Urinary
p
-cresol in autism spectrum disorder
Antonio M. Persico
⁎
, Valerio Napolioni
Child and Adolescent NeuroPsychiatry Unit, University "Campus Bio-Medico", Rome, Italy Dept. of Experimental Neurosciences, IRCCS“Fondazione Santa Lucia”, Rome, Italy
a b s t r a c t
a r t i c l e
i n f o
Article history: Received 13 June 2012
Received in revised form 4 September 2012 Accepted 4 September 2012
Available online 10 September 2012
Keywords: Autism Clostridium
Gene–environment interactions Gutflora
p-cresol p-cresylsulfate
Autism spectrum disorder (ASD) is a neuropsychiatric disorder with onset during early childhood and life-long consequences in most cases. It is characterized by impairment in social interaction and communication, as well as by restricted patterns of interest and stereotyped behaviors. The etiology of autism is highly het-erogeneous, encompassing a large range of genetic and environmental factors. Several lines of evidence sug-gest that, in addition to broader diagnostic criteria and increased awareness, also a real increase in incidence primarily due to greater gene–environment interactions may also be occurring. Environmental exposure to the organic aromatic compoundp-cresol (4-methylphenol) is relatively common and occurs through the skin, as well as the gastrointestinal and respiratory systems. However, the largest and most widespread source of this compound is represented by some gut bacteria which expressp-cresol synthesizing enzymes not found in human cells. Urinaryp-cresol and its conjugated derivativep-cresylsulfate have been found el-evated in an initial sample and recently in a replica sample of autistic children below 8 years of age, where it is associated with female sex, greater clinical severity regardless of sex, and history of behavioral regression. Potential sources ofp-cresol excess in ASD, such as gut infection, chronic constipation, antibiotics, abnormal intestinal permeability, and environmental exposure, are being investigated.P-cresol may contribute to worsen autism severity and gut dysfunction, often present in autistic children. It may also contribute to a multibiomarker diagnostic panel useful in small autistic children.
© 2012 Elsevier Inc. All rights reserved.
1. Introduction
Autism spectrum disorder (ASD) is a neuropsychiatric disorder with onset during early childhood and with life-long consequences in the vast majority of cases. It is characterized by impairment in so-cial interaction and communication, as well as by restricted patterns of interest and stereotyped behaviors. Clinical signs and symptom display great interindividual differences in pattern, severity, develop-mental trajectory and treatment response. ASD incidence has dra-matically risen during the last two decades from 2–5/10,000 to approximately 1–2/1000 children for strict autism (Fombonne, 2009) and 6–10/1000 for broad ASD (Baron-Cohen et al., 2009). Hence this once rare disease has now become one of the most fre-quent conditions in child neuropsychiatry. Genetics strongly contrib-utes to autism pathogenesis. However, heritability estimates above 90% in the early nineties (Steffenburg et al., 1989; Bailey et al., 1995) have now dropped down to 37%, while the relative weight of shared environmental factors has risen to explain as much as 55% of phenotypic variance in a recent twin study (Hallmayer et al., 2011). Furthermore, only in rare cases is autism fully explained byde novo
high-penetrance mutations or by chromosomal rearrangements affecting genes such asNLGN3/4,SHANK3,NRXN1, andMECP2. Recent whole-exome sequencing studies have instead detected a highly heterogeneous collection ofde novo mutations distributed in many autism-related genes, collectively increasing disease risk by 5- to 20-fold but nonetheless incompletely penetrant, meaning they are not sufficient to cause the disease (Neale et al., 2012; O'Roak et al., 2011; Sanders et al., 2012). In summary, increasing prevalence rates, decreas-ing heritability estimates, and the incomplete penetrance of mutations and/or CNVs often inherited from either parent or evende novostrongly suggest that ASD pathogenesis may be changing over time. The majority of ASD patients are most compatible with a “multiple hit” model, encompassing gene–gene and gene–environment interactions, as well as epigenetic contributions related to several factors, such as increasing parental age at the time of conception (Persico and Bourgeron, 2006; Leblond et al., 2010; Persico, 2013).
In order to determine whether exposure to a given environmental toxicant may contribute to autism pathogenesis in a sizable subgroup of patients, it is important to consider its timing and functional consequences relative to developmental processes. Abnormalities in neurodevelopment must seemingly start during the I–II trimester of prenatal life to yield the spectrum of behavioral abnormalities later diag-nosed as ASD (Miller et al., 2005; Bauman and Kemper, 2005). Hence early prenatal exposure should represent our primary concern. This is well exemplified by at least three evidence-based gene–environment ⁎ Corresponding author at: Child and Adolescent Neuropsychiatry Unit, Lab. of
Mo-lecular Psychiatry & Neurogenetics, University“Campus Bio-Medico”, Via Alvaro del Portillo 21, I-00128 Rome, Italy. Tel.: +39 06 225419155; fax: +39 06 501703333.
E-mail address:a.persico@unicampus.it(A.M. Persico).
0892-0362/$–see front matter © 2012 Elsevier Inc. All rights reserved. http://dx.doi.org/10.1016/j.ntt.2012.09.002
Contents lists available atSciVerse ScienceDirect
Neurotoxicology and Teratology
interaction models, recently summarized elsewhere (Fatemi et al., 2012; Bal-Price et al., 2012): (a)RELNandPON1gene variants +prenatal expo-sure to organophosphate pesticides and insecticides; (b) METgene variants+prenatal exposure to polycyclic aromatic hydrocarbons; and (c) SLC25A12 and ATP2B2 gene variants +prenatal exposure to polychlorinated biphenyls. Secondly, ASD should not be viewed strictly as a“brain disease”: the pathophysiological abnormalities underlying autism indeed are not limited to the central nervous system (CNS), but often involve the immune system and the digestive tract. Many autistic patients display altered T helper 1/T helper 2 ratio, abnormal cytokine profiles, reduced number of lymphocytes and reduced T cell mitogen re-sponse (Goines and Van de Water, 2010); autoimmune disorders cluster in many families with autistic probands (Comi et al., 1999); leukocyte gene expression is abnormal, especially for natural killer (NK) cell-related transcripts (Enstrom et al., 2009; Lintas et al., 2012); immune genes are the most overexpressed in post-mortem brains of autistic individuals (Garbett et al., 2008); anti-brain autoantibodies detected in many autistic children and their mothers can produce behavioral abnor-malities in several animal models, including primates (Singh et al., 1997; Singh and Rivas, 2004; Goines et al., 2011; Braunschweig et al., 2011). Also gastrointestinal issues are often noticed by parents in their autistic children, including: (a) constipation; (b) diarrhea; (c) abdominal bloating, discomfort, or irritability; (d) gastro-esophageal reflux or vomiting; (e) feeding issues or food selectivity (Buie et al., 2010). In-terestingly, dysregulated innate immune defenses have recently been linked with gastrointestinal issues in a subgroup of autistic children who may be especially vulnerable to common microbial dysbiosis (Jyonouchi et al., 2011).
2.P-cresol: general toxicology and implications for human health
Information about the general toxicology ofp-cresol, including its physico-chemical properties, environmental distribution, use and ex-posure, metabolism, and implications for human health, is available through the Screening Information Data Set (SIDS) of the Organiza-tion for Economic Co-operaOrganiza-tion and Development (OECD), published by the United Nations Environment Program (OECD, 2003).
2.1. Physico-chemical properties
P-cresol (4-methylphenol) belongs to the cresol class of organic ar-omatic compounds, which also includes ortho-cresol, meta-phenol, and other mixed formulations. The main physico-chemical properties ofp–cresol are reported inTable 1. Particularly important for environ-mental behavior and ecotoxicity are its octanol–water partition coeffi -cient (logKow=1.94), high vapor pressure, water solubility, and pKa
value of 10.26, indicating that at environmentally relevant pH values ranging between 5 and 9,p-cresol is largely non-dissociated in aqueous solution.
2.2. Environmental exposure
Cresols (o-, m-, p-cresol) are produced in nature through the photo-oxidation of toluene in the atmosphere. Environmental exposure
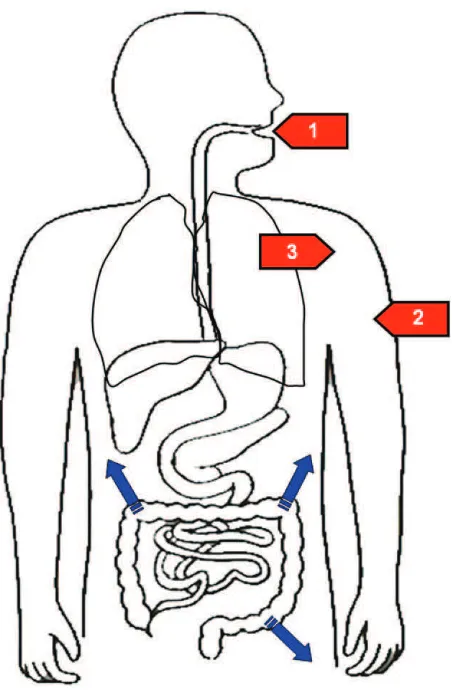
top-cresol through inhalation, skin contact, ingestion of food and bever-ages is relatively common (Mandel, 1971; DeBruin, 1976; Roberts et al., 1977) (Fig. 1, red arrows). The main natural and artificial sources of p-cresol exposure are listed inTable 2.P-cresol is readily biodegradable in aerobiosis, while anaerobic degradation plays a marginal role (OECD, 2003).
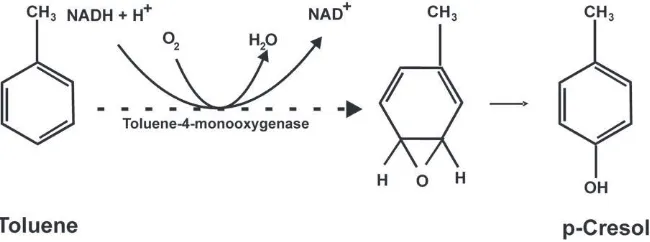
Another important source ofp-cresol exposure in humans is repre-sented by some gut bacteria, able to express synthetic enzymes not pres-ent in human cells (Fig. 1, blue arrows). Two distinct synthetic pathways have been elucidated in gut bacteria: (a)Clostridium difficile, an anaerobic bacterium involved in the most severe forms of antibiotic-associated di-arrhea occasionally leading to pseudomembranous colitis and even to death, expressesp-hydroxyphenylacetate (p-HPA) decarboxylase, able to push the fermentation of tyrosine up to the formation of p-cresol (Elsden et al., 1976; Selmer and Andrei, 2001) (Fig. 2). AlsoC. scatologenes and one genre ofLactobacillusemploy this synthetic pathway (Yokoyama and Carlson, 1981); (b)Pseudomonas stutzeri(Cafaro et al., 2005) and Pseudomonas mendocina(Whited and Gibson, 1991) producep-cresol from toluene by expressing toluene monooxygenase (Fig. 3). The former pathway is predicted to be significantly more important than the latter, given the much greater availability of L-tyrosine compared to toluene as a substrate in the gut lumen and the broader distribution ofp-cresol producing clostridial strains. In fact, exposure to toluene primarily occurs through breathing and skin contact, whereas oral ingestion can occur only through food or water contaminated with this oil derivative.
Table 1
Physico-chemical properties ofp-cresol (OECD, 2003).
Melting point 35.5 °C
Boiling point (1 atm) 201.9 °C
Density (20 °C) 1.0178 g/cm3
Vapor pressure (25 °C) 0.147 mbar
LogKow(exp.) 1.94
Water solubility (25 °C) 21.5 g/l
Dissociation constant pKa 10.26
Kow: octanol–water partition coefficient.
Fig. 1.Routes of entry forp-cresol into the human body. Red arrows represent environmen-tal exposure through [1] ingestion of food and beverages, [2] skin contact, and [3] inhalation, followed byp-cresol absorption through the upper digestive tract, skin and lungs, respec-tively. Blue arrows representp-cresol synthesized by gut bacteria from tyrosine or toluene mainly in the colon.
2.3. Metabolism and toxicity in animals
As partially lipophilic compound,p-cresol travels in the blood most-ly protein-bound (Bergé-Lefranc et al., 2010) and hypoalbuminemia can indeed increase freep-cresol plasma levels (De Smet et al., 2003). Only 0.5%–1% of total plasmap-cresol is in free form; approximately 95% of total plasmap-cresol is metabolized top-cresylsulfate through O-sulfonation, which occurs primarily in colonic epithelial cells and also in the liver; the remaining 3%–4% is metabolized to p-cresylglucuronide through glucuronidation, which takes place only in the liver (Mandel, 1971; DeBruin, 1976; Ramakrishna et al., 1991). Free plasma p-cresol and its conjugation derivatives p-cresylsulfate andp-cresylglucuronide arefiltered by the renal glomeruli and can be found in the urines of all individuals in small amounts, primarily origi-nating from gut bacteria (Bone et al., 1976; Renwick et al., 1988). It is
thus important to underscore that total urinaryp-cresol, whose mea-sure is reported in most studies, indeed represents the sum of p-cresylsulfate,p-cresylglucuronide and freep-cresol, approximately in a 95:4:1 ratio. The pivotal role of gut bacteria in synthesizing these urinary compounds is underscored by their significant increase with fasting or slow intestinal transit (Kawakami et al., 2007), and conversely by their decrease withfiber-rich diets or probiotics (Cummings et al., 1979; Kawakami et al., 2005; Nakabayashi et al., 2011).
Signs of acute toxicity in animals typically include hypoactivity, sal-ivation, tremors and convulsions. The lethal dose 50 (LD50) of oral undiluted p-cresol in rats is 207 mg/kg (Andersen, 2006). Clinical signs of toxicity following inhalation include irritation of mucous mem-branes, excitation and convulsions, hematuria at highp-cresol concen-trations, and death.P-cresol is corrosive to the skin and can cause serious eye damage by contact: the LD50 for dermal application of undilutedp-cresol is 300 mg/kg in rabbits. The“No Observed Adverse Effect Levels”(NOAEL) forp-cresol in mice and rats are generally at or above 50 mg/kg/day, depending on oral, respiratory or dermal absorp-tion (Andersen, 2006). Fertility is not compromised byp-cresol, which can instead cause fetotoxicity in the form of delayed ossification and re-duced body weight when administered at toxic levels to pregnant rats, but not to rabbits. In vitro,p-cresol does not cause gene mutations in bacterial cells and in mammalian cell systems.
2.4. Acute and chronic toxicity in humans
Voluntary or accidental intoxication withp-cresol in humans re-sults in irritation of mouth and throat, abdominal pain, vomiting, he-molytic anemia, cardiovascular disturbances, renal and liver damage, headache, facial paralysis, seizures, coma and death (Wu et al., 1998; Seak et al., 2010). Also skin contact can be potentially fatal, causing skin corrosion and discoloration, gastrointestinal bleeding, and toxic effects on the nervous system, liver and kidneys (Lin and Yang, 1992). P-cresol was initially identified as a uremic toxin, but it was later shown that it is instead the accumulation of its conjugated derivative p-cresylsulfate that causes many signs and symptoms of chronic renal disease (seeSection 5below). Using cellular, animal and human pro-tocols, many damaging effects of chronically elevated p-cresol in humans were described, but for at least some of them it is not entirely clear whether and to what extent freep-cresol or p-cresylsulfate are responsible. These effects include:
– hepatotoxicity, likely due to inhibition of mitochondrial respira-tion. This would result from the cytochorme P450-mediated oxidation of either the methyl group or the benzene ring of p-cresol, leading to the formation of two reactive intermediates, Table 2
Natural and artificial sources ofp-cresol exposure.
Natural sources of exposure: • rainwater • plants • petroleum • tar
• products of volcanic activity
• urines (produced from tyrosine by some gut bac-teria in many mammals, including humans) Artificial sources of exposure: • disinfectants and preservatives
• stabilizers in washing and cleaning products • paints
• fillers • solvents
• adhesives for surface treatment • corrosion inhibitors
• impregnation materials • perfumes and cosmetics
• combustion from incinerators and cigarette smoke Chemical synthetic processes
employing p-cresol:
• butylhydroxytoluene and other antioxidants employed as preservatives in foods or as supple-ments
• 4-anisaldehyde (a vanillin-like compound used in theflavor industry)
• intermediates employed in the manufacture of pharmaceutical products
• protective agents for plants • dyes and pigments Foods containing p-cresol: • tomatoes, ketchup
• asparagus • cheese, butter • bacon and smoked foods • red wine
• roasted coffee • black tea
a quinone methide and a ortho-benzoquinone, respectively, able to produce toxic effects by alkylating cellular proteins and nucleic acids (Thompson et al., 1996; Kitagawa, 2001; Yan et al., 2005); –increased endothelial permeability and cardiovascular disease
(Cerini et al., 2004; Meijers et al., 2008; Meijers et al., 2009); –immunosuppression and increased susceptibility to infections,
caused by blunted production of reactive oxygen species by granulocytes, diminished release of IL-12 by immunostimulated macrophages, and hampered adhesion of monocytes to endotheli-al cells (Vanholder et endotheli-al., 1995; Dou et endotheli-al., 2002; De Smet et endotheli-al., 2003; Faure et al., 2006; Kawakami et al., 2009);
–inhibition of arachidonic acid-induced platelet aggregation (Chang et al., 2011);
– growth retardation in weanling pigs (Yokoyama et al., 1982); – increased susceptibility to hearing seizures in mice (Yehuda et al.,
1994);
– membrane depolarization through blockade of the delayed-rectifier RCK1 (Kv1.1) potassium channel (Elliott and Elliott, 1997), which is widely expressed in the CNS (Beckh and Pongs, 1990);
– increased lipid peroxidation in rat brain (Calderón-Guzmán et al., 2005);
– decreased Na+-K+ATPase activity in rat brain (Calderón-Guzmán
et al., 2005);
– inhibition of the conversion of dopamine to noradrenaline (Goodhart et al., 1987);
– reduced acetaminophen sulfation capacity, due to competition of p-cresol for sulfate conjugation (Clayton et al., 2009).
Many of the effects described using cellular and animal models, such as neuronal depolarization, clearly underlie clinically-relevant signs and symptoms of acute intoxication or chronic overexposure top-cresol in humans, including some potentially relevant to ASD.
3. Autism spectrum disorder and the gutflora
The gutflora is a complex microbial ecosystem that significantly influences human health (Holmes et al., 2011; Kinross et al., 2011). Several studies have assessed the fecalflora of autistic and control in-dividuals, reporting an overgrowth of potentially pathogenic gut mi-crobial species in a sizable subgroup of autistic patients. Some of these bacterial strains are known to producep-cresol:
[1] Finegold et al. (2002)initially reported an excess ofRuminococcus andClostridium species, includingC. difficile, in fecal samples from 13 ASD patients compared to 8 controls. These Clostridia groups were then characterized bySong et al. (2004)using a TaqMan real-time PCR-based approach. Later the same group assessed 33 ASD children, 7 unaffected siblings and 8 controls by pyrosequencing,finding Bacteroidetes at high levels in se-verely autistic individuals, whereas Firmicutes were predomi-nant among controls (Finegold et al., 2010).
[2] Parracho et al (2005)found a higher incidence of theClostridium histolyticumgroup (Clostridium clusters I and II) in the fecal flora of 58 ASD children compared to 10 healthy children.
Interestingly, 12 unaffected siblings of ASD probands displayed intermediate levels. Several members of the C. histolyticum group are known toxin-producers which could both contribute to gut dysfunction and exert systemic effects.
[3] Adams et al. (2011)found lower levels ofBifidobacteriaand higher levels ofLactobacillusspecies in 58 ASD children com-pared to 39 controls. Children with autism had significantly lower levels of total short chain fatty acids. Gastrointestinal symptoms were positively correlated with autism severity. The sensitivity of the microbiological bacterial cultivation methods employed in this study is lower compared to the ap-proaches applied in the studies summarized above and this methodological discrepancy has likely influenced the consis-tency of the results.
[4] Similarly reduced levels ofBifidobacteriawere found byWang et al. (2011a,b)in faecal samples obtained from 23 individ-uals with ASD compared to 9 controls, while 22 unaffected siblings displayed intermediate levels. A significant three-fold reduction in the mucin-degrading bacterium Akkermansia muciniphilawas recorded both in autistic indi-viduals and in their unaffected siblings. Interestingly, the p-cresol producer Clostridium difficile displayed a dramatic ten-fold mean elevation in ASD patients only (and not in their unaffected siblings), with prominent interindividual variability preventing this result from reaching statistical sig-nificance.
[5] Williams et al. (2011)performed ileal biopsies on 15 autistic and 7 control children both with gastrointestinal symptoms. Bioptic tis-sue from ASD children displayed significantly reduced mRNA levels encoding for disaccharidases and hexose transporters. In addition, pyrosequencing analysis revealed reduced Bacteroidetes and relatively increased titres of Firmicutes, especially Clostridia. The same group subsequently detected a significant association between the presence of Sutterella species in ileal biopsies and gastrointestinal symptoms in autistic children (Williams et al., 2012).
[6] Indirect evidence of abnormal gutflora was obtained byYap et al. (2010), using a metabolomic approach based on1H NMR
spec-troscopy and pattern recognition methods on urine samples of Australian autistic and control individuals. Reduced levels of phe-nolic compounds including hippurate, phenyacetylglutamine, andp-cresylsulfate characterize the urinary metabolic profile of autistic patients. This result strongly points toward significant differences in gut microbiomes between patients and controls, since the precursors of these three compounds are produced by bacterial metabolism in the gut lumen. The same conclusion is also supported by the higher concentrations of urinary 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA) found in ASD children compared to age- and sex-matched controls, as well as in an adult with recurrent diarrhea due toC. difficile infec-tion (Shaw, 2010). HPHPA derives from dietary phenylalanine which, in addition to acting as substrate for phenylalanine Fig. 3.Synthesis ofp-cresol from toluene by gut bacteria expressing toluene monooxygenase (hyphenated arrow).
hydroxylase, is also metabolized by gut bacteria into either phenylpropionic acid orm-tyrosine (3-hydroxyphenylalanine). The latter compound induces catecholamine brain depletion yielding in rats a characteristic behavioral syndrome including forepaw padding, head weaving, backward walking, splayed hind limbs, wet dog shakes, hyperactivity and hyper-reactivity (Dyck et al., 1982).
The studies outlined above generally provide no behavioral descrip-tion of their patient sample, make no attempt to clinically characterize patients with an abnormal gut microbiome and typically disregard the importance of the developmental trajectory of children, which would impose accurate age- and sex-matching between patients and controls. Unfortunately these limitations raise great difficulties in interpreting in-consistencies between different data sets. Nonetheless, collectively these results support the hypothesis that an abnormal gastrointestinal coloni-zation may occur in a subgroup of ASD children. Several factors have been proposed as plausible contributors to this phenomenon, including hypochlorhydria (i.e., absent or low gastric acid production), abnormal gut motility, frequent use of antibiotics, and IgA deficiency. Also inherited orde novogenetic/genomic defects could be involved in some cases. In turn, gut bacteria could exert pathological actions through the direct or indirect production of systemic toxins and neurotoxins, and/or by trig-gering the production of auto-antibodies eventually leading to neuronal damage. To date, several autoantibodies have been found in autistic pa-tients, targeting a variety of endogenous antigens including anti-neuron-axon filament protein, anti-glial fibrillary acidic protein and anti-myelin-basic protein (Singh et al., 1997; Singh and Rivas, 2004; Goines et al., 2011; Braunschweig et al., 2011). Finally, in addition to ab-normal gut colonization, also excessive gut permeability in some autistic children has been reported by several (D'Eufemia et al., 1996; De Magistris et al., 2010), though not all studies (Robertson et al., 2008).
In summary, the relatively high frequency and variable spectrum of gastrointestinal symptoms reported by many parents of autistic children (Buie et al., 2010) could conceivably stem from a complex combination of abnormal gut microbiome, excessive intestinal per-meability, local immune dysreactivity and possibly pleiotropic roles of autism genes in nervous and gut tissue.
4. Urinaryp-cresol in autism spectrum disorder
4.1. Initialfindings
The results summarized in Section 3, spurred our interest into assessing urinary levels ofp-cresol in 59 non-syndromic autistic children and in 59 tightly age- and sex-matched controls (Altieri et al., 2011). Uri-naryp-cresol was measured infirst morning urines by high performance liquid chromatography-ultraviolet (HPLC-UV) with multi-wavelength diode array detector (DAD). Urinary concentrations ofp-cresol were significantly higher in autistic children compared to controls (123.5± 12.8 vs. 91.2±8.7μg/ml, Pb0.05). This elevation was surprisingly
age-dependent, as it was clearly detectable only up until and including age 7 (134.1±20.1 vs. 70.3±6.7μg/ml, P=0.005), with urinary p-cresol levels normalizing at age 8 and beyond. Levels of p-cresol were correlated neither with body mass index nor with urinary cotinine levels, excluding spurious contamination from passive smoking. Instead, p-cresol levels were significantly higher among:
(a) femaleautistic children compared to males (Pb0.05);
(b) more severely affected autistic children, regardless of sex (Pb0.05);
(c) children who underwentregression at autism onset, based on parents reporting loss of language skills after acquisition of more than 5 spoken words and loss of social abilities after ini-tial acquisition (Pb0.05).
4.2. Preliminary replicationfindings
A replication study is being undertaken on an independent sample of ASD children and controls recruited at the Center for Child and Adolescent Psychiatry of the Hopital Bretonneau in Tours (France). Preliminary anal-yses run on 34 French children already replicate significantly higher uri-naryp-cresol levels in 17 ASD cases compared to 17 matched controls (Pb0.05). This significant increase occurs only in 8 case-control pairs aged 7 or below (Pb0.01), with no significant difference beyond 7 years
of age. Urinaryp-cresol is again significantly correlated with clinical sever-ity, as documented using several clinical scales (Antonio Persico, Andrea Urbani, Sonia Cerullo, Catherine Barthelemy, Frederique Bonnet-Briault and Gabriele Tripi, unpublished observation). In addition to measuring totalp-cresol, as in our initial study (Altieri et al., 2011), here separately measured its three fractions, namely freep-cresol,p-cresylsulfate and p-cresylglucuronate. These compounds account for 0.06%, 95.26% and 4.68%, respectively, of total urinaryp-cresol in controls. Statistically signif-icant elevations in ASD children compared to controls were found for uri-nary p-cresylsulfate and p-cresylglucuronate (Pb0.05), whereas free
p-cresol displays at this stage a non-significant trend (P=0.086).
4.3. Potential sources of elevated urinary p-cresol in ASD
Several factors could independently contribute to the elevated uri-naryp-cresol levels detected in two ethnically-distinct samples:
[1] The excess inp-cresol could largely come fromenvironmental exposure. For example, low-functioning ASD children could bring to their mouth objects painted with cresol-containing gloss,“picky eaters”could absorb greater amounts of this com-pound when selecting cresol-enriched foods, while some fam-ilies could be administering cresol-enriched antioxidants to their autistic children, although this practice in relatively un-common in Italy;
[2] Gut infection with cresol-producing bacterial strainswould result in enhanced synthesis of p-cresol from tyrosine in the gut lumen, absorption through the gut wall, and urinary excretion of greater amounts of the compound. An abnormal gut microbiome could represent a spontaneous ASD-related event in some children, while in others it could follow frequent antibiotic treatments. Within this framework, the puzzling age-dependency of urinary p-cresol, elevated both in Italy and in France only among autistic children younger than 8 year of age, could be due to the maturation of the gastroin-testinal immune system and to its increasing ability to control the overgrowth of cresol-producing bacterial strains;
[3] Greater amounts of endogenous proteins in the cecumhave been shown to boostp-cresol production, absorption, and urinary ex-cretion rates. Cresol-producing gut bacteria are primarily located in the colon and essentially use tyrosine-containing proteins present in the gut lumen as their substrate. The colon lumen con-tains relatively little polypeptidic material of dietary origin, as this is largely digested and absorbed in the small intestine. In-stead, the colon lumen contains almost entirely endogenous pro-teins, encompassing secreted digestive enzymes, mucus and shed epithelial cells (Kawakami et al., 2007). Importantly, fasting results in elevated serump-cresol levels positively correlated with cecal protein concentrations in the rat (Kawakami et al., 2007).
derivatives, soy products,fish, chicken, etc.), and (e) pharmaco-logical inhibition of gastric acid secretion. Interestingly, chronic constipation is one of the most frequent gastrointestinal issues reported by parents with regard to their autistic children (Buie et al., 2010). Furthermore, modulating intestinal transit time ei-ther with fasting (Kawakami et al., 2007), or with fiber-rich diets and probiotics (Cummings et al., 1979; Kawakami et al., 2005; Nakabayashi et al., 2011) does result in enhanced and re-duced urinaryp-cresol levels, respectively, both in rodents and humans;
[4] Excessive intestinal permeability (the“leaky gut”),which was pre-viously described in a consistent subgroup of ASD children (D'Eufemia et al., 1996; De Magistris et al., 2010), could also fa-cilitatep-cresol absorption. In turn, excessivep-cresol produc-tion and absorpproduc-tion could conceivably alter permeability either directly or through inflammatory mechanisms.
Studies are ongoing to determine which routes are involved in the enhanced urinaryp-cresol excretion we recorded in young autistic chil-dren. Unfortunately urinaryp-cresol measures are not correlated with cecal and faecalp-cresol levels, likely due to interindividual variability in liver metabolic rates (Birkett et al., 1995). However, while urinary p-cresol does not allow immediate inferences about gut-related mech-anisms, it does, however, parallel plasmap-cresol levels, which are tox-icologically active over the CNS and other peripheral tissues (Birkett et al., 1995).
4.4. Other gut-derived bacterial metabolic products possibly relevant to autism
P-cresol can essentially originate either from the gut or from environ-mental exposure (Fig. 1). Interesting parallels can be drawn between p-cresol and other behaviorally active, gut-derived bacterial metabolic or break-down products. To date, the best-described example possibly relevant to autism is propionic acid (PPA), a short chain fatty acid produced in the gut by anaerobic bacteria including Clostridia and Propionibacteria, through fermentation of dietary carbohydrates and several aminoacids (Al-Lahham et al., 2010). PPA can also derive from environmental exposure (it is used as a food preservative in many wheat and dairy products) and, differently fromp-cresol, it is an endog-enous compound, namely an intermediate of human fatty acid metabo-lism (Al-Lahham et al., 2010). Intracerebroventricular administration of propionic acid in young rats yields behavioral abnormalities reminiscent of ASD, including perseverance in object-directed behavior, impaired re-versal learning in the T-maze and reduced interaction with a novel rat compared to a novel object (MacFabe et al., 2011). The hippocampus and white matter of these same animals display neuroinflammation in the form of activated microglia and reactive astrogliosis (MacFabe et al., 2011), similar to those detected in post-mortem autistic brains (Vargas et al., 2005). Also developmental delay and cognitive deficits have been documented in rodents prenatally exposed to PPA (Brusque et al., 1999).
In summary,p-cresol should not be viewed as necessarily unique, but may rather represent one among several gut bacteria-derived compounds able to negatively influence human development and be-havior. Nonetheless, the human clinical data summarized above sug-gest that even in this scenariop-cresol may exert particularly sizable effects, especially in small autistic children.
5. Isp-cresol orp-cresylsulfate the true toxicant?
The conjugated derivativep-cresylsulfate represents over 95% of total urinaryp-cresol, as measured in our replica sample of French ASD chil-dren (seeSection 4.2). If pharmacologically active,P-cresylsulfate could thus conceivably represent the true“toxicants”, in addition to or instead of freep-cresol, which by comparison is found only in minute amounts.
Converging evidence from studies of chronic renal failure begins to sup-port this hypothesis. For many years,p-cresol was regarded as one of the main uremic toxins (Vanholder et al., 1999): it was believed to accumu-late in the body of patients suffering from chronic renal failure and to produce many signs and symptoms of the disease. It was later realized that a preliminary step in the procedures used to measurep-cresol was the strong acidification necessary for deproteinization; this step resulted in the hydrolysis ofp-cresylsulfate andp-cresylglucuronate, spuriously boosting the concentration of freep-cresol (Vanholder et al., 2011; Lin et al., 2011).
P-cresylsulfate, now believed to represent the real uremic toxin, interestingly yields functional abnormalities in some cases similar, but in other cases different, or even opposite, to those produced by p-cresol. For example,p-cresol impairs oxygen-derived free radical production by granulocytesin vitro(De Smet et al., 2003), whereas p-cresylsulfate activates free radical production by leukocytes yield-ing excessive oxidative stress (Schepers et al., 2007; Meert et al., 2011). Elevatedp-cresylsulfate levels in chronic kidney disease have been conclusively associated with poor clinical outcome, due to endo-thelial damage and vascular calcifications eventually leading to coro-nary heart disease (Meijers et al., 2009;Liabeuf et al., 2010;Wang et al., 2010, 2012). Similar results were found in diabetic nephropathy (Chiu et al., 2010). A greater role forp-cresylsulfate rather than for freep-cresol in autism is more compatible with our preliminary re-sults, indicating that the vast majority of total urinaryp-cresol excess in ASD children is actually due top-cresylsulfate. The definition of the functional consequences of excessivep-cresylsulfate blood levels re-quires further investigation, in order to understand whether and to what extent excessivep-cresylsulfate may exert clinically-relevant ef-fects in ASD children, possibly contributing for example to enhanced oxidative stress (Chauhan and Chauhan, 2006).
6. Urinaryp-cresol as a potential biomarker for ASD in small children
(Holmes et al., 2008). Conceivably, depending on baseline gutflora composition, similar pathophysiological gut abnormalities in Italian, French and Australian autistics could well yield overgrowth of different bacterial strains, possibly resulting in different urinary patterns of gut-derived compounds.
Despite these caveats, interest into urinaryp-cresol and/or its conju-gated derivativep-cresylsulfate, is spurred not only by their negative effects on multiple biological systems, but also by their potential for in-clusion into a multibiomarker diagnostic panel in small autistic chil-dren. Our original data set (Altieri et al., 2011) shows that using urinaryp-cresol as a diagnostic biomarker in children 2–7 years old by establishing an arbitrary threshold at the highest level recorded in our controls (i.e., > 150μg/ml), the test would have been positive in 9/32 autistics vs. 0/32 controls (P=0.002). Among these nine autistic children, 5/8 (62.5%) were females vs. 4/24 (16.7%) males (Pb0.05). A
diagnosis of severe Autistic Disorder was made in 8/9 (88.9%) whereas only 1/9 (11.1%) satisfied DSM-IV diagnostic criteria for Pervasive Developmental Disorder Not Otherwise Specified (PDD-NOS). Similarly, all 15 items of the Children Autism Rating Scales (CARS) (Schopler et al., 1986), were positively correlated with urinaryp-cresol concentrations among ASD children aged≤7 years, confirming the link between urinaryp-cresol and autism severity regardless of sex. It will be interest-ing to verify whether urinaryp-cresol holds similar degrees of informa-tiveness in our replication study, once patient recruitment is completed.
7. Cellular and systemic actions ofp-cresol/p-cresylsulfate potentially relevant to ASD
P-cresol and/orp-cresylsulfate could modulate autism severity in small children acting through several mechanisms, possibly includ-ing, but not necessarily limited to the following:
1. P-cresol blocks the growth of many anaerobic bacteria. A few strains, in some casesp-cresol producers but not necessarily, instead tolerate concentrations as high as 0.5% (Dawson et al., 2011). Elevated p-cresol concentrations in the intestinal lumen, perhaps in conjunc-tion with excessive carbohydrate availability (Williams et al., 2011), could thus contribute to shape the gutflora in ways yielding gastro-intestinal symptoms in a sizable subgroup of ASD children (Buie et al., 2010).
2. The pro-inflammatory effects ofp-cresylsulfate could contribute to enhanced oxidative stress and risk of coronary heart disease in ASD (Chauhan and Chauhan, 2006; Tyler et al., 2011).
3. Pharmacological intervention in autistic children could be directly influenced by plasmap-cresol levels. Some autistic children appear exquisitely sensitive to develop side effects to psychoactive drugs at very low doses.Clayton et al. (2009), using the analgesic and an-tipyretic drug acetaminophen, found that individuals with high pre-dose urinary levels ofp-cresylsulfate had low post-dose uri-nary ratios of acetaminophen sulfate to acetaminophen glucuro-nide. A reduction in liver sulfation capacity, specifically tested using acetaminophen, has also been recorded in low functioning autistic individuals (Alberti et al., 1999). Thus, competition of p-cresol for both hepatic sulfotransferases and for albumin could impact drug clearance, leading to increased free drug plasma levels and to adverse side effects at the onset of therapy (Wilson, 2009). 4. The membrane depolarizing properties ofp-cresol are especially intriguing (Elliott and Elliott, 1997), in light of its frequent co-morbidity with hyperactivity and with epilepsy, the latter diag-nosed in as many as 30% of autistic individuals (Tuchman and Rapin, 2002).
5. P-cresylsulfate has been identified as the main component of uri-nary myelin basic protein (MBP)-like material (Cao et al., 2000). In other words, urinaryp-cresylsulfate shares conformational fea-tures with MBP so that it cross-reacts with antibodies targeted against MBP peptide 83–89. Urinary MBP-like material increases
in progressive multiple sclerosis and molecular mimicry could conceivably trigger autoimmunity also in a subset of autistic chil-dren and of mothers, who produce anti-brain autoantibodies (Singh et al., 1997; Singh and Rivas, 2004; Goines et al., 2011; Braunschweig et al., 2011).
The behavioral effects of non-toxicological doses ofp-cresol in an-imals have not been reported. We are currently performing this study: preliminary data support prominent behavioral effects in BTBR mice following acute i.v. administration ofp-cresol doses com-patible with urinary excretion rates recorded in autistic children and in adults with chronic kidney disease (Tiziana Pascucci and Antonio Persico, unpublished data). On the other hand, exploratory cellular studies addressing whetherp-cresol andp-cresylsulfate may directly enhance calcium release from intracellular stores or increase calcium entry have yielded negative results (Roberto Piacentini, Claudio Grassi and Antonio Persico, unpublished data), although indi-rect effects could still be mediated by the depolarizing properties of p-cresol (Elliott and Elliott, 1997). Further studies aimed at assessing at the cellular level howp-cresol andp-cresylsulfate can influence mi-tochondrial function, neurite outgrowth and synaptogenesis are warranted. Moreover, animal models could unveil negative effects on CNS function and development exerted byp-cresol either directly or through its influence on immune parameters and on endothelial permeability (Vanholder et al., 1995; De Smet et al., 2003; Cerini et al., 2004). Rodent models of autism, such as BTBR mice or transgenic animals carrying autism-causing human mutations (McFarlane et al., 2008; Ey et al., 2011) should be especially informative in this regard.
8. Conclusions
The currently available evidence summarized in this review pro-vides initial support forpostnatalexposure to elevatedp-cresol and/or p-cresylsulfate as a pathoplastic contributor to the severity of behavior-al abnormbehavior-alities and cognitive impairment in autistic children. In partic-ular,p-cresol and/orp-cresylsulfate seemingly belong to a restricted set of gut- or environmentally-derived compounds potentially able to worsen behavioral abnormalities and cognitive impairment in small autistic children. Studies performed in specific cellular and animal models, as well as prospective follow-up studies involving baby-siblings (i.e.,“high-risk”neonates born to parents with one grown-up child already diagnosed with ASD) will be instrumental in determining whether early prenatal exposure to environment- or maternal gut-derivedp-cresol may provide pathogenic contributions, significantly in-creasing the risk of autism spectrum disorder in the offspring. It will also be important to determine the precise origin of elevatedp-cresol in small autistic children and to define its influence on the spectrum and intensity of clinical signs and symptoms of ASD, on developmental tra-jectories, and on endophenotypic subgroupings of small children with ASD. Replication studies will also need to determine whether elevated urinaryp-cresol/p-cresylsulfate in ASD is specific to some racial and eth-nic groups or represents a generalizedfinding. If positive, these studies spur hope into the design of cresol-resistant probiotics possibly able to improve behavioral abnormalities when targeted to ASD children with elevated urinaryp-cresol.
Conflict of interest statement
I have no conflict of interests.
Acknowledgments
Italy), Autism Aid ONLUS (Naples, Italy), Autism Speaks (Princeton, NJ), the Autism Research Institute (San Diego, CA), and the European Union (IMI project EU-AIMS).
References
Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinalflora and gastro-intestinal status in children with autism—comparisons to typical children and cor-relation with autism severity. BMC Gastroenterol 2011;11:22.
Alberti A, Pirrone P, Elia M, Waring RH, Romano C. Sulphation deficit in“low-functioning” autistic children: a pilot study. Biol Psychiatry 1999;46:420–4.
Al-Lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta 2010;1801:1175–83.
Altieri L, Neri C, Sacco R, Curatolo P, Benvenuto A, Muratori F, et al. Urinaryp-cresol is elevated in small children with severe autism spectrum disorder. Biomarkers 2011;16:252–60.
Andersen A. Final report on the safety assessment of sodium p-chloro-m-cresol, p-chloro-m-cresol, chlorothymol, mixed cresols, m-cresol, o-cresol, p-cresol, isopropyl cresols, thymol, o-cymen-5-ol, and carvacrol. Int J Toxicol 2006;25(Suppl. 1):29-127. Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, et al. Autism as a
strongly genetic disorder: evidence from a British twin study. Psychol Med 1995;25:63–77.
Bal-Price AK, Coecke S, Costa L, Crofton KM, Fritsche E, Goldberg A, Grandjean P, Lein PJ, Li A, Lucchini R, Mundy WR, Padilla S, Persico AM, Seiler AEM, Kreysa J. Conference Report: Advancing the Science of Developmental Neurotoxicity (DNT) Testing for Better Safety Evaluation. ALTEX 2012;29:202–15.
Baron-Cohen S, Scott FJ, Allison C, Williams J, Bolton P, Matthews FE, et al. Prevalence of autism‐spectrum conditions: UK school‐based population study. Br J Psychiatry 2009;194:500–9.
Bauman M, Kemper T. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci 2005;23:183–7.
Beckh S, Pongs O. Members of the RCK potassium channel family are differentially expressed in the rat nervous system. EMBO J 1990;9:777–82.
Bergé-Lefranc D, Chaspoul F, Calaf R, Charpiot P, Brunet P, Gallice P. Binding of p-cresylsulfate and p-cresol to human serum albumin studied by microcalorime-try. J Phys Chem B 2010;114:1661–5.
Birkett AM, Jones GP, Muir JG. Simple high-performance liquid chromatographic anal-ysis of phenol and p-cresol in urine and feces. J Chromatogr B Biomed Appl 1995;674:187–91.
Bone E, Tamm A, Hill M. The production of urinary phenols by gut bacteria and their possible role in the causation of large bowler cancer. Am J Clin Nutr 1976;29:1448–54. Braunschweig D, Duncanson P, Boyce R, Hansen R, Ashwood P, Pessah IN, et al.
Behav-ioral correlates of maternal antibody status among children with autism. J Autism Dev Disord 2012;42:1435–45.
Brusque AM, Mello CF, Buchanan DN, Terracciano ST, Rocha MP, Vargas CR, et al. Effect of chemically induced propionic acidemia on neurobehavioral development of rats. Pharmacol Biochem Behav 1999;64:529–34.
Buie T, Campbell DB, Fuchs III GJ, Furuta GT, Levy J, Vandewater J, et al. Evaluation, di-agnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics 2010;125:1-18.
Cafaro V, Notomista E, Capasso P, Di Donato A. Mutation of glutamic acid 103 of toluene o-xylene monooxygenase as a means to control the catabolic efficiency of a recom-binant upper pathway for degradation of methylated aromatic compunds. Appl Environ Microbiol 2005;71:4744–50.
Calderón-Guzmán D, Hernández-Islas JL, Espítia Vázquez IR, Barragán-Mejía G, Hernández-García E, Del Angel DS, et al. Effect of toluene and cresols on Na+, K+-ATPase, and serotonin in rat brain. Regul Toxicol Pharmacol 2005;41:1–5. Cao L, Kirk MC, Coward LU, Jackson P, Whitaker JN. p-cresol sulphate is the dominant
component of urinary myelin basic protein like material. Arch Biochem Biophys 2000;377:9-21.
Cerini C, Dou L, Anfosso F, Sabatier F, Moal V, Glorieux G, et al. P-cresol, a uremic reten-tion solute, alters the endothelial barrier funcreten-tion in vitro. Thromb Haemost 2004;92:140–50.
Chang MC, Wang TM, Yeung SY, Jeng PY, Liao CH, Lin TY, et al. Antiplatelet effect by p-cresol, a uremic and environmental toxicant, is related to inhibition of reactive oxygen species, ERK/p38 signaling and thromboxane A2 production. Atherosclero-sis 2011;219:559–65.
Chauhan A, Chauhan V. Oxidative stress in autism. Pathophysiology 2006;13:171–81. Chiu CA, Lu LF, Yu TH, Hung WC, Chung FM, Tsai IT, et al. Increased levels of total
P-Cresylsulphate and indoxyl sulphate are associated with coronary artery disease in patients with diabetic nephropathy. Rev Diabet Stud 2010;7:275–84. Clayton TA, Baker D, Lindon JC, Everett JR, Nicholson JK. Pharmacometabonomic
iden-tification of a significant host–microbiome metabolic interaction affecting human drug metabolism. Proc Natl Acad Sci U S A 2009;106:14728–33.
Comi AM, Zimmerman AW, Frye VH, Law PA, Peeden JN. Familial clustering of autoim-mune disorders and evaluation of medical risk factors in Autism. J Child Neurol 1999;14:388–94.
Cummings JH, Hill MJ, Bone ES, Branch WJ, Jenkins DJ. The effect of meat protein and dietaryfiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am J Clin Nutr 1979;32:2094–101.
Dawson LF, Donahue EH, Cartman ST, Barton RH, Bundy J, McNerney R, et al. The anal-ysis of para-cresol production and tolerance inClostridium difficile027 and 012 strains. BMC Microbiol 2011;11:86.
De Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr 2010;51:418–24.
De Smet R, Van Kaer J, Van Vlem B, De Cubber A, Brunet P, Lameire N, et al. Toxicity of free p-Cresol: a prospective and cross-sectional analysis. Clin Chem 2003;49:470–8. DeBruin A. Metabolism of occupational agents. In: De Bruin A, editor. Biochemical
tox-icology of environmental agents. Amsterdam: Elsevier/North-Holland Biomedical Press; 1976. p. 87-170.
D'Eufemia P, Celli M, Finocchiaro R, Pacifico L, Viozzi L, Zaccagnini M, et al. Abnormal intestinal permeability in children with autism. Acta Paediatr 1996;85:1076–9. Dou L, Cerini C, Brunet P, Guilianelli C, Moal V, Grau G, et al. P-cresol, a uremic toxin,
de-creases endothelial cell response to inflammatory cytokines. Kidney Int 2002;62: 1999–2009.
Dyck LE, Kazakoff CW, Dourish CT. The role of catecholamines, 5- hydroxytryptamine andm-tyramine in the behavioural effects ofm-tyrosine in the rat. Eur J Pharmacol 1982;84:139–49.
Ecker C, Marquand A, Mourão-Miranda J, Johnston P, Daly EM, Brammer MJ, et al. Describ-ing the brain in autism infive dimensions—magnetic resonance imaging-assisted diag-nosis of autism spectrum disorder using a multiparameter classification approach. J Neurosci 2010;30:10612–23.
Elliott AA, Elliott JR. Voltage-dependent inhibition of RCK1 K+ channels by phenol, p-cresol, and benzyl alcohol. Mol Pharmacol 1997;51:475–83.
Elsden SR, Hilton MG, Waller JM. The end products of the metabolism of aromatic amino acids by Clostridia. Arch Microbiol 1976;107:283–8.
Enstrom AM, Lit L, Onore CE, Gregg JP, Hansen RL, Pessah IN, et al. Altered gene expres-sion and function of peripheral blood natural killer cells in children with autism. Brain Behav Immun 2009;23:124–33.
Ey E, Leblond CS, Bourgeron T. Behavioral profiles of mouse models for autism spec-trum disorders. Autism Res 2011;4:5-16.
Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, et al. Consensus paper: pathological role of the cerebellum in autism. Cerebellum 2012;11:777–807. Faure V, Cerini C, Paul P, Berland Y, Dignat-George F, Brunet P. The uremic solute p-cresol decreases leukocyte transendothelial migration in vitro. Int Immunol 2006;18:1453–9.
Finegold SM, Molitoris D, Song Y, Liu C, Vaisanem ML, Bolte E, et al. Gastrointestinal mi-croflora studies in late-onset autism. Clin Infect Dis 2002;35:6-16.
Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010;16:444–53.
Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res 2009;65: 591–8.
Garbett K, Ebert PJ, Mitchell A, Lintas C, Manzi B, Mirnics K, et al. Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiol Dis 2008;30: 303–11.
Goines P, Van de Water J. The immune system's role in the biology of autism. Curr Opin Neurol 2010;23:111–7.
Goines P, Haapanen L, Boyce R, Duncanson P, Braunschweig D, Delwiche L, et al. Auto-antibodies to cerebellum in children with autism associate with behavior. Brain Behav Immun 2011;25:514–23.
Goodhart PJ, DeWolf WE, Kruse LI. Mechanism-based inactivation of dopamine beta-hydroxylase by p-cresol and related alkylphenols. Biochemistry 1987;26: 2576–83.
Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritabil-ity and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 2011;68:1095–102.
Holmes E, Loo RL, Stamler J, Bictash M, Yap IK, Chan Q, et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature 2008;453:396–400. Holmes E, Li JV, Athanasiou T, Ashrafian H, Nicholson JK. Understanding the role of gut microbiome-host metabolic signal disruption in health and disease. Trends Microbiol 2011;19:349–59.
Jyonouchi H, Geng L, Streck DL, Toruner GA. Children with autism spectrum disorders (ASD) who exhibit chronic gastrointestinal (GI) symptoms and markedfluctuation of behavioral symptoms exhibit distinct innate immune abnormalities and transcrip-tional profiles of peripheral blood (PB) monocytes. J Neuroimmunol 2011;238:73–80. Kawakami K, Makino I, Asahara T, Kato I, Onoue M. Dietary galacto-oligosaccharides mixture can suppress serum phenol and p-cresol levels in rats fed tyrosine diet. J Nutr Sci Vitaminol (Tokyo) 2005;51:182–6.
Kawakami K, Kojima K, Makino I, Kato I, Onoue M. Fasting enhances p-cresol produc-tion in the rat intestinal tract. Exp Anim 2007;56:301–7.
Kawakami K, Makino I, Kato I, Uchida K, Onoue M. p-Cresol inhibits IL-12 production by murine macrophages stimulated with bacterial immunostimulant. Immunopharmacol Immunotoxicol 2009;31:304–9.
Kinross JM, Darzi AW, Nicholson JK. Gut microbiome–host interactions in health and disease. Genome Med 2011;3:14.
Kitagawa A. Effects of cresols (O-, M-, and P-isomers) on the bioenergetic system in isolated rat liver mitochondria. Drug Chem Toxicol 2001;24:39–47.
Leblond CS, Heinrich J, Delorme R, Proepper C, Betancur C, Huguet G, et al. Genetic and functional analyses of SHANK2 mutations suggest a multiple hit model of autism spectrum disorders. PLoS Genet. 2012 Feb;8(2):e1002521. Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, Temmar M, Choukroun G, Vanholder R, Massy ZA; European Uraemic Toxin Work Group (EUTox). Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 2010;25:1183–91.
Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 2010;25:1183–91.
Lin CH, Yang JY. Chemical burn with cresol intoxication and multiple organ failure. Burns 1992;18:162–6.
Lin CJ, Chen HH, Pan CF, Chuang CK, Wang TJ, Sun FJ, et al. p-Cresylsulfate and indoxyl sul-fate level at different stages of chronic kidney disease. J Clin Lab Anal 2011;25:191–7. Lintas C, Sacco R, Persico AM. Genome-wide expression studies in autism spectrum
dis-order, Rett syndrome, and Down syndrome. Neurobiol Dis 2012;45:57–68. MacFabe DF, Cain NE, Boon F, Ossenkopp KP, Cain DP. Effects of the entericbacterial
metabolic product propionic acid on object-directed behavior, social behavior, cog-nition, and neuroinflammation in adolescent rats: relevance to autism spectrum disorder. Behav Brain Res 2011;217:47–54.
Mandel HG. Pathways of drug biotransformation: biochemical conjugations. In: LaDu BN, Mandel HG, Way EL, editors. Pathways of drug biotransformation. Baltimore: Williams and Wilkins; 1971. p. 149–86.
McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like be-havioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav 2008;7:152–63. Meert N, Schepers E, Glorieux G, Van Landschoot M, Goeman JL, Waterloos MA, et al. Novel
method for simultaneous determination of p-cresylsulphate and p-cresylglucuronide: clinical data and pathophysiological implications. Nephrol Dial Transplant 2012;27: 2388–96.
Meijers BK, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 2008;73:1174–80.
Meijers BK, Van Kerckhoven S, Verbeke K, Dehaen W, Vanrenterghem Y, Hoylaerts MF, et al. The uremic retention solute p-cresyl sulfate and markers of endothelial dam-age. Am J Kidney Dis 2009;54:891–901.
Miller M, Strömland K, Ventura L, Johansson M, Bandim J, Gillberg C. Autism associated with conditions characterized by developmental errors in early embryogenesis: a mini review. Int J Dev Neurosci 2005;23:201–19.
Nakabayashi I, Nakamura M, Kawakami K, Ohta T, Kato I, Uchida K, et al. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: a prelim-inary study. Nephrol Dial Transplant 2011;26:1094–8.
Neale BM, Kou Y, Liu L, Ma'ayan A, Samocha KE, Sabo A, et al. Patterns and rates of ex-onic de novo mutations in autism spectrum disorders. Nature 2012;485:242–5. OECD. m-/p-Cresol category, screening information data set, initial assessment report.
Paris: UNEP Publications; 2003. Available atwww.chem.unep.ch/irptc/sids/oecdsids/ m-p-cresols.pdf.
O'Roak BJ, Deriziotis P, Lee C, Vives L, Schwartz JJ, Girirajan S, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet 2011;43:585–9.
Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut mi-croflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol 2005;54:987–91.
Persico AM. Autisms. In: Comprehensive Developmental Neuroscience, Rakic P and Rubenstein J, editors. San Diego: Elsevier; 2013.
Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenet-ic and environmental clues. Trends Neurosci 2006;29:349–58.
Ramakrishna BS, Roberts-Thomson IC, Pannall PR, Roediger WE. Impaired sulphation of phe-nol by the colonic mucosa in quiescent and active ulcerative colitis. Gut 1991;32:46–9. Renwick AG, Thakrar A, Lawrie CA, George CF. Microbial amino acid metabolites and bladder
cancer: no evidence of promoting activity in man. Hum Toxicol 1988;7:267–72. Roberts MS, Anderson RA, Swarbrick J. Permeability of human epidermis to the
pheno-lic compounds. J Pharm Pharmacol 1977;29:677–89.
Robertson MA, Sigalet DL, Holst JJ, Meddings JB, Wood J, Sharkey KA. Intestinal perme-ability and glucagon-like peptide-2 in children with autism: a controlled pilot study. J Autism Dev Disord 2008;38:1066–71.
Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 2012;485:237–41.
Schepers E, Meert N, Glorieux G, Goeman J, Van der Eycken J, Vanholder R. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant 2007;22:592–6.
Schopler E, Reichler RJ, Rochen Renner BR. The Childhood Autism Rating Scale for diag-nostic screening and classification of autism. New York: Irvington; 1986. Seak CK, Lin CC, Seak CJ, Hsu TY, Chang CC. A case of black urine and dark skin - cresol
poisoning. Clin Toxicol (Phila) 2010;48:959–60.
Selmer T, Andrei PI. p-Hydroxyphenylacetate decarboxylase fromClostridium difficile. A novel glycyl radical enzyme catalysing the formation of p-cresol. Eur J Biochem 2001;268:1363–72.
Shaw W. Increased urinary excretion of a 3-(3-hydroxy-phenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylanine metabolite ofClostridiaspp. In the gastro-intestinal tract, in urine samples from patients with autism and schizophrenia. Nutr Neurosci 2010;13:135–43.
Singh VK, Rivas WH. Prevalence of serum antibodies to caudate nucleus in autistic chil-dren. Neurosci Lett 2004;355:53–6.
Singh VK, Warren RP, Averett R, Ghaziuddin M. Circulating autoantibodies to neuronal and glialfilament proteins in autism. Pediatr Neurol 1997;17:88–90.
Song Y, Liu C, Finegold SM. Real-time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol 2004;70:6459–65.
Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, Jakobsson G, et al. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry 1989;30:405–16.
Thompson DC, Perera K, London R. Studies on the mechanism of hepatotoxicity of 4-methylphenol (p-cresol): effects of deuterium labeling and ring substitution. Chem Biol Interact 1996;101:1-11.
Tuchman R, Rapin I. Epilepsy in autism. Lancet Neurol 2002;1:352–8.
Tyler CV, Schramm SC, Karafa M, Tang AS, Jain AK. Chronic disease risks in young adults with autism spectrum disorder: forewarned is forearmed. Am J Intellect Dev Disabil 2011;116:371–80.
Vanholder R, De Smet R, Waterloos MA, Van Landschoot N, Vogeleere P, Hoste E, et al. Mechanisms of uremic inhibition of phagocyte reactive species production: char-acterization of the role of p-cresol. Kidney Int 1995;47:510–7.
Vanholder R, De Smet R, Lesaffer G. p-cresol: a toxin revealing many neglected but rel-evant aspects of uraemic toxicity. Nephrol Dial Transplant 1999;14:2813–5. Vanholder R, Bammens B, de Loor H, Glorieux G, Meijers B, Schepers E, et al. Warning: the
unfortunate end of p-cresol as a uraemic toxin. Nephrol Dial Transplant 2011;26: 1464–7.
Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 2005;57: 67–81.
Veenstra-VanderWeele J, Blakely RD. Networking in autism: leveraging genetic, bio-marker and model system findings in the search for new treatments. Neuropsychopharmacology 2012;37:196–212.
Walsh P, Elsabbagh M, Bolton P, Singh I. In search of biomarkers for autism: scientific, social and ethical challenges. Nat Rev Neurosci 2011;12:603–12.
Wang CP, Lu LF, Yu TH, Hung WC, Chiu CA, Chung FM, et al. Serum levels of total p-cresylsulphate are associated with angiographic coronary atherosclerosis sever-ity in stable angina patients with early stage of renal failure. Atherosclerosis 2010;211:579–83.
Wang L, Angley MT, Gerber JP, Sorich MJ. A review of candidate urinary biomarkers for autism spectrum disorder. Biomarkers 2011a;16:537–52.
Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Low relative abundances of the mucolytic bacteriumAkkermansia muciniphilaandBifidobacterium spp. in Feces of Children with Autism. Appl Environ Microbiol 2011b;77:6718–21. Wang CP, Lu LF, Yu TH, Hung WC, Chiu CA, Chung FM, et al. Associations among chronic
kidney disease, high total p-cresylsulfate and major adverse cardiac events. J Nephrol Mar 2012;23.http://dx.doi.org/10.5301/jn.5000111. [Epub ahead of print]. Whited GM, Gibson DT. Toluene-4-monoxygenase, a three component enzyme system
that catalyzes the oxidation of toluene to p-cresol inPseudomonas mendocinaKR1. J Bacteriol 1991;173:3017–20.
Williams BL, Hornig M, Buie T, Bauman ML, Paik MC, Wick I, et al. Impaired carbohy-drate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One 2011;6:e24585. Williams BL, Hornig M, Parekh T, Lipkin WI. Application of novel PCR-based methods
for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal distur-bances. mBio 2012;3:e00261-11.
Wilson ID. Drugs, bugs, and personalized medicine: pharmacometabonomics enters the ring. Proc Natl Acad Sci U S A 2009;106:14187–8.
Wu ML, Tsai WJ, Yang CC, Deng JF. Concentrated cresol intoxication. Vet Hum Toxicol 1998;40:341–3.
Yan Z, Zhong HM, Maher N, Torres R, Leo GC, Caldwell GW, et al. Bioactivation of 4-methylphenol (p-cresol) via cytochrome P450-mediated aromatic oxidation in human liver microsomes. Drug Metab Dispos 2005;33:1867–76.
Yap IK, Angley M, Veselkov KA, Holmes E, Lindon JC, Nicholson JK. Urinary metabolic phenotyping differentiates children with autism from their unaffected siblings and age-matched controls. J Proteome Res 2010;9:2996–3004.
Yehuda S, Carasso RL, Mostofsky DI. Essential fatty acid preparation (SR-3) raises the seizure threshold in rats. Eur J Pharmacol 1994;254:193–8.
Yokoyama MT, Carlson JR. Production of skatole and p-cresol by a RumenLactobacillus sp. Appl Environ Microbiol 1981;41:71–6.