.. :?:.:
=l!'.t -:
!=-j
-=:
:::!: . - i:'rl:);':,:.
--'
)--,:
.=:::
-: i J:::: tl': :i::4..
a:
-:-ia .
i;:
l _),
.-t -,tr ,..
.-t:
-"
-I:
an
ii.,,
Thm
frm{;wr*ffiffi&4ffiff'tffiH ffimro$mtrffir*effiffi
ffiffi
Mw&wr*$m$m
ffi
r.mffiffiffifr
rnffi
T"mmhmm$mffiy
5-6
Januany,
e0l
O
Wesbin
Gnande
Sukhumv'i
Hotel,
Bangkok, Thailand
o.n.n,redby
@Hn
uNlvEFslTY "'t
I
fl
T.
I
tuE0n Eu[ulnu doa$trt ttt'l-n
Intemational Conference on Materials Proccssing Technology 2010 January 5-6, 2010, Bangkok, THAILAND
Preparation of Neodymium Substituted Bismuth Titanate by
a
Fuel-liee
Combustion
Process
[Jmor
Al-Amani Azlan*, Srimala
Sreekantan, AhrnqdFauzi M. N., Khairunisak
A.R.School of
Materials
andMineral
ResourcesEngineering, (Jniversiti
SainsMalaysia,
14300Nibong
Tebal, Penang, MalaYsia.E -mail
:
ibnuazlan2 0 0 5 @gmail. comFine powders of neodymium substituted-bismuth titanate, Bi4-,Nd,Ti3O12 @NT) with x ranging from 0 to
1.0 were prepared by the fue1-free combustion process at 1ow combustion temperature of about 200"C The influence
of Nd
addition on the bismuth titanate system (BIT) was sh:died and characterizedby
themethods of XRD, Rieweld method and SEM.
It
was found that the intermediate phase of Bi12TiO26 had reduced with addition of Nd. Crystallinity and orthorhombicity of the system was found to be decreased asthe Nd content increases. The size of platey grains is strongly influenced by the Nd added to the system.
Ke.t v,ords;fuel-free combustion; neodymium; bismuth titanate; crystallinity
Introduction
Bismuth titanate
or
generally calledBIT,
alsooften abbreviated as BiaTi3O12
to
clearly
defineits composition, is a ferroelectric material which
has a perovskite structure (ABO3 structure) that
possesses
high
dielectric constant-200
and lowdissipation
factor -0.017
[1].
Recently,BIT
isbelieved to be the most promising candidate for
dielectric material
for
wireless application [2].Previously,
this
system
has
been used
forferroelectric
random
accessmemory
(FRAM),optical display, high
temperature piezoelectricapplication, pyroelectric sensors
and
electrooptic devices
[3-5].
The
performanceof
thissystem
is
comparablewith
other
perovskitestlucfure systems
such
as
barium
titanate(BaTi03),
lead
titanate (PbTiO3),
strontium titanate (SrTiO3) and calcium titanate (CaTiO3). Therefore,it
is
reasonableto
say thatBIT is
analternative
material
in
ceramic rndustry andit
can be used to replace PbTiO3 due to its
toxicity
and very harmful to the human body [6].
From a material
view point, BIT is
composedof
mixed
oxides
of
Bi2O3
and TiOz
andlanthanide atoms are frequently introduced to the
A
site
in
perovskite
BIT
matrix
to
replacebismuth
(Bi)
atoms,thus
it
is
also known
aslanthanide
doped-BlT.
The
substitution of
lanthanides element
in
this svstem has been usedin
many
studies
in
order
to
enhance theferroelectric properties.
For
instance,Kan
:t
alU)
showed
that the
dissipation
factor
of
lanthanum doped-BlT was successfully reduced
by
about 8-10 times lower than
of
pure
BIT.Besides that, the polarization properties
of
BIT
material were greatly improved
with
the additionof
Nd
[8].
in
this work,
however, the trivalentcation
of
neodymium
(Nd'*)
is
used
as
asubstitution element in
BIT
system.BIT
can be preparedby
a varietyof
methods such as mixed oxide route which based on solidstate reaction,
soi-gel route,
and hydrothermal[9-11]. Each
of
these routes has their merits anddrawbacks. However,
in
present study, a newlyprocess
which
is known as fuel-free combustion synthesis has been usedfor
the
preparationof
BIT. It
has
been
developed
from
self-propagating high-temperature synthesis (SHS)
combined
with wet
chemical techniquesfor
thesynthesizing
of
metal
oxide
based
ceramicpowders [12]. Having
this
is
mind, there
are three factors to generateignition
and combustiouactiviti,s
and
these factors
are
oxidizer,temperature,
and
fuel [13]. A
fuel-free combustion process, which does not employ anyfuel
agents such ascitric
acid, urea,or
glycine, makes useof
low-cost precursors resultingin
abased on the fact that a
wet
chemical technique (onetype
of
fuel-free combustion process) canform a stabie solution
with
better ion distribution[14].
This Drocessis
capableof
producing finepowder
after
combustion, andthe
experimentalsetup is very simple [15, 16]' Hence, in this work,
pure and
neodymium substituted-BlT
ate slmthesizedby
a
fuel-free
combustion processusing
aqueoussolutions
of
nitrate salts
andtitanium
(IV)
isoproPoxide.MethodologY
Bi4,Nd*Ti3Or2
(BNT)
systems where x -- 0,0.2,0.6 and
I
were
synthesized
via
fuel-freecombustion
process.
Bismuth
nitratepenthahydrate, Bi(NO3)3.5HzO and neodymium
nitrate
hexahydrate, Nd(NO3)3.6H2O
wereinitially
dissolved
in
2-Methaoxyethanol, CH3OCH2CHzOHat
40"C
on hot
plate
andstired
for
about 30
min.
Separately, titanium(N)
isopropoxide,
Ti[OCH(CH:)z],
wasdissolved
in
a
homogeneoussolution
of
2-MEand acetylacetone, CsHeO2 at
room
temperature and stirredfor
30 min. TheTi
solution was thenpoured
into
the
Bi
solution
with
continuousstiring
at 40oCfor
another2 h. After
that, thetemperature
of
hot plate was then set to reach at-130"C
to
for:rna
sticky
gel.
Within
a
fewseconds,
the
temperaturewas then
increasedrapidly,
andwhen
it
reachedto
-200oC, large amountof
gases(NH,,
Hz, CO2 and H2O) were liberated and a darkfluffy
powder was producedafter
the
combustion process. The process wasconsistently repeated
for
other powder
ratios.Their phase formation was determined
by XRD
(Bruker
D8
Advanced)
with
Cu
ko
(hor
= 1.5405A
and l.t*z:
1.5443A,
20 range between10o
and
90o,step size
of
0.034" (20),
fixeddivergence
slit:
0.2o, receiving slit = 0.2mm).In
addition,
the crystallite
sizewas
calculated byRietveld
method.
A
Zeiss
Supra
55VPPGT,IIKL Field
Emission
Scanning ElectronMicroscope (FE-SEM) was used
to
observe themicrostructure
and
morphology
in
the
bulkceramics. The process
flow
for the preparationof
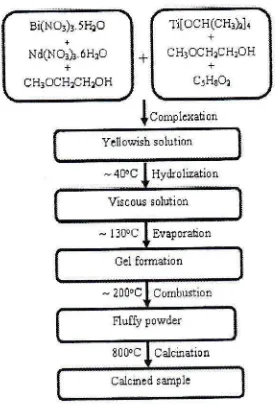
those powders is shown in Figure 1.
lnternational Conference on Materials Processing Technology 2010 January 5-6, 2010, Bangkok, THAILAND
[image:3.595.352.491.428.631.2]Result and discussion
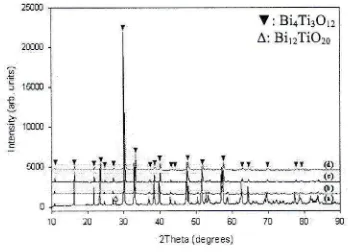
Figure
2
shows
the XRD
pattem
of
Bi+--Nd-Ti3O12 after calcination at 800oCfor
3h'
It
was found that
a
small amount
of
Bi12TiO2o intermediate phase was formedin
the pureBIT
sample, while no such an intemediate phase was observed
in
the sampleswith Nd
addition" Thisresult
indicatesthat the
peak corresponding tointermediate phase
had
reduced
as the
Ndcontent increases.
It
was similarly
reported inother work that the incorporation of
Nd
intoBIT
lattice
had
reduced
the
formation
of
theintermediate phase [8]. Other factor such as heat-treated at a higher temperature could reduce the
intermediate
phase
while
increase
thecrystallinity
of
BIT
phase.
Kan
et al.
ll7l
reported
that the
presence
of
BirzTiOzointermediate phase at 825oC was greatly reduced
after being heated
to
1000"C.
Tkough
thisanalysis,
the
intermediate
phase
in
BIT
compound can
be
reducedat a
temperature aslow
as 800"Cin
a
conditionof
introducing thelanthanide element.
Yellowish solutioq
-4loc I Hydrolsahon Viscous soiution - 1300C I Evaporahon
Ge1 fomation
- 2000C I Combustrot
Fluf$ powder
8!0oC I Calcinatiotr Calcucd smple
Figure 1 Process
flow
for the preparation of Bi+"Nd,Ti:Orz via fuel-free combustion process.
-zoo-2Theta (degrees)
Figure
2
XRD
patterns
of
Bi4Nd,Ti:Orz
powders calcined at 800"C
for
3h:
(a) pureBIT,
(b) 0.2BNT, (c) 0.6BNT and (d) i.OBNT.
It
was also found that
the
(117) peak
hadshifted
to high
angleof 20
(Figure3).
In
otherwords, the peak position at (117) had changed its characteristic as
a result of
Nd
addition. There were rwo things that could be observed from this characteristic.Firstly,
the peak intensityof
pureBIT
is relatively high as compared to other BNTsamples.
This
indicatesthat the crystallinity
of
pure
BIT is
the highest amongall.
However,it
was found
to
be decreased whenBi
atoms werethen
substitutedwith
Nd
atoms
in
bismuthtitanate system. Secondly, the peak
position
of
all
powders
varies
from the
Nd
(or
Bi)
concentration
in
accordanceto
Vegard's
Law,which
meansthat
the crystal lattice
parameter and the concentration of the constiruent elementshave
a
linear relation
that
exists
at
constant temperature [18].To
estimate qualitatively the lattice parameter aswell
as other structure, the Rietveld method was used from the TOPAZ software with yersion3.0
provided
by
Bruker
Advanced
X-RaySoiutions.
The
Nd
content dependenceof
thelattice parameter
'a', 'b',
'c'
andthe unit
cellvolume,'V'
of the powders is shown in Table 1.According
to
Table 1,BIT
and 0.2BNT had anorthorhombic strucrure by considering the lattice constant
of 'a'
is larger than thatof 'b'.
However,this
sttucture hashigher
tendencyto
form
intetragonal-type
with
additionalof Nd
content,It
was confirmed
by
the
lattice
of
'a'
and 'b'
Intemational Conference on Materials Processing Technology 2010 January 5-6, 2010, Bangkok, THAILAND
become closer" Besides
that, the shift
of
structural system
is
dueto
the
changein
ionicradius and atomic mass of Nd ions.
Having this is mind, the ionic radius of Nd3* is
about 0.1 11nm which is smaller than that of Bi3*
(0.117nm)
[19].
Thus, the
replacementof
Bi
atoms with Nd atoms would lead to the structural distortion from orthorhombic to tetragonal which
also resulted in reducing of cell unit volume, V.
In
Table 2, the crystallite size of those samplesprepared
in this
work was
significantlydecreased as increase the
Nd
contentin
bismuth titanate compound. The crystallite size measuredby
Scherrer's formula for pureBIT
is about 69.1nm
andit
is
also considered larger than otherBNT
samples"Upon
increasing theNd
content, the vacancy site that alreadyleft
byBi
atoms is then occupied byNd
atoms whichfinally
results the decreasein
degreeof crystallinity
aswell
ascrystallite
size.
This
is
parallel
with
thediffraction profile
obtainedby XRD
shown inFigure 3.
Table
I
Structure and parameter refinementusing Rietveld Method
Smple Pue BIT 0.281'{T 0 6BI'IT 1 0BNT
Space group Lattice prameter
Crystal sysleru
t rB ^6 v,.&
Densrty, g/rm' Refinemcnt index
R.-% D
B2cb
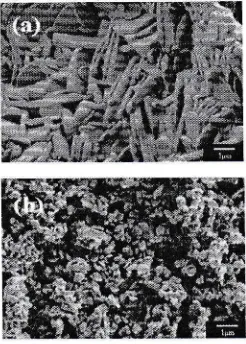
Figure
4
showsthe SEM
imagesof
pureBIT
and 1.0BNT which were calcined at 800"C
for
3h.
It
can be clearly seen that pureBIT
exhibits aplate-like
structure andits
sizeis
over
lpm
asshown
in
Figure
4(a).
On the
other hand,
a remarkable decreasein the grain
sizewith
less than 1pm was observedin
1.0BNT (Figure a(b)). From the size comparison between both samples,Orthorhombic Onhorhonibic Tetragorral Tetragonal
5.4432 5.4320 5.4052
5.40015,4015 5.4050 5.4175
5.403032.8127
328161
32.843'7 32.1928965.80 963.41 961.15
956.90'7.8354 7.4040
-7422']
'7.459610.65 12.15
4t2 t40
10.00
9 94I t5 000
-16t-it
is
reasonableto
say thatthe
substituted Nd3*ions act as a grain-growth
inhibitor in
BIT-typesystems. These results
are
consistentwith
theXRD
results where a decrease in peak sharpness and intensity was observed with Nd addition.Table 2: Crystallite size calculated
by
Scherrer's formulaPwe BIT 0 2BNI () 6BNT I,OBNI
lntemational Conltreec: oi,:. \ !a:ena-s P:oc es si:l-i
T:;:: :'.
:1'Ja:-'la;r j-,:. l'i - t-, Ba::-<;.'
li'.
-Conclusion
In
presentu'ork. pure
BIT
anil
B\-T
';':::
successfully prepared b1' a fue1-tree
conb.s:ii-synthesis.
The
influence
of \d
aioitr.-rl
o::bismuth
titanate-type system rvasclearlr'
see" through the eliminationof
intermediate phase.ri
BirzTiOzo in theXRD
pattern and the changedoi
peak characteristic at peak position (1 17). Uponincreasing of
Nd
content, the strucfural systemof
bismuth titanate
was also
changed
fromorthorhombic
to
fotm
in
tetragonal.
Suchinfluence
with this
addition was also shown inthe grain
morphologywhich
the grain
sizeof
pure
BIT
was recordedto
be over
lpm
but
it
tends
to
changeto
be
iessthan
ipm
with
Ndaddition.
Acknowledgement
The
authors
appreciatethe
technical support providedby
the School of Materials andMineral
ResourcesEngineering,
USM"
Thisresearch
was
supportedby
the
E-science Fund 305/Pbahan/6013357 and the USM Fellowship"References
lL)
Z.Lazarevi6,B. D.
Stojanovi6, J.A.
Varela,An
Approach
to
Analyzing
Synthesis,Structure
and
Properties
o.f
BismuthTitanate
Ceramics,
Sci.
Sintering,
3'7(200s) r99-216.
A.U.
A1-Amani,
S.
Sreekantan, M.N.Ahmad Fauzi, Ef.fect of
Bi
and Ti additionson
the phaseformation of
BiaTi3O12 usingfuel-free
combustion
method,
4'hIntemational
Conference
on
RecentAdvances
in
Materials,
Minerals,Environment and, 2"d
Asian
Symposium onmaterials
and
Processing
(RAMM
&
ASMP), Bayview Beach
Resort,
BatuFerringi, Penang, Malaysia, 1-3 Jun 2009.
A.M.
Umabala,
M.
Suresh),
A.V.
Prasadarao,
Bismuth titanate
.fro*
coprecipitated stoichiometric
hydroxideprecursors, Mater. Lett. 44 (2000) 175-1 80.
D.
Xie, Z,
Zhang, T. Ren,T. Liu, Y.
Dong,L.
Liu,
Properties
of
l,leodymium-DopedBiaTi jO
12 Thin
films
for
ferroelectric
Smple
Crysralilre ,lze, nfl 09. I 61.0
29 0 298 30 0 31.5
[image:5.595.77.280.227.415.2]2Theta (degrees)
Figure
3
XRD
patternsof
(a)
pure0.2BNT, (c) 0.6BNT
and(d)
1.OBNT powders at the (1 1 7) peak position. [image:5.595.126.249.484.656.2]41.9
Figure 4 Grains morphology
(a) pure
BIT
and (b) 1.0BNT.31 0
BIT,
(b)calcined
of caicined powder
12)
t3l
Random Access MemorY,
Integrated Fenoeiectric , 84 (2006) 6'7 ''73 .t5l
Q. Yang,
Y. Li,
Q.
Yin,
P. Wang, Y.B,
Cheng,
Bi[itOtz
nanoparticles ptepared by hydrothermal synthesis, J. Eur. Ceram' Soc',23 (2003)
l6l-166
t6l
H.L.W.
Chan, S.H. Choy, C.P. Chong, H.L.Li,
P.C.K. Lilu, Bismuth sodium
titanqtebased lead-free
ultrasonic
transducerfor
micro electronics wirebonding applications, Ceramics International, 34 (2008) 713-777.
11) Y. M.
Kan,
X.
H.
Jin, G. J. Zhang,P.
L.Wang,
Y.B.
Cheng,
and D.S.
Yan,Lanthanum
modified bismuth
titanateprepared by a hydrolysis method. J. Mater.
Chem. 2004, 14, 3566-357 0.
i8l
Y.M.
Kan,
G.J. Zhang,P.L. Wang,
Y.B.Cheng, Preparation
and
properties
of
neodymium-modified
bismuth
titanateceramics,
J. Eur.
Ceram.Soc.
28
(2008)164t-164',t.
t9l
L.B.
Kong,
J.
Ma,
W.
Zhu, O.K.
Tan,Preparation
of
BiaTijOl2
ceramicsvia
ahigh-energy
ball
milling process)
Mater.Lett. 51 (2001) 108-114.
t10l
X.Q.
Chen,
H.Y.
Qi,
Y.J.
Qi,
C.J.
Lu,Ferroelectric and dielectric properties
of
bismuth
neodymium
titanate
ceramicsprepared
using sol-gel
derived
-finepowders, Phys" Lett.
A
346 (2005) 204-208't11l
D.
Chen,X.
Jiao, Hydrothermal synthesisand characterization
of
Bi{i:Op
powdersfrorn dffirent
precursors, Mater. Res. Buil.36 (2001) 3ss-363.
[12]
S.Luo, Z.
Tang,W. Yao, Z.
Zhang,Low-temperature combustion synthesis
andcharacterization
of
nanosized tetragonalbarium
titanate powdets,
Microelectron.Eng. 66 (2003) 14'7-152.
[13]
K.C.
Patil,
M.S.
Hedge,
T.
Rattan,
S.T"Aruna, Chemistty of
Nanooystalline
OxideMaterials Combustion Synthesis, Properties
Intemational conference on Mnefi_f
:
i;iS:Hli:;Tli'trli$tj
and
Applications,
World
Scientif,rcPublishing Co. Pte.
Ltd.,
Singapore, 2008,pp-42.
t14l
A.
Ries,A.Z.
Simo'es,M.
Cilense,M.A'
Zaghete,
J.A.
Varela, Barium
strontiumtitonate
powder
obtained
by
polymericprecursor
method,
Mater
Charact'
50(2003)
2r1-22t.
[15]
P.Y.
Goh,
K.A.
Razak,
S.
Sreekantan,Structural
and
morphology studies
of
praseodymium-doped
bismuth
titanateprepared using
a
wet
chemicalroute,
J'Alloys
ComPd. 415 (2009)758-761,[16]
J.C. Baeet
al. Ferroelectricproperties
of
lanthanum-doped
bismuth titanate
thin.films grown
by
a
sol-gel
method,Thin
Solid Films 412 (200s) 90-95.
[17]Y.
Kan,X,
Jin, P. Wang,Y. Li, Y.B.
Cheng,D.
Yan,
Anisotropic
grain
growth
ClBiqTisOtz
in
molten salt Jluxes, Mater. Res.Bull.
38 (2003) s67-s76'[18]
P.Y.
Goh,
K.A.
Razak,
S"
Sreekantan,Structural
and
morphology studies
"J'praseodymium-doped
bismuth
titqnateprepared using
a
wet
chemicalroute,
I'
Alloys
Compd. 475 (2009)758-761,t19l
X.
Hu,
A.
Garg,Z. H.
Barber, Structutaland
electrical properties
of
samarium-substituted bismuth titanate
ferroelectricthin
films
on
Pt/TiOx/SiO2/Si substrates,