 88.46 mm in SL, Sepi Bay, Sekotong distric-western Lombok. (A) First and second dorsal fins, first sipine at D1 elongate, (B) Ventral view of head showing black branchiostegal membranes and ventral fins with frenum. ... 36
30 Pelvic disc (a) not adherent to belly in Stiphodon and (b) adheren in
Sicyopterus. ... 37
32 (A) Sicyopterus cyanocephalus, 65.33 mm in SL, Belimbing River, eastern Lombok, (B) Ventral view of head showing clefts in upper lip and frenum at ventral fins with fleshy lobes. ... 38
33 (A) Sicyopterus hageni, 51.35 mm in SL, Sidutan River, northern Lombok, (B) Ventral view of head showing clefts in upper lip and frenum at ventral fins with fleshy lobes. ... 39
34 (A) Sicyopterus microcephalus, 45.72 mm in SL, Jangkuk River, Mataram, (B) Ventral view of head showing clefts in upper lip and frenum at ventral fins with fleshy lobes. ... 40
LIST OF APPENDIXES
Page
1 Clustering of gobies based on morphomrtric data. ... 51
2 Clustering of gobies based on meristic data. ... 53
3 Morphometric and Meristic Data of Acentrogobius audax (n=5). ... 55
4 Morphometric and Meristic Data of Acentrogobius janthinopterus (n=20). ... 56
5 Morphometric and Meristic Data of Acentrogobius viridipunctatus (n=21). ... 57
6 Morphometric and Meristic Data of Amoya gracilis (n=13). ... 58
7 Morphometric and Meristic Data of Amoya moloana (n=11). ... 59
8 Morphometric and Meristic Data of Amoya raveni (n=12). ... 60
9 Morphometric and Meristic Data of Cristatogobius rubripectoralis (n=2). .... 61
10 Morphometric and Meristic Data of Glossogobius celebius (n=1). ... 62
11 Morphometric and Meristic Data of Oxyurichthys tentacularis (n=14). ... 63
12 Morphometric and Meristic Data of Oxyurichthys ophthalmonema (n=3). ... 64
13 Morphometric and Meristic Data of Pseudogobius javanicus (n=6). ... 65
14 Morphometric and Meristic Data of Boleophthalmus boddarti (n=12). ... 66
15 Morphometric and Meristic Data of Periophthalmus argentilineatus (n=14).. 67
16 Morphometric and Meristic Data of Periophthalmus gracilis (n=5). ... 68
17 Morphometric and Meristic Data of Periophthalmus malaccensis (n=1). ... 69
18 Morphometric and Meristic Data of Periophthalmus minutus (n=3). ... 70
19 Morphometric and Meristic Data of Periophthalmus novemradiatus (n=6). ... 71
20 Morphometric and Meristic Data of Sicyopterus cyanocephalus (n=12). ... 72
21 Morphometric and Meristic Data of Sicyopterus hageni (n=12). ... 73
22 Morphometric and Meristic Data of Sicyopterus microcephalus (n=25). ... 74
INTRODUCTION
Gobies (family Gobiidae) are bottom-dwelling fishes with flattened head,
rounded snout, and bulbous cheeks that having a characteristic of two soft-spined
dorsal fins and united ventral fins (disc-like) adapted to bottom-living life style.
These modified fins are sucker device which enable the fish to grasp on surfaces
and prevent from drifting in fast water current (Koumans 1931; Larson & Lim
2005). They have a wide spectrum habitats covering the sea-floor at a depth of
over 300m below surface to mountain streems. Adaptations to these habitats lead
to various morphological specializations. Gobies lives on rock bottoms have short
round ventral fins sucker, whereas gobies lives on sandy and unstable bottoms
have large ventral fins sucker (Akihito et al. 2000).
Gobies dominate ocean islands. This condition was inferred from
amphidromous larvae stage, euryhalinity, small size, an excellent climbing ability,
a wide range of trophic level, frequent lack of gas bladder, and an associated
bottom-living life style (Ryan 1991).
Gobies comprise of approximately 2000 species in over 200 genera (Nelson
2006). Larson et al. (2008) reported 134 species of gobies found in Singapore;
Kottelat & Whitten (1993) found 18 species of freshwater gobies from Sumatera,
17 species from Borneo (5 species were endemic), 19 species from Java (2 species
were endemic) and 22 species from Celebes (9 species were endemic); Haryono &
Tjakrawidjaja (2004) found 12 species of freshwater gobies in Northern Celebes.
New Guinea was reported to have 300 species of gobies, 50 species of them were
found in freshwater (Allen 1991), and 11 species of freshwater gobies were found
in Timika region (Allen et al. 2000).
Lombok is one of oceanic islands in Lesser Sunda archipelago, located in
the southern Wallacea. Geologically, it was formed from under sea mountain
(Monk et al. 2000) and never be a part of mainland in Sundaland or Sahulland (Cracraft 1988), so all fauna (including freshwater fishes) living in Lombok were
migrated through the sea.
The morphological adaptations give variation in morphological
characterize the phenetic distance between them. The distance may makes each
taxon uniquely identifiable. The focus of this research is observing and analysing
variations on morphological data in Lombok gobies.
The samples were collected from six locations in Lombok. They were three locations at rivers (Jangkuk, Sidutan and Belimbing) and three locations at mangrove forests (Selindungan, Sepi Bay, and Labuan Treng). Electric fishing gear (12 V, 10
A) was employed for about one hour per location in rivers, whereas in mangrove
forest sampling was carried out using anaesthetic agent during low tide in pools
and creeks. A mixture of one part clove oil and three parts of 50% alcohol was
used as anaesthetic.
These specimens could be classified based on morphometric variations into
seven clusters. They were Acentrogobius (1st cluster), Amoya (2nd cluster),
Periophthalmus argentilineatus (3rd cluster), Stiphodon elegans (4th cluster),
Sicyopterus (5th cluster), Boleophthalmus boddarti (6th cluster), and Oxyurichthys
tentacularis (7th cluster). These specimens had six morphometric characters (snout to ventral fins length, caudal peduncle length, 2nd dorsal fin base length, anal fin
base length, ventral fin length, and postorbital length) and two meristic characters
(lateral scale rows and predorsal scale rows) gave higher variation and good as
LITERATURE REVIEW
Taxonomic Remarks of Gobies
Taxonomy of Gobiidae are highly complex and volatile. Gobies were
identified by Linnaeus (1759) based on “Caput poris 2 inter oculos approximatos : altero anteriore, membr. branch. radiis IV, pinnae ventrales unitae in ovatam 12-radiatam.”. He grouped these fishes into division III Pisces Thoracici under genus Gobius. Seven species were included into this genus, they were G. anguillaris, G. aphya, G. eleotris, G. jozo, G. niger, G. paganellus, and G. pectinirostris. The earliest classification system of gobies interrelationship was proposed by Günther
(1861). He identified four groups of gobies, they were Amblyopina, Callionymina,
Gobiinae, and Trypauchenina
Regan (1911) identified these fishes based on osteology. This system
divided gobies into three families: Eleotridae, Gobiidae, and Psammichthyidae.
The Gobiidae were divided into two subfamilies, Gobiinae and Periophthalminae.
Major revision on family Gobiidae was carried out by Koumans (1931) who analyzed the family by the presence of united ventral fins. He divided the extant species into six subfamilies: Apocrypteinae, Gobiinae, Gobiodontinae, Periophthalminae, Sicydiaphiinae, and Tridentigerinae. Partial taxonomy revision of these fishes were studied by Murdy (1989) for Oxudercinae; Pezold (1993) for Gobiinae; Parenti & Maciolek (1993) and Keith et al (2010) for Sicydiinae.
In the latest holistic study of these families, Larson & Murdy (2001) identified approximately 500 species of 100 genera in the Western Central Pacific. They identivied five subfamilies: Amblyopinae, Gobiinae, Gobionellinae, Oxudercinae, and Sicydiinae.
Lombok Gobies
Geologically, Lombok is one of oceanic islands in the Wallacea region
which was formed from undersea mountains located in the middle of Indonesia,
between Sundaland and Sahulland (Monk et al. 2000). Based on geological time, the islands in Wallace region (include Lombok island) are classified as very young
islands (15-1 mya) and never be a part of mainland in Sundaland or Sahulland.
The flora and fauna between the continent of Asia and Australia have never been
linked (Cracraft 1988). At least faunas living in Wallacea region (include Lombok
Island) migrated through the sea.
The distribution of fishes (especially of freshwater fishes) in oceanic
islands are strongly correlated with the islands geology (Allen 1991). All fishes
inhabit these islands must migrated from the main islands through the sea. So, all
fishes living in this area have a diadromy lifestyle. Diadromy is migrated of fishes
between the sea and freshwater (McDowall 2007). Gobiidae is one of fish family
which diadromy lifestyle. Some gobies were reported found at Lesser Sundas by
Monk et al. (2000). The authours recorded several genera that founded in Lesser Sundas, i.e. Acentrogobius, Awaous, Bathygobius, Glossogobius, Hemigobius, Oxyurichthys, Pseudogobius, Sicyopterus, Stenogobius, and Stiphodon, but there
is no specific data which explain that gobies in this location.
Morphological Analysis
Gobies have many variations in size and shape. These variations can be
used as data to study of morphology between individuals, populations, species,
and higher taxa. Morphological data can be divided into morphometric and
meristic. Morphometric is continuous data from measured structure whereas
meristic is discrete data from countable structure (Helfman et al 2002).
Both morphometric and meristic data can be clustered by many methods,
one of them is K-means. K-means is method of cluster analysis which aims to
deviden observations into k clusters, so that the data have similar character within cluster and different from the other clusters (Agusta 2007). K-means method is
better than other methods to analyze large and continuous data type. This method
MATERIALS AND METHODS
Study Site
This study was conducted during January-October 2010. Fish samples were
collected from rivers and mangrove forests ecosystem in Lombok Island. In
rivers, samples were collected from three locations. They were Jangkuk river in
Mataram City, Sidutan river in northern Lombok, and Belimbing river in eastern
Lombok. Sampling location in mangrove forests were Sepi Bay and Selindungan
Village in Sekotong district, and Labuan Treng in Labuan district. Both Sekotong
and Labuan are located in the western Lombok (Figure 1). Gobies identification
and data analysis were conducted at Laboratory of Elementary Biology on
Mathematics and Natural Science Faculty in Mataram University and
Biosystematic and Animal Ecology Laboratory, Biology Department,
Mathematics and Natural Science Facultyof Bogor Agricultural University.
Sample Collection
Sampling was carry out in the morning and afternoon at river and mangrove
forest. Electric fishing gear (12 V, 10 A) was employed for about one hour per
location in rivers, whereas in mangrove forest sampling was carried out using
anaesthetic agent during low tide in pools and creeks. A mixture of one part clove
oil and three parts of 50% alcohol was used as anaesthetic. The gobies were
subsequently caught using a hand net (Wen et al. 2005; Imanpoor et al. 2010; Robertson & Smith-Vaniz 2010). Gobies were fixed by formalin 4% and
Figure 1 Map of sampling areas: (A) Jangkuk river, (B) Sidutan river, (C) Belimbing river, (D) Labuan Treng mangrove forest, (E) Selindungan mangrove forest, and (F) Sepi Bay mangrove forest.
Gobies Identification
Identification to genera based on Larson & Murdy (2001). Identification to
species based on Koumans (1953), Whitley (1953), Akihito & Meguro (1975),
Akihito & Meguro (1976), Sakai & Nakamura (1979), Murdy (1989), Kottelat &
Whitten (1993), Allen (1991), Allen et al. (2000), Akihito & Meguro (2000), Akihito et al. (2003), Haryono & Tjakrawidjaja (2004), Larson & Takita (2004), Larson & Lim (2005), and Jaafar & Larson (2008). Based on morphological
identification result, 214 specimens of gobies from Lombok could be
distinguished into 21 species in 10 genera and 4 subfamilies. In this study, 12
species (B, C, D, E, F, G, K, L, R, S, T, and U) were included in analysis and 9
species (A, H, I, J, M, N, O, P, and Q) were excluded because consisting of only
excluded in analysis were plotted in principal component space to find their
relation with other clusters.
Table 1 Twenty one species in this study
Code Amount of specimens
Species Code Amount of
specimens
Species
A B C D E F G H I J K
5 20 21 13 11 12 12 2 1 3 14
Acentrogobius audax Acentogobius janthinopterus Acentrogobius viridipunctatus Amoya gracilis
Amoya moloana Amoya raveni
Boleophthalmus boddarti Cristatogobius rubripectoralis Glossogobius celebius Oxyurichthys ophthalmonema Oxyurichthys tentacularis
L M N O P Q R S T U
14 5 1 3 6 6 12 12 25 16
Periophthalmus argentilineatus Periophthalmus gracilis Periophthalmus malaccensis Periophthalmus minutus Periophthalmus novemradiatus Pseudogobius javanicus Sicyopterus cyanocephalus Sicyopterus hageni Sicyopterus microcephalus Stiphodon elegans
Total 214
Morphometric data
Method of morphometric measurement followed Murdy (1989). The gobies
were measured using digital caliper with resolution of 0.01 mm. These study used
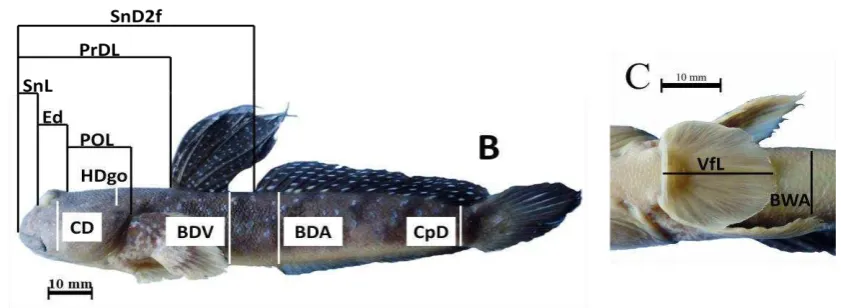
22 morphometric characters and their abbreviation were presented in Figure 2 and
Table 2. Measurement of all characters were expressed as a percent of Standard
Length (SL).
Figure 2 Morphometric characters of gobies (continued).
Table 2 List of morphometric characters of gobies used in this study
NO MORPHOMETRIC CHARACTERS ABBREVIATION
Comparison with Standard length (SL)
1 Head length HL
2 Predorsal length PrDL
3 Snout to 2nd Dorsal Fin Length SnD2f
4 Snout to ventral fin length SnVf
5 Snout to anal fin length SnAf
6 Caudal peduncle length CpL
7 Caudal peduncle depth CpD
8 1st Dorsal Fin Base D1b
9 2nd Dorsal Fin Base D2b
10 Anal fin base Ab
11 Pectoral fin length PfL
12 Ventral fin length VfL
13 Body depth at ventral BDV
14 Body depth at anal BDA
15 Body width at anal BWA
16 Ventral fin to anal VfA
17 Snout length SnL
18 Eye diameter Ed
19 Check depth CD
20 Postorbital length POL
21 Head width at maximum HDm
Meristic data
Methods of meristic count followed Murdy (1989). The first element of anal
and second dorsal fin may be a soft spine or a segmented ray. Anal and second
dorsal fin did not discriminate between spines and rays. The last two rays of each
fins were very close together and shared ultimate pterygiophore, so these rays
were counted as a single element (Springer 1978, 1983).
Scale count in logitudinal series (LSR) was begun from dorsoposterior
attachment of opercular membrane, continued on posteroventral diagonal to the
tip of the pectoral fin, and then in a straight line along the midline of the body to
the posterior edge of the hypural plate, determined externally. Transverse scale
count (TSR) was counted from anal fin origin dorsoanteriorly to the first dorsal
fin base. Predorsal scales (PSR) extended just anterior to the first dorsal spine.
These study used 8 meristic characters and their abbreviation were presented in
Figure 3 and Table 3.
Table 3 List of meristic characters of gobies used in this study
No MERISTIC CHARACTERS ABBREVIATION
Fin-ray counts
1 First dorsal fin D1
2 Second dorsal fin D2
3 Anal fin A
4 Pectoral fin P
5 Segmented caudal fin rays C
Scale counts
6 Longitudinal scale row LSR
7 Transverse scale row TSR
8 Predorsal scale row PSR
Data Analysis
The morphometric measurements were standardized on Standard Length
(SL) to remove size effect (Lleonart et al. 2000). Morphometric data were log transformed because the equation log (x/y) = log x – log y is a linear function of log x and log y whereas x/y is not a linear function of x and y (Murdy 1989).
Principal Components Analaysis (PCA) was employed in multivariate comparison
using covariance matrix to eliminate the correlation between variables.
K-means clustering algorithm function was used to determine a prespecified
number of cluster centres to minimize the within-class sum of squares from those
centres (Venables & Ripley 1999). The analysis was followed by elbow criterion
method to estimate the best number of clusters. This methods used the ratio of
within-group to the total variance. The best number of clusters was estimated
when adding a group did not improve the explained variance (Claude 2008). The
closeness between clusters were estimated use Minimum Spanning Tree (MST)
CLUSTER ANALYSIS
Clustering of Gobies Based on Morphometric Characters
Mathematically 22 morphometric measurements build a character space within which individual gobies floated. If the measurement values were same or similar, they would cluster together. PCA was used to remove any correlation between measurements, so the axes (Principal Components/ PCs) of the space were spanned orthogonally.
In the character space, the true number of clusters is not known. So we used elbow criterion method to determine the best number of clusters. Iteratively, adding a number of clusters will add information (explain a proportion of variance) but at some point the marginal gain will drop, giving an angle in the graph of number of cluster versus variances (Twarakavi et al. 2010). Elbow criterion use the ratio of within-cluster variance to total variance. The best number of clusters would be estimated when adding a group did not improve the explained variance (Claude 2008). The number of clusters are chosen at this elbow (Twarakavi et al. 2010).
Iteration to cluster gobies was done using K-means method (Venables and Ripley 1999). Table 4 suggested that the best number of clusters was seven because adding a cluster did not increase the explained variance. These partition were showed in principal-component space in Appendix 1. The best partition was showed in Figure 4.
Table 4 Percentage of variance explainable with adding number of clusters
Number of Clusters Percentage of Explained Variance
2 28.41%
3 19.37%
4 12.19%
5 6.25%
6 5.8%
7 5.5%
8 1.61%
9 1.22%
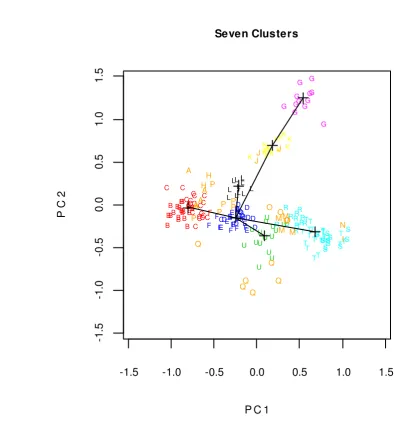
Figure 4 Plotting of all speciments in two first PC indicate centroid of clusters and their minimum spanning tree
Figure 4 showed seven clusters of gobies based on morphometric data. This
clustering referred to genera level. They were Acentrogobius (B and C) as 1st cluster, Amoya (D, E, and F) as 2nd cluster, Periophthalmus (L) as 3rd cluster,
Stiphodon (U) as 4th cluster, Sicyopterus (R, S, and T) as 5th cluster,
Boleophthalmus (G) as 6th cluster, and Oxyurichthys (K) as 7th cluster. The closeness between clusters were estimated using Minimum Spanning Tree (MST),
which is defined as a tree with the minimal sum of the edge weights among all the
trees than contain all clusters (Xu & Wunsch II, 2009). The weight of the edge
-1.5 -1.0 -0.5 0.0 0.5 1.0 1.5
-1 .5 -1 .0 -0 .5 0 .0 0 .5 1 .0 1 .5
P C 1
P C 2 C C C C
CC C
between each pairs of clusters is the distance between those two clusters, where
the distance can be estimated using Euclidean distance method. Based on MST
result, the distance of 1st to 2nd cluster is 0.700; 2nd to 3rd cluster is 1.008; 2nd to 5th
cluster is 1.015; 2nd to 7th cluster is 1.064; 2nd to 4th cluster is 1.216; and 7th to 6th
cluster is 1.256.
Figure 4 is plotting specimens in principal component space using two
first PCs. Actually, the space has 22 PCs and each PC bear different sizes of
variance information. The first PC informed the higher score in eigen value
(0.3372) and this score contributed to 36.61% variation of data. The score of eigen
value in second PC was 0.2029 and the score contributed 22.03% variation of data
[image:31.595.115.506.327.572.2](Table 5).
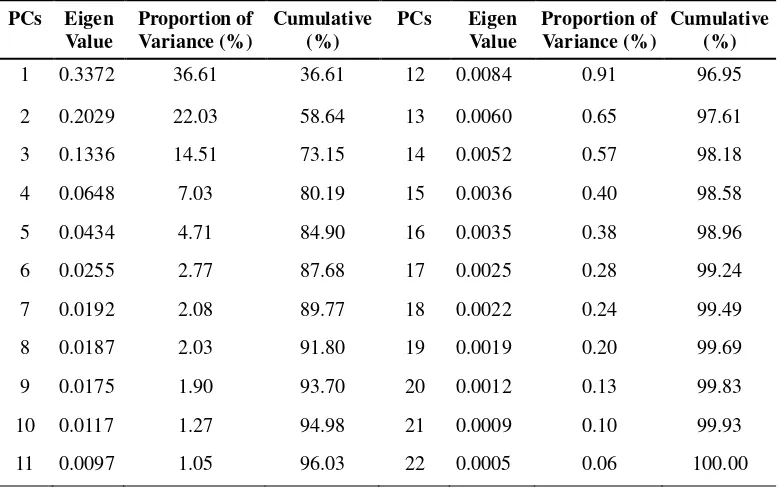
Table 5 Eigen value, proportion of variance, and cumulative of variance.
PCs Eigen Value
Proportion of Variance (%)
Cumulative (%)
PCs Eigen Value
Proportion of Variance (%)
Cumulative (%)
1 0.3372 36.61 36.61 12 0.0084 0.91 96.95 2 0.2029 22.03 58.64 13 0.0060 0.65 97.61 3 0.1336 14.51 73.15 14 0.0052 0.57 98.18
4 0.0648 7.03 80.19 15 0.0036 0.40 98.58
5 0.0434 4.71 84.90 16 0.0035 0.38 98.96
6 0.0255 2.77 87.68 17 0.0025 0.28 99.24
7 0.0192 2.08 89.77 18 0.0022 0.24 99.49
8 0.0187 2.03 91.80 19 0.0019 0.20 99.69
9 0.0175 1.90 93.70 20 0.0012 0.13 99.83
10 0.0117 1.27 94.98 21 0.0009 0.10 99.93
11 0.0097 1.05 96.03 22 0.0005 0.06 100.00
Plotting of PC1 and PC2 account for 58.64% of cumulative variance.
Because the two PCs have more than half of total information, these PCs informed
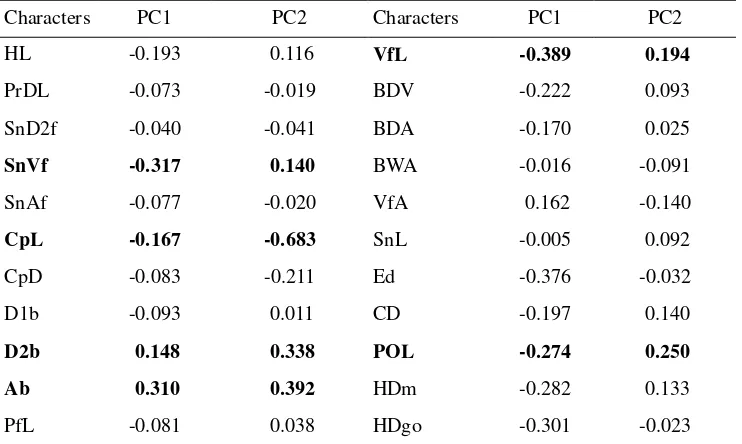
high contribution of each characters in data separation. Tabel 6 showed eigen
vector score of each characters in PC1 and PC2. Six characters (SnVf, CpL, D2b,
Ab, VfL, and POL) have larger scores than others, so these characters should be
sufficient to be used as taxonomic character to separated gobies based on
Table 6 Eigen vector of each character on first two PCs.
Characters PC1 PC2 Characters PC1 PC2
HL -0.193 0.116 VfL -0.389 0.194
PrDL -0.073 -0.019 BDV -0.222 0.093
SnD2f -0.040 -0.041 BDA -0.170 0.025
SnVf -0.317 0.140 BWA -0.016 -0.091
SnAf -0.077 -0.020 VfA 0.162 -0.140
CpL -0.167 -0.683 SnL -0.005 0.092
CpD -0.083 -0.211 Ed -0.376 -0.032
D1b -0.093 0.011 CD -0.197 0.140
D2b 0.148 0.338 POL -0.274 0.250
Ab 0.310 0.392 HDm -0.282 0.133
PfL -0.081 0.038 HDgo -0.301 -0.023
Note: The bold characters showed higher score in cumulative variation of PC1 and PC2 than their 3rd quartile.
Clustering of Gobies Based on Meristic Characters
There are eight meristic characters (D1, D2, A, P, C, LSR, TSR, and PSR)
to clustering of gobies collected in the present study. Iteration to cluster was done
using K-means method and their result were showed in principal component space
(Appendix 2). Iteratively, adding a number of cluster will add explain a proportion
of variance and the best number of clusters would be estimated when adding a
group did not improve the explained variance. Based on this method, the best
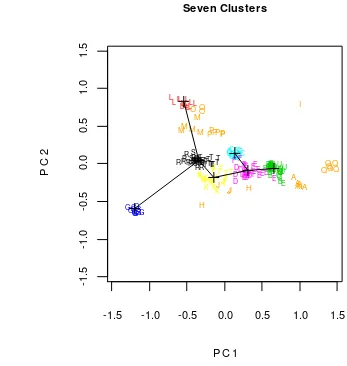
[image:32.595.114.485.99.321.2]number of gobies cluster used meristic count was seven (Table 7 and Figure 5).
Table 7 Percentage of variance explainable with adding number of clusters.
Number of Clusters Percentage of Explained Variance
2 41.56%
3 19.10%
4 13.88%
5 9.00%
6 3.44%
7 2.95%
8 1.28%
9 1.22%
Figure 5 Plotting of all speciments in two first PC indicate centroid of clusters and their minimum spanning tree
Figure 5 showed seven clusters of gobies based on meristic data. They were
G (1st cluster); L (2nd cluster);C (3rd cluster); D, F, and 8 of E (4th cluster); B, U,
and 3 of E (5th cluster); K and 10 of T (6th cluster); and R, S, and 15 of T (7th
cluster). Based on MST result, 4th cluster more close to 5th cluster than the other
clusters with distence 0.407; after that 6th to 7th cluster with distance 0.418; 4th to
6th cluster with distance 0.518; 3rd to 4th cluster with distance 0.663; 2nd to 7th
cluster with distance 0.968; and 7th to 1st cluster with distance 1.238.
Actually, these analysis has 8 PCs and each PC bear different sizes of
variance information. But we used two first PCs to plotting specimens (Figure 5)
because these PCs have more than half of total information of variance. The first
-1.5 -1.0 -0.5 0.0 0.5 1.0 1.5
-1 .5 -1 .0 -0 .5 0 .0 0 .5 1 .0 1 .5
P C 1
P C 2 B B BB B B BBB B BBBB BB B B BB CCCC
C C C C C C C
CCCCCC CCC
C
D
D DDD
D D D D D D
D EEEEEEE
E E E E F F F F F FF F F F F G G G G GG GG G G G G K K K K KK K K K K K K K K L L L L L L L L L L L L L L R R R R R R R R R RRRSS
S SS SSSS SS ST
T T T T
TT T T T TT TT TT T T T T T T T TT
UUU U UU
U UUU U
U U U UU Seven Clusters A A AA A H H I J J J M M M M M N O OO
PC informed the higher score in eigen value (0.272) and this score contributed to
57.56% variation of data. Whereas, second PC informed 0.089 of eigen value
(18.84% variation of data). So plotting of PC1 and PC2 account for 76.40% of
cumulative variance (Table 8).
Table 8 Eigen value, proportion of variance, and cumulative of variance.
PC Eigen Value Proportion of Variance (%) Cumulative (%)
1 0.272 57.56 57.56
2 0.089 18.84 76.40
3 0.054 11.37 87.77
4 0.034 7.19 94.97
5 0.012 2.51 97.49
6 0.006 1.16 98.65
7 0.004 0.90 99.56
8 0.002 0.44 100.00
Tabel 9 showed eigen vector score of each characters in PC1 and PC2.
These score informed high contribution of each characters in data separation Two
characters (LSR and PSR) have larger scores than others, so these characters
should be sufficient to be used as taxonomic character to separated gobies based
on meristic data.
Table 9 Eigen vector of each character on PC1 and PC2.
Characters PC1 PC2
D1 -0.086 0.684
D2 -0.271 -0.343
A -0.361 -0.389
P -0.087 -0.279
C -0.036 -0.113
LSR -0.593 0.109
TSR -0.488 -0.068
PSR -0.436 0.395
IDENTIFICATION
Gobiidae are small fishes (up to 30 cm, usually less than 10 cm) with united ventral fins, which are sometimes totally or posteriorly incised, basal membrane present or absent. Two dorsal fins, separated or connected with their bases, sometimes first dorsal fin absent. Body scaled with ctenoid or cycloid scales, sometimes partly or totally naked. Gobiidae can be separated into five subfamilies, they are Amblyopinae (worm-gobies), Gobiinae (true gobies), Gobionellinae (brackish-water gobies), Oxudercinae (mudskippers), and Sicydiinae (freshwater gobies) (Koumans 1931; Larson & Murdy 2001; Larson & Lim 2005).
Key to the subfamilies of Gobiidae from Lombok Island
1a. Lower jaw typically possessing only single row of teeth ...2
1b. Lower jaw typically possessing more than 1 row of teeth ...3
2a. Pelvic frenum with fleshy lobes over spines (Fig. 6c); eyes lateral and without
eyelid (Fig. 7b) ...Sicydiinae
2b. Pelvic fins without frenum (Fig. 6a) or if frenum present without fleshy lobes
over spines (Fig. 6b); body elongate; eyes located mostly dorsally and with a
free lower eyelid (Fig. 7a) ...Oxudercinae
a b c
Figure 6 Ventral view of pelvic fins (a) Without frenum; (b) Frenum without fleshy lobes; (c) Frenum with fleshy lobes
No fleshy lobes Ventral fins
without frenum
a b
Figure 7 (a) Ventral fins with frenum and fleshy lobes; (b) eyes lateral and without eyelid
3a. Paired anterior interorbital pores and with a pore immediately behind anterior
nostril (Fig. 8a) ...Gobionellinae
3b. A single anterior interorbital pore and without a pore immediately behind
anterior nostril (Fig. 8b) ...Gobiinae
a b
Figure 8 Head pores type (a) A pair and (b) A single of anterior interorbital pore(s)
Subfamily Gobiinae
Key to the genera and species of Gobiinae from Lombok Island
1a. Thin dermal crest on top of head anterior to first dorsal fin (Fig. 9); body
deep; second dorsal fin and anal fin with I spine and 9 soft rays (estuaries)
...Cristatogobius (with a single species known in the area, Cristatogobius rubripectoralis)
1b. No dermal crest anterior to first dorsal fin ...2
Eyes with eyelid Without eyelid
Anterior nostril
Figure 9 Dermal crest on top of head.
2a. Tongue distinctly bilobed (Fig. 10) ...Glossogobius (with a single species known in the area, Glossogobius celebius)
2b. Tongue variable, usually rounded or concave ...3
Figure 10 Tongue distinctly bilobed.
3a. Opercles full scaled ...Acentrogobius (4)
3b. Opercle naked ... Amoya (6)
4a. Transverse papilla pattern on cheek ...Acentrogobius viridipunctatus 4b. Longitudinal papilla pattern in cheek ... 5
5a. Anal spine rays I,8; the horizontal dark stripes across the cheek and pectoral
base ...Acentrogobius janthinopterus 5b. Anal spine rays I,9; dark bar on caudal peduncle and caudal base ...
...Acentrogobius audax 6a. Total D2 elements usualy I,10; LSR less than 40 (usually 30-36) ...
...Amoya moloana 6b. Total D2 elements usualy I,11; LSR more than 40 (usually 39-48) ...7
7a. First dorsal fin with filamentous extension ...Amoya raveni 7b. First dorsal fin without filamentous extension ...Amoya gracilis
Dermal crest
Genus Acentrogobius Bleeker
Acentrogobius audax (Smith, 1959)
Figure 11 Acentrogobius audax, 43.33 mm in SL, Selindungan, Sekotong distric-western Lombok.
Material Examined
Five specimens from Selindungan, Sekotong. Size ranges are 29.94 to 43.33 mm
in SL.
Diagnosis:
D. VI; I,9-10; A. I,9; P. 16-17; longitudinal scales 27-29; transverse scales 8; predorsal scales 9-12. Body is elongate and compressed. Scale on nape is cycloid. Preopercle and opercle totally scaled. The large, rounded blackish-brown blotches along the side are very distinctive, as are this species 'large eyes', making it easy to
identify the fish from a distance.
Distribution: Western Indian Ocean: Ibo, Mozambique and Natal; South Africa;
Remarks : -
Acentrogobius janthinopterus (Bleeker, 1852)
Acentogobius janthinopterus: Koumans, 1953: 59-60 (Singapore) Acentogobius janthinopterus: Kottelat & Whitten, 1993: 196, Pl. 64 (Indonesia)
Acentrogobius janthinopterus: Larson & Lim, 2005: 60 (Singapore)
Material Examined
Twenty specimens, three specimens from Sepi bay, eleven specimens from
Selindungan, Sekotong distric; and six specimens from Labuan Treng, Labuan
distric. Size ranges are 35.22 to 59.75 mm in SL.
Diagnosis
D. V-VI; I,10; A. I,8; P. 15-18; longitudinal scales 30-32; transverse scales
10-11; predorsal scales 14-18. Body is elongate and compressed. Upper half of
cheek and opercle fully scaled, and predorsal scaled up to behind eyes.
Longitudinal papillae rows on cheek. The horizontal dark stripes across the cheek
and pectoral base are characteristic.
Distribution:
Western Pacific, Sumatra to Japan (Larson & Lim 2005).
Remarks : -
Acentrogobius viridipunctatus (Valenciennes, 1837)
Acentrogobius viridipunctatus: Koumans, 1932: 134 (Indo-Australian) Acentrogobius viridipunctatus: Kottelat & Whitten, 1993: 196, Pl. 64 (Indonesia)
Acentrogobius viridipunctatus: Larson & Lim, 2005: 64 (Singapore)
Figure 13 Acentrogobius viridipunctatus, 66.14 mm in SL, Selindungan, Sekotong distric-western Lombok.
Material Examined
Twenty one specimens. Five specimens from Selindungan, Sekotong distric and
sixteen specimens from Labuan Treng, Labuan distric. Size ranges are 49.84 to
Diagnosis:
D. VI; I,9-10; A. I,8-9; P. 16-20; longitudinal scales 33-39; transverse
scales 11-13; predorsal scales 28-34. Body is elongate and compressed. Opercles
and uppermost part of cheek behind eyes scaled. Transverse papillae rows below
eye. The spots above the pectoral base may be most distinct.
Distribution:
Indonesia, India, Philippines, Thailand, Hongkong, South Africa, and Japan
Remarks : -
Genus Amoya
Amoya gracilis (Bleeker, 1875)
Figure 14 Amoya gracilis, 42.35 mm in SL, Labuan Treng, Labuan distric-western Lombok.
Material Examined
Thirteen specimens from Labuan Treng, Labuan distric. Size ranges are 41.60 to
53.80 mm in SL. Diagnosis
D. VI; I,11; A. I,10; P. 15-17; longitudinal scales 39-48; transverse scales
12-14; predorsal scales 10-21. Body is elongated and compressed. Caudal fin
lanceolate.
Distribution: Indo-Pacific.
Amoya moloana
Amoya moloana : Kottelat & Whitten, 1993, 196 Pl. 64 (Indonesia)
Figure 15 Amoya moloana, 44.83 mm in SL, Selindungan, Sekotong distric-western Lombok.
Material Examined
Eleven specimens from Selindungan, Sekotong. Size ranges are 36.56 to 46.61
mm in SL.
Diagnosis
D. VI; I,10; A. I,8; P. 14-17; longitudinal scales 30-36; transverse scales
11-17; predorsal scales 9-13. Body elongated, a row of drak on the upper side of
body and midlateral row of drak elongated spots. A row of widely placed spot on
the lower part of body (not always distinct).
Distribution :
Celebes, Philippines, and Japan (Kottelat & Whitten 1993).
Remarks : -
Amoya raveni (Bleeker, 1875)
Material Examined
Twelve specimens, two specimens from Selindungan, Sekotong and ten specimens
from Labuan Treng, Labuan. Size ranges are 35.54 to 53.56 mm in SL. Diagnosis
Body elongated, compressed, covered with 39 to 47 ctenoid scales on
longitudinal scale row, 9 to 10 scales on transverse scales row, and 9 – 15 on predorsal scale row. Dorsal fin VI, I 10-12, anal fin I, 9 and pectoral fin 13-16
rays. Caudal fin lanceolate. First dorsal fin with filamentous extension.
Distribution: Indo-Pacific
Remarks : -
Genus Cristatogobius Herre, 1927
Cristatogobius rubripectoralis Akihito et all 2003
Cristatogobius sp. : Kottelat et al., 1993 :199, pl.66 ( Southern Java, Indonesia). Cristatogobius sp. : Larson & Williams, 1997: 368 (Northern Territory, Australia).
Cristatogobius rubripectoralis : Akihito et al., 2003: 117 (northeastern Australia)
Figure 17 Cristatogobius rubripectoralis, 49.80 mm in SL, Selindungan, Sekotong distric- western Lombok.
Material Examined
Two specimens, one specimen from Selindungan, sekotong distric and one
specimen again from Labuan Treng, Labuan distric, western Lombok. Size ranges
are 37.86 to 49.80 mm in SL.
Diagnosis:
D. VI; I,10; A. I,9; P. 16-17; longitudinal scales 37-64; transverse scales
21-26; predorsal scales 9-10. Head and Body compressed. Dorsal profile sloping
nuchal crest with rounded margine extending from above eye to origin of firs
dorsal fin. First dorsal fin distally rounded witout folamentous spines. Posterior
margin of caudal fin rounded.
Distribution:
Western Central Pacific: southern Java (Kottelat et al 1993), western Lombok
(this research), Indonesia and northern Australia (Akihito et al 2003).
Remarks :
Maybe Cristatogobius rubripectoralis in this research is a new record for Lasser Sundas territory.
Genus Glossogobius Gill 1862 Glossogobius celebius (Velenciennes, 1837)
Glossogobius celebius : Allen, 1991 : 180 Pl. 17 No.15 (New Guinea) Glossogobius celebius : Kottelat and Whitten, 1993 : 200 Pl. 66 (Indonesia)
Glossogobius celebius : Haryono and Tjakrawidjaja, 2004 : 92 Fig. 52 (northern Sulawesi : Indonesia)
Figure 18 Glossogobius celebius, 47.00 mm in SL, Sidutan River, Kayangan distric- northern Lombok.
Material Examined
One specimen from Sidutan River, Kayangan. Diagnosis
canal with pores K' and L'. Preopercular canal with pores M', N, and O'. Lines of pit organs mostly in single rows. Five longitudinal lines (7, 8, 9, 10, and 11) below line 5. Line 6 in long single row. Lines 9 and 10 in single rows. Line 13 in single low. Lines 20, 21, and 22 unbranched.
Distribution:
Widespread in the western tropical Pacific including northern Australia, New
Guinea, Solomon Islands, Indonesia, Philippines, Taiwan and Ryuku Islands.
Remarks : -
Subfamily Gobionellinae
Key to the genera and species of Gobionellinae from Lombok Island
1a. Predorsal scale fewer than 11 (usually 6-11); None or 1 pair of pores present
on snout (Fig. 19a); without dermal crest on nape; without tentacle at margine
of eyes; caudal fins rounded ...Pseudogobius (with a single species known in the area, Pseudogobius javanicus)
1b. Predorsal scale more than 11 (usually 13-22); Two pairs of pores present on
snout (Fig. 19b); nape with narrow dermal crest; tentacle at margine of eyes;
caudal fins lanceolate ...Oxyurichthys (2)
Figure 19 Dorsal view of head
2a. A tentacle at upper margine of eyes; scales on upper part of body without
black spot; LSR 55-60 ...Oxyurichthys tentacularis 2b. A tentacle at above of eyes; upper part of body with distinct black spot on
Genus Oxyurichthys Bleeker 1860
Oxyurichthys tentacularis (Cuvier & Valenciennes 1837)
Oxyurichthys tentacularis : Koumans, 1932 : 126 (Indo-Australian) Oxyurichthys tentacularis : Kottelat & Whitten, 1993 : 203, Pl. 68 (Indonesia)
Figure 20 Oxyurichthys tentacularis, 66.96 mm in SL, Labuan Treng, Labuan distric-western Lombok.
Material Examined
Fourteen specimens, both Labuan Treng and Selindungan have each seven
specimens of gobies. Size ranges are 41.95 to 66.96 mm in SL.
Diagnosis:
D. VI-VII; I,12-13; A. I,11-13; P. 17-21; longitudinal scales 55-60;
transverse scales 13-17; predorsal scales 13-22. Body elongate and compressed.
Scales of head and anterior part of body cycloid, posteriorly pointed and
increasing in height. Preopercle and opercle naked, tentacle at upper margin of
eye. Upper lip constricted in the middle. Caudal fin lanceolate, teeth in the upper
jaw with single row. Snout obtuse, as long as or shorter than eye; tip before lower
margin of eye. Anterior nostril in a short tube. Mouth oblique, lower jaw
prominent. Maxillary extends to below posterior part of eye.
Distribution: Indo-West Pacific
Oxyurichthys ophthalmonema (Bleeker)
Oxyurichthys ophthalmonema : Akihito, 1972 : 103-110 (Japan)
Oxyurichthys ophthalmonema : Kottelat & Whitten, 1993 : 203, Pl. 68 (Indonesia)
Figure 21 Oxyurichthys ophthalmonema, 41.51 mm in SL, Selindungan, Sekotong distric-western Lombok.
Material Examined
Three specimens from Selindungan mangrove forest, Sekotong. Size ranges are
39.55 to 49.81 mm in SL.
Diagnosis
D. VI; I,12; A. I,13; P. 17-18; longitudinal scales 49-52; transverse scales
14-15; predorsal scales 13-16. Body elongate and compressed. A tentacle above
eye. Upper lip not constricted in the middle. Body have five round dark spots,
placed in longitudinal row and upper part of body with distinct black spot on each
scale. First spine at first dorsal fin is elongate ( two to six times in SL).
Distribution: Indo-West Pasific
Remarks:
Tomiyama (1936) consedered that G. ophthalmonema Bleeker is synonymous with his O. tentacularis (not of Cuvier and Valenciennes). But referred Akihito (1972), O. ophthalmonema (Bleeker) can be distinguised from O. tentacularis
(Cuvier and Valenciennes) by (1) In O. ophthalmonema, the upper and lower rims of the upper lip are almost parallel with each other, whereas upper lip of O. tentacularis
Genus Pseudogobius
Pseudogobius javanicus (Bleeker, 1856)
Pseudogobius javanicus : Kottelat & Whitten, 1993 : 208, Pl. 70 (Indonesia) Pseudogobius javanicus : Chen & Shou : 1993 : 76 Fig.1 (southern Taiwan) Pseudogobius javanicus : Ng et al. : 1999 : 182 Fig.8 (Pulau Tioman : Malaysia)
Pseudogobius javanicus : Larson & Lim, 2005 : 141 (Singapore)
Figure 22 Pseudogobius javanicus, 27.75 mm in SL, Selindungan, Sekotong distric-western Lombok.
Material Examined
Six specimens from Selindungan, Sekotong. Size ranges are 21.00 to 31.35 mm
in SL.
Diagnosis:
D. VI; I,7; A. I,7; P. 14-16; longitudinal scales 24-31; transverse scales 6-7; predorsal scales 7. Body elongate, with numerous minute spots and laterally 2 longitudinal rows, each alternate 5 blotches. Second dorsal and caudal fin with several rows of tiny spots. Caudal fin base with 2 vertically black spots. Maxillary extends to the vertical of anterior half of eye, upper jaw prominent. Lips thin. Snout obtuse, overhanging the upper lips.
Head scaled above behind eye and opercle. Scale on head, breast and belly cycloid, on lateral side of body ctenoid. Middle spines of first dorsal fin are the longest. Second dorsal and anal fins higher than first dorsal fin.
Distribution :
Western Pasific from Indonesia to Philippines (Larson & Lim 2005), Southern Taiwan (Chen & Shao 1993).
Remarks :
Its specimen similar with P. masago (Tomiyama 1936), but refer to Chen & Shao (1993), P. javanicus can be distinguised with P. masago by first dorsal fin of P. javanicus has a black blotch, whereas first dorsal fin of P. masago all whitish and P. javanicus has two black spots on the caudal fin base, but in P. masago is a wedge-
Subfamily Oxudercinae Günther, 1861
Key to the genera and species of Oxudercinae from Lombok Island
1a. Two canine teeth internal to lower jaw symphysis; anal fin base and second
dorsal fin base 34% SL or greater ...Boleophthalmus (with a single species known in the area, Boleophthalmus boddarti)
1b. No canine teeth internal to lower jaw symphysis; a single row of teeth in upper
jaw; anal fin base and second dorsal fin base 27% SL or less...
... Periophthalmus (2)
2a. Frenum present ...3
2b. Frenum absent ...4
3a. D1 with few or no white spots; D2 with one dark band; blackish to grey stripe
across the branchiostegal membranes on the underside of the head (Fig. 23a)
...Periophthalmus novemradiatus 3b. D1 with many white spots; D2 with two dark bands the branchiostegal
membranes pale (not pigmented) (Fig. 23b)...Periophthalmus malaccensis
a b
Figure 23 Ventral view of head showing (a) black branchiostegal membranes and (b) pale brachiostegal membrans.
4a. D1 rounded with not more than X spines ...Periophthalmus gracilis 4b. D1 pointed with more than X spines...5
5a. D1 with black stripe inframarginally; peritoneum densely black ventrally; LSR
75 or more ...Periophthalmus argentilineatus 5b. D1 with light brown stripe inframarginally; peritoneum lightly pigmented
ventrally; LSR 75 or fewer ...Periophthalmus minutus
Black branchiostegal
membranes
Pale branchiostegal
Genus Boleophthalmus Valenciennes in Cuvier & Valenciennes, 1837 Boleophthalmus boddarti (Pallas 1770)
Boleophthalmus boddarti : Koumans, 1953 : 259-260 (Singapore) Boleophthalmus boddarti : Murdy, 1989 : 14 (Malaysia)
Boleophthalmus boddarti : Kottelat & Whitten, 1993 : 198, Pl. 65 (Sumatra and Borneo : Indonesia) Boleophthalmus boddarti : Larson & Lim, 2005 : 73 (Singapore)
Figure 24 Boleophthalmus boddarti, 89.94 mm in SL, Labuan Treng, Labuan district-western Lombok. (A) Male 1st dorsal fin, (B) Female 1st dorsal fin, and (C) Ventral fins with frenum.
Material Examined
12 specimens, all specimens from Mangrove forest at Labuan Treng,
Labuan-western Lombok. Size ranges are 73.14 to 98.57 mm in SL.
Diagnosis:
D. V; I,23-24; A. I,23-24; P. 18-20; longitudinal scales 71-80; transverse
scales 21-26; predorsal scales 28-35. Head and dorsal half of body grey brown,
ventral half of body greenish white, head and trunk with white speckles. Body
and dorsal fins with bright blue spots and seven black diagonal bars on body (1st
at D1, 2nd to 6th beneath D2, and 7th on caudal peduncle). Four first spine of first
dorsal fin are elongate in female. Pectoral fin yellow distally, dark brown
proximally with a brown margin. Anal fin is transparent except for black stripe
[image:49.595.102.506.177.419.2]Distribution:
India, Burma, Thailand, Vietnam, and Singapore (Koumans 1953; Murdy 1989;
Larson & Lim 2005), Sumatra and Borneo (Kottelat & Whitten 1993), and
Lombok (this research)
Remarks: This species probably is new record for Lasser Sundas
Genus Periophthalmus Bloch and Schneider, 1801 Periophthalmus argentilineatus (Valenciennes, 1837)
Periophthalmus vulgaris vulgaris : Koumans, 1953 : 212 (Ekos[=Ekas] and Labuan Hadji : Lombok) Periophthalmus dipus dipus : Koumans, 1953 : 214 (Larantuka and Mbawa : Flores)
Periophthalmus argentilineatus argentilineatus : Koumans, 1953 : 215 (Labuan Tring[=Labuan Treng] : Lombok)
Periophthalmus argentilineatus : Murdy, 1989 : 32, Fig. 32 (Laser Sundas Islands : Indonesia) Periophthalmus argentilineatus : Allen, 1991 : 190, Pl. 17 no.18 (New Guinea) Periophthalmus argentilineatus : Kottelat & Whitten, 1993 : 205, Pl. 69 (Indonesia)
Periophthalmus argentilineatus : Ng et al., 1999 : 181 (Pulau Tioman : Malaysia) Periophthalmus argentilineatus : Allen et al., 2000 : 127 (Timika : Indonesia)
Periophthalmus argentilineatus : Larson & Lim, 2005 : 130 (Singapore)
Figure 25 Periophthalmus argentilineatus, 37.85 mm in SL, Sepi Bay, Sekotong distric-western Lombok. (A) First and second dorsal fins and (B) Ventral fin without frenum.
Material Examined
14 specimens, eight specimens from Mangrove forest at Selindungan village and
six specimens from Sepi bay, Sekotong. Size ranges are 37.85 to 51.66 mm in SL.
Diagnosis
Body elongate, a little compressed, covered with 66-79 small cycloid
scale. Eyes in anterior half of the head, close together and prominent above the
dorsal profile, lower eyelid well developed. Mouth inferior. Pectoral fins with a
No frenum between ventral spines; ventrals fin without membrane uniting
both posterior rays; first dorsal fin pointed and grey to blackish with
white-bordered sub-marginal black stripe, no elongate spines. The second dorsal fin also
has a similar broad stripe. This species can be identified from a distance by the
bright silvery-white vertical lines along the sides of the body.
Distribution:
In The World : South Africa, Tanzania, Kenya, Somalia, Seychelles, Mandagascar,
Pakistan, Burma, Malaysia, Singapore, Philippine, Japan, Papua New
Guinea, Australia, Solomon Islands, Vanuatu, and Fiji.
Indonesia: Sumatra, Java, Madura, Lombok, Sumbawa, Flores, Timor, Borneo,
Celebes, Halmahera, Ternate, Batjan, Buru, Ceram, Ambon, Saparua,
Remarks:
Periophthalmus vulgaris (Koumans 1953: 210) and P. dipus (Koumans 1953: 212) are synonim of P. argentilineatus
Periophthalmus gracilis (Eggert, 1935)
Periophthalmus gracilis: Koumans, 1953: 210 (Java, Sumatra, Nias: Indonesia) Periophthalmus gracilis: Murdy, 1989: 37, Fig. 35 (Sumatra: Indonesia) Periophthalmus gracilis: Kottelat & Whitten, 1993: 206, Fig. 302 (Indonesia)
Periophthalmus gracilis: Larson & Lim, 2005: 132 (Singapore)
Figure 26 Periophthalmus gracilis, 33.45 mm in SL, Sepi Bay, Sekotong distric-western Lombok. (A) First and second dorsal fins and (B) Ventral fin without frenum.
Material Examined
Diagnosis
D. IX-X; I,12; A. I,11; P. 11-14; longitudinal scales 69-78; transverse
scales 13-17; predorsal scales 22-30. Slender mudskippers are small and not
conspicuously marked. Ventral fins are completely separated, and the frenum is
absent. D1 is short and rounded, no elongated spines. D2 with single dusky stripe
mesially. Dorsal fin not connected by membran.
Distribution:
Indo-west Pacific from Malaysia to northern Australia
Remarks: -
Periophthalmus malaccensis Eggert, 1935
Periophthalmus malaccensis : Koumans, 1953 : 205 (Singapore) Periophthalmus malaccensis : Murdy, 1989 : 39, Fig. 38 (Moluccas : Indonesia) Periophthalmus malaccensis : Kottelat & Whitten, 1993 : 206, Pl. 69 (Indonesia)
Figure 27 Periophthalmus malaccensis, 69.28 mm in SL, Sepi Bay, Sekotong distric-western Lombok. (A) First and second dorsal fin, (B) Ventral view of head showing pale branchiostegal membranes and ventral fin with frenum.
Material Examined
Diagnosis:
Ventral fins partially united by basal membrane with frenum prominent. First dorsal fin height moderate, its margin slightly rounded, a brown stripe inframarginally with numerous white spots proximally, first spine elongate, second dorsal fin with single dusky stripe mesially, dorsal fins not connected by membrane. Trunk with eight saddle-like blotches dorsally : 1st at anterior of first dorsal fin origin, 2nd across first dorsal fin base, 3rd at first dorsal fin terminus, 4th at second dorsal fin origin, 5th across second dorsal fin base, 6th at secon dorsal fin terminus, 7th at origin of dorsal procurrent rays, and 8th at caudal fin base.
Distribution:
Singapore (Koumans 1953); Philippines: Iloilo and Mariveles and Indonesia: Moluccas (Murdy 1989) and Lombok (this research).
Remarks: The type of this species was destroyed in WWII (Murdy, 1989).
Periophthalmus minutus Eggert, 1935
Periophthalmus minutus : Murdy, 1989 : 40, Fig. 39 (Java : Indonesia in part) Periophthalmus minutus : Kottelat & Whitten, 1993 : 206, Fig. 303 (Indonesia)
Figure 28 Periophthalmus minutus, 41.55 mm in SL, Sepi Bay, Sekotong. (A) First and second dorsal fin, (B) Ventral fin without frenum.
Material Examined
[image:53.595.86.484.435.694.2]Diagnosis:
Frenum at ventral fin lacking or only visible with magnification. Ventral
fins united posteriorly by a slight to moderate membrane. First dorsal fin pointed,
its margin straight to slight convex, a brown stripe mesially and no elongate
spines. Second dorsal fin with single dusky stripe mesially. Trunk with six
saddle-like blotches dorsally : 1st at anterior of first dorsal fin origin, 2nd across first
dorsal fin base, 3rd at first dorsal fin terminus, 4th across second dorsal fin base, 5th
at secon dorsal fin terminus, and 6th at origin of dorsal procurrent rays.
Distribution:
Java, Andamans, Philippine, Thailand, Australia (Murdy 1989; Kottelat & Whitten
1993) and Lombok (this research)
Remarks: -
Periophthalmus novemradiatus (Hamilton, 1822)
Periophthalmus variabilis variabilis : Koumans, 1953 : 206 (Tjilatjap[=Cilacap] : Indonesia in part) Periophthalmus novemradiatus : Murdy, 1989 : 42, Fig. 42 (Peninsular, Sabah, Sarawak : Malaysia)
Periophthalmus novemradiatus : Kottelat & Whitten,