EFFECTS OF CPPU AND CoSO
4ON POSTHARVEST
QUALITY OF MANGOSTEEN FRUIT (G
arcinia mangostana
L.)
DURING STORAGE
CHEA SINATH
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
DECLARATION
I declare that this thesis titled “Effects of CPPU and CoSO4 on postharvest quality
of mangosteen fruit (Garcinia mangostana L.) during storage.” was entirely
completed by myself with resourceful help from the Department of Agronomy
and Horticulture, Bogor Agricultural University. Information and quotes which
were sourced from journals and books have been acknowledged and mentioned
where in the thesis they appear. All complete references are given at the end of the
paper.
Bogor, September 2010
Chea Sinath
ABSTRACT
CHEA SINATH. A252098171. Effects of CPPU and CoSO4 on Postharvest Quality
of Mangosteen Fruit (Garcinia mangostana L.) during Storage. (Under supervision of ROEDHY POERWANTO, DARDA EFENDI, and SUTRISNO)
Mangosteen (Garcinia mangostana L.) fruit is one of the most delicious
tropical fruits, known as “queen of tropical fruit” with short shelf life during
storage. The research objective was to study the effects of CPPU and CoSO4 on
postharvest quality and physiological changes of mangosteen fruit during storage.
The experimental design was arranged in a completely randomized block design
(CRBD) with two factors, i.e., cobalt sulphate and CPPU. Postharvest treatment
with CPPU was found to be effective to promote lightness of mangosteen fruit at
day 4 and day 6, while CoSO4 tended to accelerate the darkening in both fruit and
sepal. The resistance of mangosteen fruit pericarp was considered as low (less
than 2 kgf/cm2), representing that fruit were still easily to be opened during 30
days of storage. CPPU and CoSO4 were less effective in inhibiting fruit
hardening. Titratable acidity was significantly reduced in CPPU-treated fruit at the
end of storage, while total soluble solids were not clearly affected by the
treatment. TSS/TA ratio was significantly higher in fruit treated with CPPU at the
end of storage. Respiration rate of mangosteen fruit was low and remained
constant with prolonged storage period. CPPU and/or CoSO4 could considerably
decrease the respiration rate, but less effective in inhibiting ethylene production
during storage at 14oC-16oC (78%-96% RH).
ABSTRAK
CHEA SINATH. A252098171. Pengaruh CPPU dan CoSO4 terhadap Kualitas
Pascapanen Buah Manggis (Garcinia mangostana L.) selama Penyimpanan. (Dibimbing oleh ROEDHY POERWANTO, DARDA EFENDI, dan SUTRISNO)
Buah manggis (Garcinia mangostana L.) merupakan salah satu buah
tropis yang paling lezat, yang dikenal sebagai "queen of tropical fruit" dengan
masa simpan rendah selama penyimpanan. Tujuan penelitian ini adalah untuk
mempelajari pengaruh CPPU dan CoSO4 terhadap kualitas pascapanen dan
perubahan fisiologis buah manggis selama penyimpanan. Rancangan percobaan
menggunakan rancangan acak kelompok lengkap (RAKL) dengan dua faktor,
yaitu, kobalt sulfat dan CPPU. Perlakuan pascapanen dengan CPPU ditemukan
efektif untuk mempromosikan kecerahan buah manggis pada hari ke 4 dan 6,
sedangkan CoSO4 cenderung mempercepat gelap baik pada buah atau sepal.
Resistensi pericarp buah manggis dianggap rendah (kurang dari 2 kgf/cm2),
menunjukkan bahwa buah masih mudah untuk dibuka selama 30 hari
penyimpanan. CPPU dan CoSO4 kurang efektif menghambat pengerasan buah.
Asam tertitrasi berkurang secara signifikan dalam buah pada perlakuan degnan
CPPU di akhir penyimpanan, sedangkan total padatan terlarut tidak jelas
terpengaruh oleh pelakuan. Rasio PTT/AT secara signifikan lebih tinggi dalam
buah yang diberi perlakuan dengan CPPU di akhir penyimpanan. Laju respirasi
buah manggis masih rendah dan tetap konstan dengan periode penyimpanan yang
lama. CPPU dan / atau CoSO4 dapat menurunkan laju respirasi, tetapi kurang
efektif dalam menghambat produksi etilen selama penyimpanan pada 14 oC-16 oC
(78% -96% RH).
SUMMARY
CHEA SINATH. A252098171.
Effects of CPPU and CoSO4 on Postharvest Quality of Mangosteen Fruit (Garcinia mangostana L.) during Storage. (Under supervision of ROEDHY POERWANTO, DARDA EFENDI, and SUTRISNO)Physical, chemical and physiological changes during storage are
common phenomena occurring in all commodities. Postharvest quality is
strongly associated with those changes and depends heavily on commodity
characteristics, preharvest factor, postharvest treatment and storage
condition. Physiochemical changes of climacteric products during storage are
substantially triggered by its respiratory rate and ethylene production. Since mangosteen
fruit is climacteric fruit, postharvest attributes may be related to climacteric respiration
and ethylene production. Postharvest application of substances that can suppress
respiration and ethylene production may help prolong shelf-life of mangosteen fruit
during storage. The objective of the research was to study the effects of CPPU and CoSO4
on postharvest quality and physiological changes of mangosteen fruit during storage.
The research was done at Postharvest Laboratory, Faculty of Agriculture,
Laboratory of Food and Agricultural Product Process Engineering, Laboratory of
Environmental and Agricultural Building, Faculty of Agricultural Technology, Bogor
Agricultural University (IPB), starting from February to May 2010. The research was
divided into two stages. The first experiment covered physical and chemical changes of
mangosteen fruit treated with CPPU and CoSO4 during storage, while the second one
involved the study of physiological and its relation to color development of mangosteen
fruit treated with CPPU and CoSO4 during storage. The experimental design was
arranged in a completely randomized block design with 2 factors and 3 replications. The
first factor was cobalt sulphate (CoSO4) at four concentrations 0, 500, 1000, and 2000
ppm, while the second one was CPPU at four concentrations 0, 10, 20, and 30 ppm. The
combination of the above factors provided 16 treatments with 48 experimental units. Each
experimental unit comprised 40 mangosteen fruit. For the second experiment, the design
was as in the first experiment, but only cobalt sulphate at 0, 2000 ppm, and CPPU at 0, 30
ppm were used with 5 mangosteen fruit per experimental unit. The fruits used in the
on the same day and were of similar sizes. The harvested fruit were transported at night to
laboratory. In the following morning, the fruit were sorted, and washed with tap water to
remove the dust. After washing, the fruit were air dried, and then treated with solution of
fungicide TBZ 1 ppm for 30 seconds and air dried. Following fungicide application,
air-dried fruit were dipped in the solution of CoSO4 and CPPU for 30 seconds according to
its concentrations used in the treatments. To facilitate the absorption of the solution by
fruit, tween20 (1%) was added. After application, treated fruits were air dried, then stored
at 14-16oC (76-96% RH).
Fruit resistance (FR), pericarp water content (PWC), fruit and sepal color, weight
loss (WL), total soluble solids (TSS), and titratable acidity (TA) were measured every
two days in the experiment one. Respiration rate was measured every 3 hours on the first
day, followed by 6 hours in the second day, 12 hours, and 24 hours in the following days
up to day 27. Ethylene production was measured every day up to storage day 12.
Analysis of variance was done using SPSS statistics 17.0 and treatment means were compared using Duncan‟s Multiple Range Test (DMRT) at P<0.05.
The results showed that Postharvest treatment with CPPU was found to be effective
to promote lightness of mangosteen fruit at day 4 and day 6, while CoSO4 tended to
accelerate the darkening in both fruit and sepal. The resistance of mangosteen fruit was
considered as low (less than 2 kgf/cm2), representing that fruit were still easily to be
opened during 30 days of storage. CPPU and CoSO4 were less effective in inhibiting fruit
hardening. Titratable acidity was significantly reduced in CPPU-treated fruit at the end of
storage, while total soluble solids were not clearly affected by the treatment. TSS/TA
ratio was significantly higher in fruit treated with CPPU at the end of storage. Respiration
rate of mangosteen fruit was low and remained constant with prolonged storage period.
CPPU and/or CoSO4 could considerably decrease the respiration rate, but less effective in
inhibiting ethylene production during storage at 14-16oC (78%-96% RH).
In conclusion, Postharvest treatments of either CPPU or CoSO4 were found to have
low effectiveness on the component of postharvest quality of mangosteen fruit although
sporadic significances were observed among observed times. Mangosteen fruit is a
climacteric fruit with high ethylene production after harvest. However, no clear peak of
respiration was observed during cold storage. Fruit color changes were closely associated
with ethylene production.
Keywords: Climacteric fruit, shelf life, color development, ethylene production,
© Copyright of IPB, year 2010
Copyright reserved
1. Forbidden to quote part or all of these writings without including or
mentioning the source.
a. Be cited only for educational purposes, research, writing papers,
drafting reports, writing criticism or review an issue;
b. Quotation must not harm the affairs of IPB.
2. Prohibit publication and reproduction of part or all of the paper in any
EFFECTS OF CPPU AND CoSO
4ON POSTHARVEST
QUALITY OF MANGOSTEEN FRUIT (G
arcinia mangostana
L.)
DURING STORAGE
CHEA SINATH
A Thesis
As Partial fulfillment of the Requirement to obtain
Master of Science Degree in Agronomy and Horticulture
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
Title : Effects of CPPU and CoSO4 on Postharvest Quality of
Mangosteen Fruit (Garcinia mangostana L.) during
Storage.
Name : Chea Sinath
Registration Number : A252098171
Major : Master of Science in Agronomy and Horticulture
Approved:
Advisory Committee
Prof. Dr. Ir. Roedhy Poerwanto, M.Sc
(Chairman)
Dr. Ir. Darda Efendi, M.Si Dr. Ir. Sutrisno. M.Agr
(Member) (Member)
Agreed:
Graduate Coordinator of Major Dean of Graduate School
Dr. Ir. Munif Ghulamahdi, MS Prof. Dr. Ir. Khairil Anwar Notodiputro, MS
ACKNOWLEDGEMENT
My study and thesis research would not have been accomplished without the
help of many people.
Special thanks to Prof. Dr. Ir. Roedhy Poerwanto, MSc, Dr. Ir. Darda
Efendi, MSi, and Dr. Ir. Sutrisno, M.Agr, as supervisory committee, for all of
guidance and encouragement as well as invaluable academic advices for the whole
period of my study and research at IPB.
Special thanks also are to be given to the Department of Agronomy and
Horticulture and Department of Agricultural Technology for the full support given
to enable the successful completion of this research. I am very grateful to all the
invaluable lecturing staff as well as lab and technical staff of Agronomy and
Horticulture Department who have imparted knowledge and help.
Extended thanks are expressed to all fellow students of Agronomy and
Horticulture Department and KNB Scholarship. Of these fellows, I would like to
express my deepest thanks to close friends Sanou Faye, Dahono and Herman who
always providing a helping hand and good advices. I wholeheartedly thank to
research team of mangosteen fruit postharvest such as Ismadi, Mesil, and Hatifah,
who made my master research a great experience of team working.
Further, I am highly indebted to my affectionate parents, brother (Na
Bunnan), sisters (Na Dany, Na Chantha, Na Chan Thu, Chea Veasna, Chea Ratana
and Chea Phalla) and other family members who always inspire and encourage
me for higher education, and finally to Miss Bao Lian who contributes immensely
in providing psychological supports and good constructed advice during busy time
of my study and research.
The research was financially supported by Hibah Tim Pasca Sarjana, and
additional funding was provided through my study sponsor-Dikti under
Developing Countries Partnership Program.
Bogor Agricultural University, September 2010
BIOGRAPHY
Chea Sinath was born on the 2nd of March, 1981 in Takeo province to Mr
Na Cheam and Mrs Sok Kimngy from Cambodia. Chea Sinath was born the fifth
of eight children.
In 2001, he finished Senior High School from Heng Somrin Prey Lovea
High School and continued with Bachelors Degree study in Agronomy and
graduated in August 2005, from the Royal University of Agriculture. After
Bachelors level, he had worked for Cambodian Agricultural Research and
Development Institute (CARDI) as research assistant in Agronomy and Farming
systems office for a year. In 2006, he continued with higher teacher training
(Bachelor +1) at National Institute of Education and started teaching at Prek Leap
High School located in Phnom Penh since 2007. In 2008, he was awarded by the
Indonesian Government to do Masters Degree in Bogor Agricultural University,
majoring in Agronomy and Horticulture under the Developing Countries
Partnership Program (KNB – Kemitraan Negara Berkembang) with official
permission from the Cambodian Ministry of Education, Youth and Sports for a
TABLE OF CONTENTS 2.1. Mangosteen Fruit Development and Maturity Indices ... 3
2.2. Mangosteen Fruit Quality and Color Development ... 5
2.3. Causes of Mangosteen Fruit Hardening ... 6
2.4. Fruit Ripening and Senescence ... 7
2.5. Ethylene Biosynthesis and its Physiological Effects ... 9
2.6. Ethylene Action and Methods for Inhibiting Ethylene Responses ... 11
2.7. Cytokinin and its Physiological Effects ... 12
2.8. Cobalt Sulphate (CoSO4) and its Effects ... 13
III. MATERIAL AND METHODS 3.1. Time and Place ... 14
3.2. Plant Material and Treatment... 14
3.3. Observed Variables ... 15
3.3.2. Chemical Attributes... 16
3.3.2.1.Total Soluble Solids ... 16
3.3.2.2.Titratable acidity ... 16
3.3.3. Physiological Variables ... 17
3.3.3.1.Respiration Rate ... 17
3.3.3.2.Ethylene Production ... 17
3.4.Statistical Analysis ... 18
IV. RESULTS AND DISCUSSION 4.1. Physical Changes of Mangosteen Fruit during Storage ... 19
4.1.1. Fruit Resistance ... 19
4.1.2. Pericarp Water Content ... 20
4.1.3. Weight Loss ... 21
4.1.4.1.Lightness ... 22
4.1.4.2.a/b ratio ... 24
4.1.4.3.Hue angle (ho) ... 25
4.1.5. Sepal Color ... 26
4.1.5.1.Lightness ... 26
4.1.5.2.a/b ratio and Hue angle ... 27
4.1.6. Visual Observation ... 30
4.2. Chemical Changes of Mangosteen Fruit during Storage ... 31
4.2.1. Total Soluble Solids (oBrix) ... 31
4.2.2. Titratable Acidity (%) ... 32
4.2.3. TSS/TA ratio ... 34
4.3. Physiological Changes and its Relation to Color Development ... 35
4.3.1. Respiration Rate of Mangosteen Fruit ... 35
4.3.2. Ethylene Production of Mangosteen Fruit ... 37
4.3.3. Lightness and Hue angle of Mangosteen Fruit ... 38
V. CONCLUSIONS AND SUGGESTION ... 40
REFERENCES ... 41
LIST OF TABLES
Page
1 Harveste mangosteen maturity indices developed in Malaysia ... 4
2 Effects of CPPU and CoSO4 interaction on pericarp water content (%)
of mangosteen fruit during storage at day 18 ... 21
3 Effects of CPPU and CoSO4 interaction on a/b ratio of sepal of
mangosteen fruit during storage at day 22 and day 26 ... 29
4 Effects of CPPU and CoSO4 interaction on hue angle of sepal of
mangosteen fruit during storage at day 22 and day 26 ... 30
5 Effects of CPPU and CoSO4 interaction on TSS &TA ratio of
mangosteen fruit during storage at day 18 ... 35
6 Effects of CPPU and CoSO4 interaction on respiration rate of
mangosteen fruit during storage ... 37
LIST OF FIGURES
Page
1 The ethylene synthesis pathway ... 10
2 Resistance of mangosteen fruit treated with CPPU during storage ... 20
3 Resistance of mangosteen fruit treated with CoSO4 during storage ... 20
4 Weight loss of mangosteen fruit treated with CPPU during storage ... 22
5 Weight loss of mangosteen fruit treated with CoSO4 during storage ... 22
6 Changes in skin lightness of mangosteen fruit treated with CPPU during storage ... 23
7 Changes in skin lightness of mangosteen fruit treated with CoSO4 during storage ... 23
8 Changes in skin color (a/b) of mangosteen fruit treated with CPPU during storage ... 24
9 Changes in skin color (a/b) of mangosteen fruit treated with CoSO4 during storage ... 24
10 Changes in skin color (ho) of mangosteen fruit treated with CPPU during storage ... 25
11 Changes in skin color (ho) of mangosteen fruit treated with CoSO4 during storage ... 25
12 Changes in sepal color (L) of mangosteen fruit treated with CPPU during storage ... 26
13 Changes in sepal color (L) of mangosteen fruit treated with CoSO4 during storage ... 27
14 Changes in sepal color (a/b) of mangosteen fruit treated with CPPU during storage ... 27
15 Changes in sepal color (a/b) of mangosteen fruit treated with CoSO4 during storage ... 28
16 Changes in sepal color (ho) of mangosteen fruit treated with CPPU during storage ... 28
17 Changes in sepal color (ho) of mangosteen fruit treated with CoSO4 during storage ... 29
18 Changes in total soluble solids of mangosteen fruit treated with CPPU
during storage ... 32
19 Changes in total soluble solids of mangosteen fruit treated with CoSO4
during storage ... 32
20 Changes in titratable acidity of mangosteen fruit treated with CPPU
during storage ... 33
21 Changes in titratable acidity of mangosteen fruit treated with CoSO4
during storage ... 33
22 Changes in TSS/TA of mangosteen fruit treated with CPPU
during storage ... 34
23 Changes in TSS/TA of mangosteen fruit treated with CoSO4
during storage ... 35
24 Respiration rate of mangosteen fruit treated with CPPU and CoSO4
during storage ... 36
25 Ethylene production of mangosteen fruit treated with CPPU and CoSO4
during storage ... 38
26 Changes in lightness of mangosteen fruit treated with CPPU and CoSO4
during storage ... 39
27 Changes in Hue angle (ho) of mangosteen fruit treated with CPPU
and CoSO4 during storage ... 39
LIST OF APPENDICES
Page
1 Effects of CPPU and CoSO4 on fruit resistance (kgf/cm2) of mangosteen
fruit during storage ... 47
2 Effects of CPPU and CoSO4 on pericarp water content (%) of
mangosteen fruit during storage ... 48
3 Effects of CPPU and CoSO4 on weight loss (%) of mangosteen fruit
during storage ... 49
4 Effects of CPPU and CoSO4 on lightness (L) of mangosteen fruit
during storage ... 50
5 Effects of CPPU and CoSO4 on a value (red-green) of mangosteen fruit
during storage ... 51
6 Effects of CPPU and CoSO4 on b value (yellow-blue) of mangosteen
fruit during storage ... 52
7 Effects of CPPU and CoSO4 on a/b value of mangosteen fruit during
storage ... 53
8 Effects of CPPU and CoSO4 on hue angle of mangosteen fruit during
storage ... 54
9 Effects of CPPU and CoSO4 on lightness (L) of sepal of
mangosteen fruit during storage ... 55
10 Effects of CPPU and CoSO4 on a value (red-green) of sepal
of mangosteen fruit during storage ... 56
11 Effects of CPPU and CoSO4 on b value (yellow-blue) of sepal
of mangosteen fruit during storage ... 57
12 Effects of CPPU and CoSO4 on a/b ratio of sepal of mangosteen fruit
during storage ... 58
13 Effects of CPPU and CoSO4 on hue angle of sepal of mangosteen fruit
during storage ... 59
14 Effects of CPPU and CoSO4 on total soluble solids (oBrix) of
mangosteen fruit during storage ... 60
15 Effects of CPPU and CoSO4 on titratable acidity (%) of mangosteen
fruit during storage ... 61
16 Effects of CPPU and CoSO4 on TSS &TA ratio of mangosteen fruit
during storage ... 62
17 Changes in respiration rate of mangosteen furit treated with CPPU
and CoSO4 during storage ... 63
18` Ethylene production of mangosteen fruit treated with CPPU and CoSO4
during storage ... 65
19 Images of mangosteen fruit treated with CPPU and CoSO4
during storage ... 66
I. INTRODUCTION
1.1 Background
Indonesia is one of the major mangosteen producers in Southeast Asia. The
production of mangosteen in 2007 was 112,722 ton with average yield of 9.42
ton/ha. Indonesia exports fresh mangosteen to China (including Hong Kong and
Taiwan), Japan, Singapore, the Netherlands, France and Saudi Arabia (Osman and
Milan, 2006). The volume of export was 9,093,245 kg in 2007 and increased to
9,465,665 kg in 2008, which valued at USD 4,951,442 and USD 5,832,534 in
2007 and 2008, respectively (Ditjen Hortikulura, 2009). Indonesian mangosteen
export is only 8.06% from the total production 112,722 ton in 2007. Many issues
are involved in low fruit quality and have resulted in a barrier for exports and low
export value, namely (i) gamboge, a physiological disorder evident by exudating
latex onto the fruit surface and aril rendering the fruit unsuitable for eating, (ii)
brown spot (burik) on the fruit skin, and (iii) short shelf life of mangosteen fruits
(pericarp hardening, color changes to dark blackish purple, and fruit calyx turns
brown within a few day).
Generally, the fruit will soften within a few days after harvested. On the
contrary, mangosteen fruit hardens and causes difficulty in opening after
prolonged storage. Mechanical injury of fruit during storage and handling of
mangosteen often causes fruit hardening (Kader, 2003). A drop of 10 cm can
cause slight pericarp damage, indicated as hardening at the point of impact within
24 h. Higher drops cause significantly greater damage and often lead to
downgrading of the fruit (Tongdee and Suwanagul, 1989; Ketsa and Atantee,
1998).
Azhar (2007) reported that optimal temperature for endurance of skin color
and calyx was 10 oC, and temperature storage less than 10 °C (50 oF) leads to
rapid hardening and darkening of pericarp when fruit are returned to ambient
temperature (Uthairatanakij and Ketsa, 1995). Storage at 4 °C (39.2 °F) or 8 °C
(46.4 °F) can lead to significant hardening of the pericarp (Augustin and Azudin,
1986), although the flesh may still be acceptable after 44 days. However, storage
of mangosteen fruit at temperature above 15 oC can also cause hardening and
treatment was more effective to inhibit pericarp hardening, and shelf-life of
mangosteen fruit could be kept as long as 20 days after treatment (Ekaputri,
2009). Changes of fruit color are one of maturity parameter. Inayati (2009) found
that fruits coated with wax, chitosan and coconut oil suspension could inhibit fruit
hardening from 14 days to 20 days.
In climacteric fruits, ethylene production at the onset of ripening controls
the changes of color, aroma, texture, flavour, and other biochemical and physical
attributes (Lelievre et al., 1997). Since mangosteen fruit is climacteric fruit
(Qanitah, 2004), postharvest quality changes may be related to climacteric
respiration and ethylene production. Co2+ is a potent inhibitor of ethylene
biosynthesis. Application of cobalt sulphate is expected to change the climacteric
respiration of mangosteen during storage. Williams and Golden (2002) found that
the enzyme ACC was inhibited by Cobalt sulphate (CoSO4). Forchlorfenuron
(CPPU) is a type of synthetic cytokinins, which is responsible for the maintenance
of chlorophyll, protein, and RNA levels. Although CPPU has been found to be
effective in improving postharvest quality of many fruits when applied at
preharvest, its direct postharvest application has not yet been reported. Therefore,
research on postharvest application of CPPU and CoSO4 needs to be conducted.
1.2 Research Objective
The objective of the research was to study the effects of CPPU and CoSO4
on postharvest quality and physiological changes of mangosteen fruit during
storage.
2. Application of CPPU can delay changes of sepal color because it can delay
chlorophyll degradation.
3. Combination of CPPU and CoSO4 can extend shelf-life of mangosteen fruit
II. LITERATURE REVIEW
2.1 Mangosteen Fruit Development and Maturity Indices
Mangosteen fruits are produced singly at the end of the branchlets, and
usually do not mature and ripen uniformly. The tree will bear coexisting
generations of fruit, resulting from successive generations of flowers. Therefore,
not all the fruits will reach maturity or ripen at the same time. Mangosteen fruits
take 5 to 6 months to mature from fruit set. Initially, fruit growth is dominated by
the pericarp, with aril dry matter not increasing until 20 days from anthesis and
then continuing throughout the fruit development. At 13 weeks the fruit shows
the highest percentages of pulp, rind, sugar and acid: 29.37%, 69.14%, 18% and
0.49%, respectively (Kanchanapom and Kanchanapom, 1998). During ripening, a
thick, clear green cortex changes to dark purple or red purple. Enclosed by the
rind are 4-8 edible white segments. The flavor is slightly acidic, but sweet
(Nakasone et al., 1998).
At present, there is no standard or uniform maturity index that is universally
used. Countries such as Malaysia, Thailand and Australia have developed their
own maturity indices for harvesting to meet various marketing purposes. For
harvesting maturity indices used by growers in Australia, fruit harvested at stage 1
with pale yellow-green color, pH 3.9, rind thickness 9.0 mm, Brix%<12, and
firmness index 7.9, and stage 2 with botchy pink color, pH 3.3, rind thickness
7.7mm, Brix%<14 and firmness index 6 are not acceptable. Fruit harvested at
stage 3 with pinkish red, pH 3.2, rind thickness 7.4mm, Brix%>16, and firmness
index =5; fruit harvested at stage 4 with maroon-red, pH 3.2, rind thickness
7.2mm, Brix%>16, firmness index 5; and stage 5 with maroon-violet, pH 3.2,
rind thickness 7.0mm, Brix%>16, firmness index=5 are acceptable. Fruit
harvested at stage 6 with violet-black, pH 3.6, rind thickness 6.8mm, Brix%<14,
and firmness index=5 is also unacceptable. However, Tongdee and Suwanagul
(1989) reported that fruits are at the edible, ripe stage when the skin has darkened
to a reddish purple, no latex remains in the skin, and the flesh segments separate
easily from the skin, and soluble solids content ranges from 17 to 20% and
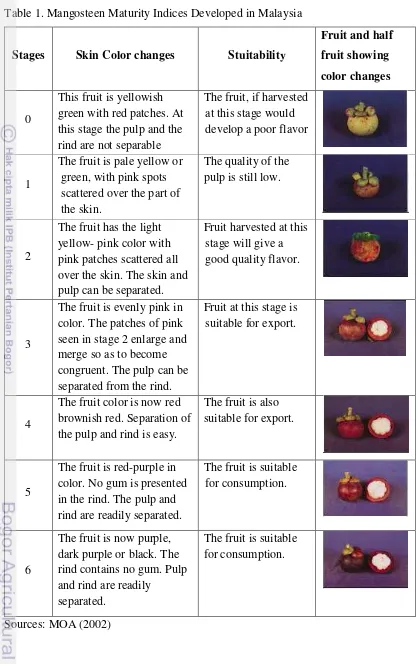
Table 1. Mangosteen Maturity Indices Developed in Malaysia
Stages Skin Color changes Stuitability
Fruit and half this stage the pulp and the rind are not separable
The fruit, if harvested at this stage would develop a poor flavor
1
The fruit is pale yellow or green, with pink spots over the skin. The skin and pulp can be separated.
Fruit harvested at this stage will give a good quality flavor.
3
The fruit is evenly pink in color. The patches of pink seen in stage 2 enlarge and merge so as to become
2.2 Mangosteen Fruit Quality and Color Development
The quality of mangosteen (Garcinia mangostana L.) fruit is measured not
only by external factors such as color, shape, size, skin blemishes, latex staining
and insect damage, but also by internal factors such as translucent flesh, yellow
gummy latex and hardening pericarp which are also very important for consumer
acceptance (Teerachaichayut et al., 2006). Fruit color is a major criterion used to
judge maturity and for grading of mangosteen fruit. The fruit are usually harvested
at different stages according to colour, from light greenish yellow with scattered
pink spots to dark purple. After harvest, the purple color continues to develop very
quickly. For high fruit quality, the minimum harvest color stage is that of distinct
irregular, pink–red spots over the whole fruit. If fruit are harvested with a light
greenish yellow with scattered pink spots, the fruit do not ripen to full flavor
(Tongdee and Suwanagul, 1989; Paull and Ketsa, 2004). `
The color of mangosteen (Garcinia mangostana L.) fruit changes from
green to purple black after harvest as the fruit ripens, and is used as a quality
guide for growers and consumers. During the postharvest period, hue angle values
and pericarp firmness decreased significantly, while soluble solids contents
increased. Anthocyanin contents in the outer pericarp were higher than in the
inner pericarp and increased to a maximum at the final color stage (Palapol et al.,
2009). Commercial production has been limited by slow growth, long juvenile
periods of 10-15 years and short shelf-life of fruits when mature (Wiebel et al.,
1992). Keeping quality of mangosteen fruits is longer compared to other tropical
fruits. Long storage of fruits leads to hardening of pericarp and opening of fruit
become difficult (Radha et al., 2007). Palapol et al. (2009) indicated that fruit
harvested at stage 1 developed rapidly to the purple black stage (stage 6) within 9
days with color development from stage 5 to stage 6 being slower than other
stages. During color development, the a*/b* value increased slightly from stage 1
to stage 3, and then increase sharply to stage 6. The increase in the a*/b* values
correlated well with color development. When fruit at the six different stages of
maturity were harvested and kept at 25 oC, each stage fully developed to the
purple black stage 6. No matter at what stage the fruit were harvested, they all
including hue angle values, firmness, soluble solids content (SSC) and titratable
acid (TA), when the fruit were accessed at stage 6 (Palapol et al., 2009).
Calyx freshness of mangosteen fruit strongly affects quality value during
periods of storage. Fresh mangosteen fruit has green and fresh calyx, but the
freshness becomes brown after a few days. Research conducted by Suryanti et al.
(1999) showed that mangosteen fruit harvested at green and fresh peel with purple
spots and calyx freshness could maintain its freshness for 6 days of storage.
Optimum temperature for retention of peel and calyx color is 10 oC (Azhar, 2007).
Ekaputri (2009) found that treatment of chitosan 1.5% could retain color of
mangosteen peel and calyx. Beeswax 6% treatment and BAP 20 ppm could retain
calyx color for 21 days after treatment, but it started wrinkling after 15 days of
treatment (Pratiwi, 2008). Anggraeni (2008) reported that combination of 0.01
mm- thick plastic wrapping and chitosan coating 1.5%, and plastic wrapping with
wax Britex gave a better effect on inhibiting changes of peel and calyx color for
30 days of storage at room temperature, and 35 days at temperature 15oC.
2.3 Causes of Mangosteen Fruit Hardening
Generally the fruit will soften during a few days after harvested, but
mangosteen fruit become harden that causes difficulty in opening fruits for
consumption. Mechanical injury of fruit during storage and handling of
mangosteen often causes fruit hardening (Kader, 2003). A drop of 10 cm can
cause slight pericarp damage, indicated as hardening at the point of impact within
24 h. Higher drops cause significantly greater damage and often lead to
downgrading of the fruit (Tongdee and Suwanagul, 1989; Ketsa and Atantee,
1998).
Storage temperature less than 10°C (50 oF) leads to rapid hardening and
darkening of pericarp when fruit are returned to ambient temperature
(Uthairatanakij and Ketsa, 1995). Storage at 4 °C (39.2 °F) or 8 °C (46.4 °F), can
lead to significant hardening of the skin (Augustin and Azudin, 1986), although
the flesh may still be acceptable after 44 days. Ideal storage temperature for
mangosteen is 15oC. Temperature above 15oC mangosteen fruit hardens and
Anggraini (2008) reported that chitosan-coated treatment gave better effects
on inhibiting pericarp hardening change, while shelf-life of mangosteen fruit
could be kept as long as 20 days after treatment (Ekaputri, 2009). Dangcham et al.
(2008) found that when pericarp hardening occurred, pericarp firmness and lignin
contents increased while total phenolics decreased and fruit at the red-brown and
red-purple maturity stages stored at 6oC had higher lignin contents than of those
stored at 12 oC. Of the phenolic acids predominant in the hardened pericarp, p
-coumaric acid declined whereas sinapic acid increased throughout the storage
time. Application of low O2 (0.25%) to red-purple fruit during storage at 6 oC
(84% RH), or at room temperature (30 oC, 71.5% RH) following storage at 6 oC,
did not reduce pericarp hardening and there were no significant differences in
firmness, lignin and total free phenolics when compared with fruit in normal air
conditions. The results also suggested that increase in pericarp firmness of
mangosteen fruits results from induction of lignin synthesis, associated with an
increase in phenylalanine ammonia (PAL) and peroxydase (POD) activity and
gene expression. Recent research conducted by Palapol et al. (2009) showed that
pericarp firmness of mangosteen fruit decreased from stage 1 to stage 6, 779.3,
201.3, 136.0, 98.4, 66.5 and 46.5 N, respectively when stored at temperature
25oC. Increases in pericarp lignin contents are at least part of the reason for the
tissue hardening. Bunsiri (2003) found that 3 hours after impact, lignin contents
increased in the damaged pericarp.
2.4. Fruit Ripening and Senescence
Ripening and senescence are the ultimate phases in the developmental
events of fruits that result in the expression of the quality characteristics inherent
to the fruit (Paliyath et al., 2008) and this phenomenon involves structural,
biochemical, and molecular changes that in many cases bear the hallmarks of
programmed cell death (Arora, 2008). Degradation of structural elements such as
the cell wall and the plasma membrane results in a loss of compartmentalization
of ions and metabolites, leading to the loss of tissue structure and ultimately
Fruit ripening is accompanied by a number of biochemical events, including
changes in color, sugar, acidity, texture, and aroma volatiles that are crucial for
the sensory quality. At the late stages of ripening, some senescence-related
physiological changes occur that lead to membrane deterioration and cell death.
All biochemical and physiological changes that take place during fruit ripening
are driven by the coordinated expression of fruit ripening-related genes. These
genes encode enzymes that participate directly in biochemical and physiological
changes. They also encode regulatory proteins that participate in the signaling
pathways, and in the transcriptional machinery that regulate gene expression and
set in motion the ripening developmental program (Bouzayen et al., 2010).
For the consumers and distributors, the process of ripening corresponds to
those modifications that allow fruit to become edible and attractive for
consumption (Bouzaye et al., 2010). Fruits have classically been categorized
based upon their abilities to undergo a program of enhanced ethylene production
and an associated increase in respiration rate at the onset of ripening. Fruits that
undergo this transition are referred to as climacteric and include tomato, apple,
peach, and banana, whereas fruits that do not produce elevated levels of ethylene
are known as non-climacteric and include citrus, grape, and strawberry (Barry and
Giovannoni, 2007). The relationship existing between the climacteric respiration
and fruit ripening has been questioned following the discovery that ripening on
the vine of a number of fruit may occur in the absence of any increase in
respiration (Salveit 1993; Shellie and Salveit 1993). More recently, it has been
reported that the presence or absence of a respiratory climacteric on the vine
depends upon prevailing environmental conditions (Bower et al. 2002). These
observations indicate that the respiratory climacteric is probably not an absolute
trigger of the ripening process, but secondary and consequential to the process of
ripening. An ethylene burst that precedes respiratory climacteric has been shown
during the ripening of banana (Pathak et al. 2003).
Senescence of leaves, flowers and fruits can be regulated by an array of
external and internal factors. Many environmental stresses (such as extreme
temperatures, drought, nutrient deficiency, insufficient light/shade or total
senescence. Internal factors influencing senescence include age, levels of plant
hormones and other growth substances, and developmental processes such as
reproductive growth (Gan, 2004). Ethylene plays a key role in promoting
senescence of climacteric fruits and flowers although it is less effective in
stimulating non-climacteric fruits and flowers to senesce. Other promotions of
senescence process include sugar, jasmonic acid (JA), salicylic acid (SA),
brassinosteroids (BRs), and abscisic acid (ABA), while cytokinins (CK),
Polyamines (PAs), Auxin, Gibberellins are considered to delay senescence
process (Gan, 2004).
2.5. Ethylene Biosynthesis and its Physiological Effects
Ethylene is synthesized by most tissues in response to stress. In particular, it
is synthesized in tissues undergoing senescence or ripening (Davies, 2004).
Chaves and Mello-Farias (2006) provide a thorough review of the ethylene
synthesis pathway that the end of the ethylene synthesis pathway involves three
enzymes to convert methionine into ethylene (Figure 1). Two of these enzymes
are involved in the formation and oxidation of the immediate precursor of
ethylene, 1-aminocyclopropane-1-carboxylic acid (ACC). ACC-synthase converts
S-Adenosylmethionine (SAM) into ACC and is the rate-limiting step in the
pathway. ACC-oxidase catalyzes the conversion of ACC to ethylene. The final
conversion of ACC to ethylene is oxygen dependent (Kende, 1993).
Ethylene is a plant hormone influencing plant processes such as the so
called triple response, maintenance of the apical hook in seedlings, stimulation of
defense responses in response to injury or diseases, release from dormancy, shoot
and root growth and differentiation, adventitious root formation, leaf and fruit
abscission, flower induction in some plants, induction of femaleness of dioecious
flower, flower opening, flower and leaf senescence, fruit ripeing (Davies, 2004).
Of particular economic importance is the role of ethylene as an inducer of
fruit ripening (Bleecker and Kende, 2000). Through this action, it induces changes
in certain plant organs, such as textural changes, color changes, and tissue
degradation. Some of these changes may be desirable qualities associated with
climacteric fruits, ethylene is generally thought to be regulate fruit ripening by
coordinating the expression of gene responsible for 1) enhancing a rise in the rate
of respiration, 2) autocatalytic ethylene production, 3) chlorophyll degradation, 4)
pigment synthesis (carotenoids and flavonoids), 5) conversion of starch to sugars,
production of aroma volatiles, and 7) increase of activities of cell wall-degrading
enzymes (pulp and peel softening), 8) changes in pH (Grey et al., 1992; Lelievre
et al., 1997; Seymour et al., 1993).
Kader (2002) recommended that respiration of mangosteen fruit kept at
20oC should be 6-10ml CO2/kg/hr. Palapol et al. (2009) measured ethylene
production of mangosteen fruit stored at temperature 25oC and found that ethylene
production at stage 1 increased linearly until stage 5 (dark purple) by 5 days, then
decreased slightly after stage 5.
2.6. Ethylene Action and Methods for Inhibiting Ethylene Responses
Ethylene inhibitors reduce or eliminate the biological activity of
ethylene.These compounds can be divided into two groups: inhibitors of ethylene
biosynthesis and inhibitors of ethylene action. The first are substances that interact
with the ethylene biosynthesis pathway through inhibition of key enzymes, ACC
synthase, and ACC oxidase. The 1-aminoethoxyvinylglycine (AVG) and the
amino-oxyacetic acid (AOA) are inhibitors of ethylene biosynthesis, while silver
thiosulfate (STS), silver nitrate, and 1-methylcyclopropene (1-MCP) are inhibitors
of ethylene action (Ferrante and Francini, 2008). Yang and Hoffman (1984)
indicated that aminoethoxyvinylglycine (AVG) and aminoethoxycetic acid (AOA)
disrupt ACC synthase, while cobalt (Co2+) and α-aminois-butyric acid (AIBA) disrupt
ACC oxidase.
Blocking ethylene effects at the receptor level is more effective as it will
protect against both endogenous and exogenous ethylenes (Serek and Reid, 1993).
The silver ion (Ag+) has proved to be a potent inhibitor of ethylene action in
ornamentals. STS is generally applied as a pretreatment solution to cut flowers.
The persistence and mobility of STS allows very short pulse treatments. In potted
plants STS is applied as an aqueous spray. Beneficial effects of STS are reported
for a great variety of cut flowers and potted plants. STS treatment prevents petal
senescence induced by ethylene and prolongs the vase life (Arora, 2008).
1-Methylcyclopropene (1-MCP) has been reported to be a non-toxic antagonist of
ethylene action (Sisler and Serek, 1997) that blocks the physiological action of
ethylene (Sisler et al.,1996). Applications of 1-MCP to delay fruit ripening and
extend the storage life have been extensively reported in both climacteric and
non-climacteric fruit (Watkins, 2006). The use of 1-MCP for harvested fruits and
vegetables represents a revolutionary advance in postharvest science and
practices. The gas works by attaching to a site (receptor) in fruit tissues that
normally binds to ethylene. Binding of ethylene to these sites is how plant tissues
perceive that ethylene is present in the environment. If ethylene binding is
prevented, ethylene no longer promotes ripening and senescence (Huber et al.,
2.7. Cytokinin and its Physiological Effects
Cytokinins are derivatives characterized by an ability to induce cell division
in tissue culture, and the most common cytokinin base in plants is zeatin. It is
biosynthesized through the biochemical modification of adenine and transported
from roots to shoots. The effects of cytokinins include cell division,
morphogenesis, growth of lateral buds, leaf expansion, delay leaf senescence,
enhancement of stomatal opening in some species, chloroplast development
(accumulation of chlorophyll and conversion of ethioplasts into chloroplasts)
(Davies, 2004).
A synthetic cytokinin N1-(2-Chloro-4-pyridyl)- N3-phenylurea (CPPU) is
known to be effective for enhancing fruit enlargement by stimulating cell division
and/or cell expansion in many kinds of fruits including Actinidia deliciosa
kiwifruit (Iwahori et al., 1988; Lewis et al., 1996; Cruz-Castillo et al., 2002). Jo et
al. (2003) reported that a single application of CPPU at the concentration of 16
mg l-1 15 days after pollination was effective for increasing the fruit size of a local
selection. The effectiveness of CPPU application on fruit development of
Actinidia deliciosa kiwifruit was largely influenced by the time of application and
concentration (Cruz-Castillo et al., 1999). Antognozzi et al. (1996) found that
spraying application of CPPU 20 ppm on Actinidia deliciosa (A. Chev.) fruitlets
inside the canopy 2 weeks after full bloom influenced fruit growth soon after
treatment and yield per vine were about 25% higher than the control. The
chlorophyll content was higher in CPPU treated fruits. During storage, the
differences in carbohydrate content disappeared and treated fruits performed as
well as control ones, maintaining good quality for up to 6 months. Cruz-Castillo et
al. (2002) reported that the cytokinin-active compound, N1
-(2-chloro-4-pyridyl)-N3-phenylurea (CPPU), applied at different flowering dates, affected final
„Hayward‟ kiwifruit size.
Recent research conducted by Kim et al. (2006) showed that fruit size of
hardy Kiwifruit was increased and fruit weight was doubled when CPPU was
applied at concentration of 5-10 mg l-1 and at 10 days after petal fall (DAPF).
Although a significant reduction in the concentrations of total soluble solids
was recorded, the TSS/TA ratio and AsA content per fruit increased by the
treatment. CPPU application at petal fall induced abnormally protruding fruit tip.
2.8. Cobalt Sulphate (CoSO4) and its Effects
Cobalt sulfate is an inorganic salt of divalent cobalt. It is the usual source of
water-soluble cobalt, because it is more economical and has less tendency to
dehydrate than cobalt chloride or cobalt nitrate (Budavari et al., 1996). Co2+ is a
potent inhibitor of ethylene biosynthesis. Williams and Golden (2002) studied
purification and characterization of ACC oxidase from Artocarpus altilis and
found that the enzyme ACC was inhibited by cobalt sulfate (CoSO4). When
applied in concentration of 0.1 mM, activity of enzyme ACC was only (0.09 ±
0.01) ×10-18 kat ml extract, and its percentage inhibition reached 97.1%, compared
to control (1.11±0.03) ×10-18 kat ml extract. Singh and Agrez (2000) reported that
single exogenous spray applications of CoSO4 (200 mg/L) to fully-grown panicles
of 'Kensington Pride' mango before anthesis, was most effective for improving
fruit set, fruit retention and yield, compared to aminoethoxyvinylglycine (AVG),
aminooxyacetic acid (AOA), and ethylene action inhibitor silver thiosulphate
III. MATERIALS AND METHODS
3.1. Time and Place
The research was done at Postharvest Laboratory, Faculty of Agriculture,
Laboratory of Food and Agricultural Product Process Engineering, Laboratory of
Environmental and Agricultural Building, Faculty of Agricultural Technology,
Bogor Agricultural University (IPB), starting from February 2010 to May 2010.
3.2. Plant Material and Treatment
The research was divided into two stages. The first experiment covered
physical and chemical changes of mangosteen fruit treated with CPPU and CoSO4
during storage, while the second one involved the study of physiological and its
relation to color development of mangosteen fruit treated with CPPU and CoSO4
during storage. The experimental design was arranged in a completely randomized
block design with 2 factors and 3 replications. The first factor was cobalt sulphate
(CoSO4) at four concentrations (0, 500, 1000, and 2000 ppm), while the second
one was CPPU at four concentrations (0, 10, 20, and 30 ppm). The combination of
the above factors provided 16 treatments with 48 experimental units. Each
experimental unit comprised 40 mangosteen fruit. For the second experiment, the
design was as in the first experiment, but only cobalt sulphate at 0, 2000 ppm, and
CPPU at 0, 30 ppm were used with 5 mangosteen fruit per experimental unit.
The fruits used in the experiment were harvested from local orchard
(Purwakarta) at stage 1(light greenish yellow with 5-50% scattered spots) on the
same day and were of similar sizes. The harvested fruit were transported at night
to Postharvest Laboratory. In the following morning, fruit were sorted, and
washed with tap water to remove the dust. After washing, the fruit were air dried,
and then treated with solution of fungicide TBZ 1 ppm for 30 seconds and air
dried. Following fungicide application, air-dried fruit were dipped in the solution
of CoSO4 and CPPU for 30 seconds according to its concentrations used in the
treatments. To facilitate the absorption of the solution by fruit, Tween 20 (1%)
was added. After application, treated fruits were air dried, then immediately stored
at 14-16oC (76-96% RH) which is an ideal storage temperature for mangosteen
3.3. Observed Variables 3.3.1 Physical Attributes
3.3.1.1.Fruit Resistance(kgf/cm2)
Mangosteen fruit was pressed until breakdown to see the level of easiness
for fruit to be opened by using fruit resistance tool. The observation was done
every two days with 16 times of observation, each of which two fruits were used.
3.3.1.2. Pericarp Water Content (%)
Pericarp sample (g) were weighed, and placed in the paper envelope. The
sample was dried in oven at 105 oC for 96 hours, then cooled down in desiccators
and weighed. PWC was calculated using formula:
Pericarp water content (%) =a−b
a × 100, where a = Fresh weight (g)
b = dried constant weight (g)
3.3.1.3. Weight Loss
Loss of fruit weight is measured based on the percentage of the reduction in
the weight since the beginning up to the end of the storage period. The weight loss
was calculated using the following formula:
Weight loss (%) =W−Wi
W × 100, where W = Weight at initial storage (g)
Wi = Weight at ith observation (g)
3.3.1.4. Fruit and Sepal Color
The observation of fruit and sepal color was conducted every two days to
see colour development during storage. Fruit and sepal color was measured using
Color Reader CR-10 that was already calibrated. The tool consists of color
notation (color system L, a, and b). Color system L means the brightness with
value 0 (black) until 100 (white). The color system a and b is chromaticity
coordinate. It means that chromatic mixture of red and green with value +a from 0
to +60 for red and –a from 0 to -60 for green color. b value means that chromatic
yellow and blue mixture with the value of +b from 0 to +60 for yellow color and
the value –b from 0 to -60 for blue color. The colour reading was measured twice
at the equatorial region of each fruit and two sepals, and averaged to give a value
0=/black, 100= white color), a/b ratio and hue angle (ho) with red-purple at an
angle of 0o, 90o representing yellow color, and 180o bluish-green color (Palapol et
al., 2009). Hue angle was calculated using the following formula:
Hue angle (ho) = arctan (b/a)
3.3.2.Chemical Attributes
3.3.2.1. Total Soluble Solids (TSS)
To measure total soluble solids on fresh juice, the white fresh of the arils
with seeds, is wrapped in cheesecloth, and squeezed by hand to separate juice
from seeds. Pulp liquid is placed on the prism of digital refractometer. TSS was
reported as oBrix.
3.3.2.2. Titratable Acidity (Titration method AOAC 1984)
Analysis of titratable acidity of the mangosteen fruit was measured in
duplicate by using the titration method. Pulp was weighed as much as 10 g and
put in a glass baker. Distilled water was added to get a solution of 100 ml, and
then filtered with filter paper. Twenty five ml of the filtrate were titrated with 0.1
N NaOH using phenolphthalein (pp) as an indicator until the solution turns pink.
Titratable acidity was calculated with following formula:
��(%) = � � × � �× 64 × × 100 � �
Where, ml NaOH = NaOH Volume
N = NaOH normality (0.1 N)
df = dilution factor
3.3.3. Physiological Variables 3.3.3.1. Respiration Rate
Five fruits were weighed and placed in 3.3 L- glass jar and sealed with wax to
prevent the entry of gas O2 and CO2 and stored in 15oC. The carbon dioxide
concentration measurement was done by using Shimadzu Infrared Gas Analyzer
Model IRA-107, while O2 was measured using Oxygen Portable Tester Model
POT-101. The measurement was done every 3 hours on the first day (after
treatment), every 6 hours on the second day, every 12 hours, and followed by
every 24 hours. The rate of respiration was calculated with the following fomula:
R = � × , where R = Respiration rate (ml kg-1 h-1)
Chromatograph (GC) with the FID system (Flame Ionization Detector) which was
connected with a chroma-integrator D-2000 was used. The measurement was done
by using the column (2000 mm x 4 mm) and the column 80-100 activated mesh
alumina. The column temperature for the measurement was 60 oC and injector
temperature was 110 oC. N2 carrier gas flow rate was 30 ml/minute and gas
pressure 5 kg/cm2. Mangsosteen fruit was incubated in the air-locked stopples,
and sample was taken as much as 1 ml. Only 0.5 ml was injected into gas
chromatograph. The measurement was done every 24 hours up to d13 of storage.
Ethylene production rate was expressed as µl kg-1 h-1.
Sample (ppm) =
Sample peak areaStandard peak area
× standard ( ppm)
�
=
�×
Where, EP = Ethylene production rate (µl/kg/h)
E = Ethylene concentration (ppm)
t = time (hour)
V = Space volume (L)
W = Product weight (kg)
3.4. Statistical Analysis
All the recorded data was entered and stored in Microsoft Excel 2007.
Analysis was performed using SPSS statistics 17.0. Analysis of variance was done
and treatment means were compared using Duncan Multiple Range Test (DMRT)
IV. RESULTS AND DISCUSSION
Experiment one: Physical and Chemical Changes of Mangosteen Fruit Treated with CPPU and CoSO4 during Storage
4.1. Physical Changes of Mangosteen Fruit 4.1.1. Fruit Resistance (kgf/cm2)
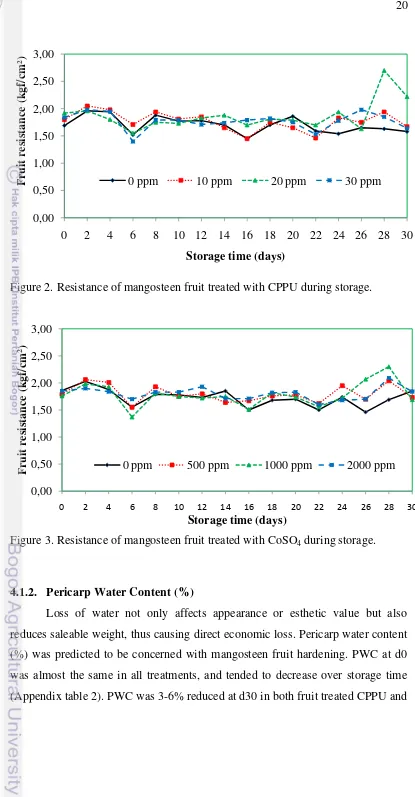
Fruit softening is closely associated with ripening process. The resistances
of mangosteen fruit were similarly noticed during storage from d0 to day 30 at
14-16oC (76-96% RH) in both fruit treated with CPPU and CoSO4 (Figure 2, 3). The
results showed that mangosteen fruit could be stored at 15oC for 30 days without
affecting fruit hardness, and treatments applied had no effect on fruit hardening
during storage period, except at day 16 and day 30 (Appendix table 1). At d16,
CPPU 20 and 30 ppm had higher fruit resistance than CPPU 10 ppm and control
fruit, while fruit treated with CPPU 20 ppm was most resistant and significantly
different from CPPU 0, 10 and 30 ppm at day 30 (Appendix table 1). High
resistant fruit treated with CPPU 20 ppm (2.70 kgf/cm2, and 2.22 kgf/cm2 at day
28 and d30, respectively) was due to fungal infection at the end of storage. Our
results were consistent with Azhar (2007), who reported that mangosteen fruit
pericarp was not difficult to be opened after 30 days of storage at 15oC. The
results also confirmed the findings by Inayati (2009), who found that BAP, a type
of cytokinins, from 0 to 40 ppm could maintain the pericarp resistance at less than
2 kgf/cm2 during 26 days of storage at 15oC. Resistance values equally less than 2
0,00
0 ppm 500 ppm 1000 ppm 2000 ppm
Figure 2. Resistance of mangosteen fruit treated with CPPU during storage.
Figure 3. Resistance of mangosteen fruit treated with CoSO4 during storage.
4.1.2. Pericarp Water Content (%)
Loss of water not only affects appearance or esthetic value but also
reduces saleable weight, thus causing direct economic loss. Pericarp water content
(%) was predicted to be concerned with mangosteen fruit hardening. PWC at d0
was almost the same in all treatments, and tended to decrease over storage time
CoSO4, and no statistical differences were observed, except day 18 which showed
significant interaction between CPPU and CoSO4. Pericarp water contents in fruit
treated with CPPU 0 ppm + CoSO4 500 ppm , CPPU 10 ppm + CoSO4 2000 ppm,
CPPU 20 ppm + CoSO4 0 ppm, CPPU 10, 20, 30 ppm + CoSO4 1000 ppm were
lowest and significantly different from control fruit at day 18 (Table 2). According
to Kondo et al. (2003), skin hardening of mangosteen fruit during storage at low
temperature was not accompanied by moisture loss.
Table 2. Effects of CPPU and CoSO4 interaction on pericarp water content (%) of
mangosteen fruit during storage at day18
CPPU (ppm) CoSO₄ (ppm)
Note: Different letters indicate significant differences among treatment means
(P < 0.05) by Duncan‟s multiple range test (DMRT).
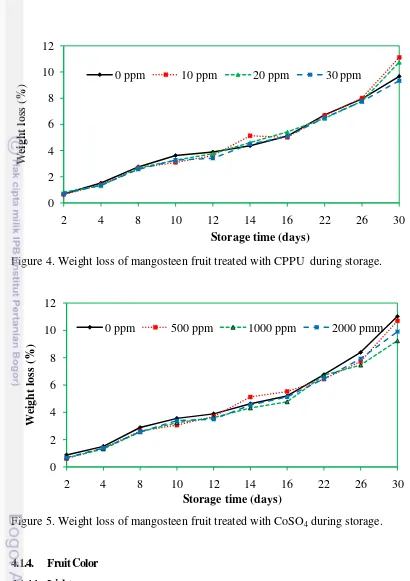
4.1.3. Weight Loss (%)
Mangoteen fruit increasingly lost weight with storage time from day 0 to
day 30 in either fruit treated with CPPU or CoSO4 (Figure 4, 5). The rate of
weight loss in CPPU-treated fruit was lower than control fruit although no
statistical significance was observed from day 2 to day 12 (Appendix table 3).
Weight loss of fruit during storage was the results of water loss through
transpiration (Yaman and Bayoindirli, 2002) and loss of carbon due to respiration
(Pan and Bhowmilk, 1992). In mangosteen fruit, pericarp water content was only
reduced 3-6% during storage time from d0 to d30 as seen in appendix table 2.
This was not proportional to weight loss which ranged from 9-12%. The results
suggested that water loss from aril and other parts of the fruit, and carbon loss by
Figure 4. Weight loss of mangosteen fruit treated with CPPU during storage.
Figure 5. Weight loss of mangosteen fruit treated with CoSO4 during storage.
4.1.4. Fruit Color 4.1.4.1. Lightness
Fruit color is one of the most important appearance quality always
employed by consumers when purchasing products. The darkening of mangosteen
fruit drastically increased from day 0 to day 4, and was constant from day 4 to day
16. The darkening was reaccelerated from day 18 in either fruit treated with CPPU 0
and CoSO4 (Figure 6, 7). Lightness of mangosteen fruit reduced (darkening
increased) from 42 to 33 (around 9%) during storage time from day 0 to day 30.
CPPU 20 ppm could most effectively delay darkening of the fruit and
significantly varied from other concentrations as shown in day 4 and day 6
(Appendix table 4).
Figure 6. Changes in skin lightness of mangosteen fruit treated with CPPU during storage.
Figure 7. Changes in skin lightness of mangosteen fruit treated with CoSO4 during
storage.
4.1.4.2. a/b Ratio
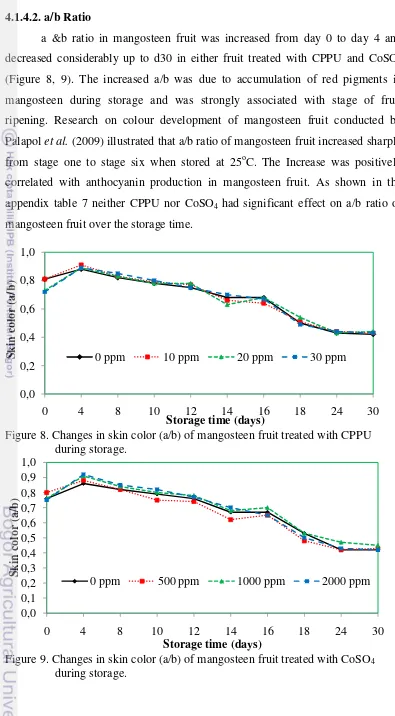
a &b ratio in mangosteen fruit was increased from day 0 to day 4 and
decreased considerably up to d30 in either fruit treated with CPPU and CoSO4
(Figure 8, 9). The increased a/b was due to accumulation of red pigments in
mangosteen during storage and was strongly associated with stage of fruit
ripening. Research on colour development of mangosteen fruit conducted by
Palapol et al. (2009) illustrated that a/b ratio of mangosteen fruit increased sharply
from stage one to stage six when stored at 25oC. The Increase was positively
correlated with anthocyanin production in mangosteen fruit. As shown in the
appendix table 7 neither CPPU nor CoSO4 had significant effect on a/b ratio of
mangosteen fruit over the storage time.
Figure 8. Changes in skin color (a/b) of mangosteen fruit treated with CPPU during storage.
Figure 9. Changes in skin color (a/b) of mangosteen fruit treated with CoSO4
during storage.
0 ppm 500 ppm 1000 ppm 2000 ppm
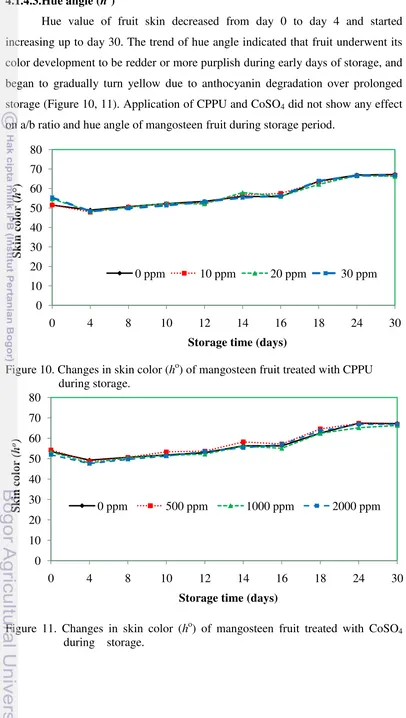
4.1.4.3.Hue angle (ho)
Hue value of fruit skin decreased from day 0 to day 4 and started
increasing up to day 30. The trend of hue angle indicated that fruit underwent its
color development to be redder or more purplish during early days of storage, and
began to gradually turn yellow due to anthocyanin degradation over prolonged
storage (Figure 10, 11). Application of CPPU and CoSO4 did not show any effect
on a/b ratio and hue angle of mangosteen fruit during storage period.
Figure 10. Changes in skin color (ho) of mangosteen fruit treated with CPPU
during storage.
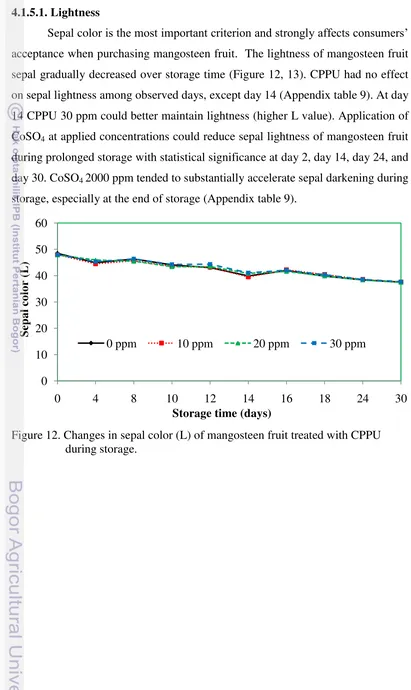
4.1.5. Sepal color 4.1.5.1. Lightness
Sepal color is the most important criterion and strongly affects consumers‟
acceptance when purchasing mangosteen fruit. The lightness of mangosteen fruit
sepal gradually decreased over storage time (Figure 12, 13). CPPU had no effect
on sepal lightness among observed days, except day 14 (Appendix table 9). At day
14 CPPU 30 ppm could better maintain lightness (higher L value). Application of
CoSO4 at applied concentrations could reduce sepal lightness of mangosteen fruit
during prolonged storage with statistical significance at day 2, day 14, day 24, and
day 30. CoSO4 2000 ppm tended to substantially accelerate sepal darkening during
storage, especially at the end of storage (Appendix table 9).
Figure 13. Changes in sepal color (L) of mangosteen fruit treated with CoSO4
during storage.
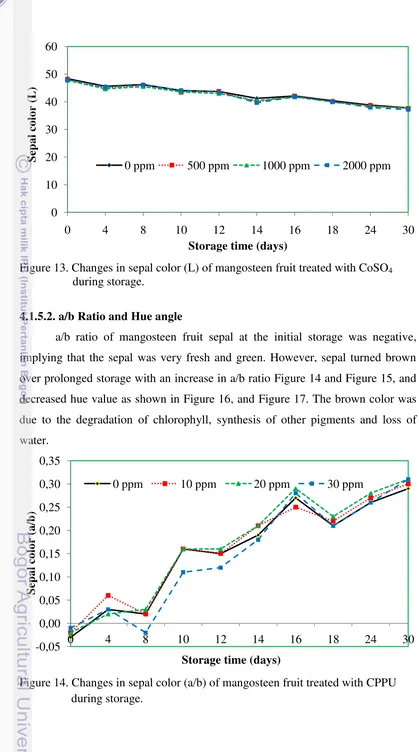
4.1.5.2. a/b Ratio and Hue angle
a/b ratio of mangosteen fruit sepal at the initial storage was negative,
implying that the sepal was very fresh and green. However, sepal turned brown
over prolonged storage with an increase in a/b ratio Figure 14 and Figure 15, and
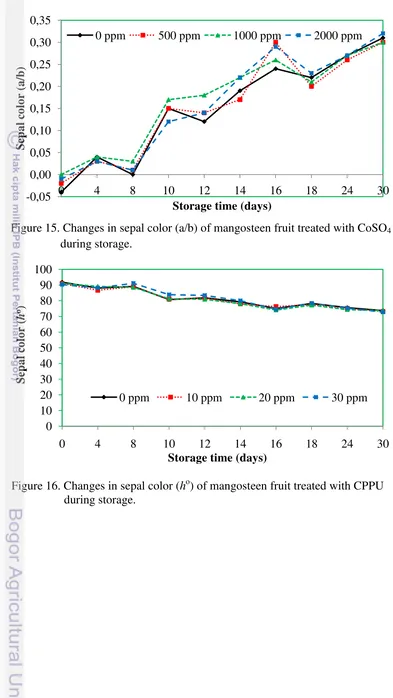
decreased hue value as shown in Figure 16, and Figure 17. The brown color was
due to the degradation of chlorophyll, synthesis of other pigments and loss of
water.
Figure 14. Changes in sepal color (a/b) of mangosteen fruit treated with CPPU during storage.
0 ppm 500 ppm 1000 ppm 2000 ppm
Figure 15. Changes in sepal color (a/b) of mangosteen fruit treated with CoSO4
during storage.
Figure 16. Changes in sepal color (ho) of mangosteen fruit treated with CPPU
during storage.
0 ppm 500 ppm 1000 ppm 2000 ppm
Figure 17. Changes in sepal color (ho) of mangosteen fruit treated with CoSO4
during storage.
Table 3 showed that there were significant interactions between CPPU and
CoSO4 on a/b ratio of mangosteen fruit sepal at day 22 and day 26. The a/b was
significantly higher in fruit treated with CPPU 0 pmm + CoSO4 2000 ppm (0.37)
than control (0.27) at day 22. At day 26, CPPU 30 ppm and CoSO4 2000 ppm
gave higher a/b (0.41) and statistically significant compared to control fruit with a/b only 0.27. The result showed that application of Cobalt Sulphate was likely to accelerate redness of the sepal (increase a value) as seen in appendix table 10.
Table 3. Effects of CPPU and CoSO4 interaction on a/b ratio of sepal of
mangosteen fruit during storage at d22 and d26
Day CPPU (ppm) CoSO4 (ppm)
Note: Different letters indicate significant differences among treatment means
(P < 0.05) by Duncan‟s ultiple range test (DMRT)
0