by Prior Subchronic Phencyclidine Administration:
Evidence for Cognitive Impulsivity
J. David Jentsch, Robert H. Roth, and Jane R. Taylor
Background: Impulsivity associated with frontal cortical dysfunction appears to be a direct consequence of chronic consumption of drugs of abuse, though few investigations in animals have attempted to directly address this issue. In this study the effects of repeated, intermittent administra-tion of a psychotomimetic drug of abuse, phencyclidine, on the acquisition and performance of a task sensitive to corticostriatal function was examined in nonhuman primates.
Methods: Monkeys were repeatedly exposed to phency-clidine (0.3 mg/kg) twice daily for 14 days. Acquisition and performance on an object-retrieval detour task was subsequently examined for up to 28 days after drug withdrawal.
Results: Animals treated with phencyclidine exhibited impaired acquisition of the task. The performance of trials requiring inhibitory control (as opposed to solely sensory-guided responding) was specifically impaired by prior phencyclidine administration. Impairments were found to be due to increased perseveration and barrier reaching. As is the case after frontal cortex ablation, the behavioral deficits were particularly evident during acquisition and appeared to be alleviated by prolonged training.
Conclusions: The current data demonstrate that sub-chronic administration of phencyclidine can produce def-icits in inhibitory response control that are manifest as impulsivity (increased control of behavior by uncondi-tioned, appetitive stimuli). These data suggest that long-term phencyclidine exposure induces frontostriatal-like cognitive impairments and may represent a potential (drug induced) model for the study of prefrontal cortical cognitive and dopaminergic dysfunction. Biol Psychiatry 2000;48:415– 424 © 2000 Society of Biological Psychiatry
Key Words: Inhibitory control, dopamine, prefrontal cortex, drug addiction, schizophrenia, animal model
Introduction
A
t present, little is known, from studies in animals or humans, about the consequences of chronic intake of or withdrawal from drugs of abuse on cognitive function mediated by the frontal cortex and its inputs to the striatum. Several studies suggest that frontal cortical dysfunction can result in impulsivity, perseverative re-sponding, and impaired planning and working memory function (Baddeley and della Scalla 1996; Damasio 1996; Fuster 1980; Goldman-Rakic 1987, 1996; Robbins 1996; Roberts et al 1998). It has been argued that impulsivity and perseveration may be due to a failure of inhibitory mod-ulation of conditioned behavior by working memory or executive processes of the frontal cortex (Dias et al 1996a, 1997; Goldman-Rakic 1987; Robbins 1996). This suggests that the capacity of the frontal cortex to actively inhibit conditioned responses, mediated largely by the striatum, may be lost after frontal cortical damage.An elegant series of studies over the past 3 decades has demonstrated that experimentally produced frontal lobe lesions in animals can produce increases in the ability of conditioned stimuli to control behavior. For example, lesions to ventral regions of the frontal cortex potently impair performance of tasks that require shifting respond-ing away from conditioned-reinforcrespond-ing stimuli (e.g., dis-crimination reversal; Butter 1968; Butter et al 1973; Dias et al 1996a; Iversen and Mishkin 1970; Ridley et al 1993). Moreover, lesions to the frontal cortex actually augment responding for an established conditioned reinforcing stimulus (Weissenborn et al 1997).
Moll and Kuypers (1977) originally showed that large frontal lesions (including the dorsolateral prefrontal, me-dial prefrontal, premotor, supplementary motor, and lateral orbital cortices) impaired performance of a task that involved inhibiting responding into a transparent barrier when reaching for a reward. The barrier negotiation strategy was subsequently adapted into an object retrieval/ detour task (Diamond 1990). Monkeys had to retrieve a reward from a transparent box, often needing to circum-vent a transparent barrier to do so successfully. Lesions of From the Section of Neurobiology (JDJ) and Departments of Psychiatry (RHR,
JRT) and Pharmacology (RHR), Yale University School of Medicine, New Haven, Connecticut.
Address reprint requests to Jane R. Taylor, Ph.D., Yale University School of Medicine, Department of Psychiatry, 333 Cedar Street, P.O. Box 208068, New Haven CT 06520-8068.
Received January 31, 2000; revised May 2, 2000; accepted May 9, 2000.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
the dorsolateral prefrontal cortex (areas 8,9,46, part of 10) impaired task performance, whereas parietal lobe lesions failed to affect successful performance of the task; hip-pocampal lesions also failed to impair performance of object retrieval (Diamond and Goldman-Rakic 1985; Di-amond et al 1989). Furthermore, performance on this task has been recently shown to be sensitive to large excito-toxic lesions of (lateral and ventral) frontal cortex of marmosets (Dias et al 1996b) or more selective lesions of the orbital frontal cortex (Wallis et al 1997).
Long-term impairments on this task are also a result of MPTP-induced reductions in corticostriatal dopamine lev-els in monkeys (Taylor et al 1990a, 1990b; Schneider and Kovelowski 1990), and deficits are additionally caused by subchronic phencyclidine (PCP) treatment (Jentsch et al 1997). Moreover, we have found selective dopaminergic dysfunction in subregions of the frontal cortex after subchronic PCP-treatment which correlate with perfor-mance on the object retrieval task (Jentsch et al 1999). In addition, children affected by phenylketonuria (PKU), who despite early and continuous treatment for PKU have average plasma phenylalanine [Phe] 6 to 10 mg/dL, are impaired on an object retrieval/detour task, whereas those that have average plasma Phe levels 2 to 5.9 mg/dL are not impaired (Diamond 1996; Diamond et al 1997). These impairments have been suggested to be due to reduced cortical dopamine levels because in an animal model of PKU, prefrontal cortical dopamine and homovanillic acid metabolite levels were found to be reduced (Dia-mond et al 1994). Thus, there are several converging lines of evidence that suggest that the object retrieval task appears to be sensitive to frontal cortical and dopaminergic function.
The aim of our study was to investigate the impact of subchronic exposure to a drug of abuse, PCP, on the acquisition and performance of the object retrieval/detour task. Long-term intake of PCP previously has been re-ported to result in neuropsychologic impairments in hu-mans (Cosgrove and Newell 1991). The focus was to extend our earlier findings by examining the behavior of PCP-treated and control monkeys on trials that taxed response inhibition versus those that required simply sensory-guided behavior to test the hypothesis that deficits are related specifically to cognitive dysfunction. We thus utilized our modified version of the object retrieval/detour (Taylor et al 1990a, 1990b) that was designed to quanti-tatively examine indices of response inhibition, persevera-tion, fine motor funcpersevera-tion, and motivation in monkeys. Behavior was examined to determine whether the ob-served impairments were persistent and whether noncog-nitive impairment(s) were evident. These distinctions are critical to characterize the specificity of the cognitive deficits to one of impulsivity, as well as to characterize the
relationship between these behavioral impairments and corticostriatal function.
Methods and Materials
Animals
Our study included as subjects 32 experimentally naı¨ve, young adult male and female African green (vervet) monkeys
(Cerco-pithecus aethiops sabaeus of the St. Kitts Biomedical Research
Foundation, St. Kitts, West Indies), divided into PCP- or control-treated groups. Sixteen subjects were sacrificed after 7 days of testing, and biochemical analyses were performed on these subjects (Jentsch et al 1999). All animals were housed in standard stainless steel primate squeeze cages in an open but covered facility under natural daylight conditions. Excess amounts of food were given following testing, and water was available ad libitum; fruit supplements were also given regularly. All animals were housed, maintained, and utilized in accordance with the National Institute of Health Guide for the Care and Use of Animals in Research, and all experimental protocols were approved by the relevant institutional committees for the care and use of animals.
Drugs
Phencyclidine hydorchloride (Research Biochemicals, Natick, MA) was dissolved in sterile saline to a concentration of 3.0 mg/mL, the solution was filtered (0.2mm filter), and animals were injected intramuscularly at a volume of 0.1 mL/kg. The resulting dose (0.3 mg/kg) was administered twice daily for 14 days (injection times: 7:30AMand 3:30PM). Control injections consisted of 0.1 mg/kg sterile saline delivered similarly.
Object Retrieval/Detour Testing
GENERAL PROCEDURES. Training and testing of monkeys on the object retrieval/detour task was performed as detailed by Taylor et al (1990a, 1990b). Testing was done in a quiet, controlled situation so that external distractions to the subject being tested were minimized. During the test, vocalizations of other animals were rare because all the subjects were acclima-tized to the human experimenters. The monkeys were tested in a random order from day to day.
The first test day was 2 days after the final dose of PCP. All subjects were tested for an initial four day training period (termed acquisition) and subsequently were tested 7 days and 4 weeks after the end of drug treatment (termed performance). The acquisition and performance periods were distinguished based on previous studies (Taylor et al 1990a, 1990b), where control subjects are found to acquire optimum performance (i.e., 80% success) by the forth day of testing using these task parameters and continue to perform subsequently at that level. The testers were blind to treatment conditions.
cage during testing so that the subject could reach into the box after a transparent screen was raised. The position of the reward in the box (sticking out, front, middle, corner), of the box on the tray (left, right, middle), and orientation of the open side of the box relative to the subject (front, left, right) were parametrically varied. Subjects were allowed unlimited time and reaches on the trials to retrieve the reward as long as they continued attempting to respond. A maximum of 3 min during which no responses were delivered would result in a scored “failure” on the trial
The first day of testing consisted of 11 trials, which were largely stimulus-driven trials (e.g., most trials involved a direct reach for the reward and did not involve the need to inhibit reaching at the barrier). Subsequently, test sessions consisted of 20 trials, in which the trials were more difficult. Trials requiring either direct reaches or barrier negotiation were intermixed on days 2 through 4 and subsequent test days. In addition, day 4 and subsequent days were the most “difficult,” consisting of sets of trials which included “runs” to bait animals into a repeated response followed by a switch of the direction of the box (e.g., left, left, left, right). These trials were included to increase the tendency for animals to exhibit perseverative responses, defined as the successive repetition of a previously rewarded reach.
Our version of the object retrieval detour/task produced performance in the 70 to 80% success range in normal animals. This allowed us the potential for seeing improvements, as well as impairments, over time. Because the location of the box and reward varies across trials and because 35% of trials involve switches of the open side from the previous trial, the task was sufficiently difficult to produce control performance in this range. In addition, the configuration of the trials included a sufficient number of both “easy” and “hard” trials so that an analysis by trial difficulty could be performed (see Difficulty Score Analysis below).
BEHAVIORAL ANALYSIS. All reaches were hand-scored by the tester at the time of testing. The data were subsequently entered into a computer file by a second technical person who was likewise blind to treatment and testing conditions. These data files were later processed for several variables by data reduction for subsequent analysis (see Statistical Analysis be-low). Measures of performance included correct (retrieval of the reward on the trial), success (retrieval of the reward on the first reach of the trial), motor problems (reaching into the open side of the box but failing to retrieve the reward or dropping the reward), response initiation latency (time from raising the screen at the start of each trial until the subject made contact with the test box or reward), total reaches (number of reaches on a trial), barrier reaches (responding at the closed, transparent, side of the box), and perseverative reaches (a trial in which the first reach was made into the barrier and was a repeat of the last reach on the previous trial).
DIFFICULTY SCORE ANALYSIS. Trials were classified as “easy” or “hard” if they satisfied several conditions (for details, see Taylor et al 1990a, 1990b). “Easy” trials were defined as ones on which the open face of the box was directed toward the monkey. In addition, if on a given trial the reward was protruding from the opening of the box, rendering it directly visible, the trial
was considered “easy.” A trial was considered “hard” if the open face of the box faced to the side and followed a trial where the open face of the box was directed toward the opposite side (e.g., left-to-right switch of the open face of the box). Some trials satisfied neither criterion. Measures of performance could then be examined on the easy or hard trials. Data were pooled from days 2 to 7 for the analyses to provide a sufficient number of trials of the two types for the analyses, as was done previously (Taylor et al 1990a, 1990b).
Statistical Analysis
All data were tabulated by an automated program using the SAS statistical analysis package (SAS Institute, Cary, NC) running on a Power Macintosh. Statistical evaluations employed analyses of variance. Preliminary tests of variance homogeneity, normality, and distributions satisfied the assumptions required for a stan-dard parametric analysis of variance with several exceptions. Data that had a binomial distribution (e.g., success, correct, and perseveration) were arc sin transformed. Analysis of variance was used to determine significant main effects (e.g., group: saline-treated vs. PCP-treated) and interactions. Post hoc analy-ses using Scheffe’s F test determined whether main (group) effects revealed by analysis of variance were significant.
Results
Successful Trial Completion
Almost uniformly, both saline-treated and PCP-treated animals completed 100% of the trials on any given day. Despite persistent and motivated performance, however, PCP-treated monkeys were markedly less successful at performing the task than were control subjects. When performance across the first 4 days was analyzed, there were significant effects of group (saline vs. PCP) on success [F(1,29) 5 4.47, p 5 .04] and a significant group 3 day interaction [F(1,29) 5 6.08, p 5 .02]. Measures of success for both groups are shown in Figure 1. No difference was evident on day 1 [when all trials were largely stimulus driven; i.e., when the box opening faced toward the monkey: F(1,28)5 1.09, p 5 .30] or day 2 [when both groups performed poorly because of first experience with the need to negotiate the barrier: F(1,29) 5 1.73, p 5 .20]. In contrast, by day 3, the performance of saline-treated control subjects dramati-cally improved, whereas that of PCP-treated monkeys did not. Thus, significant differences between saline-treated and PCP-treated monkeys on successful performance of the object retrieval/detour task was present on day 3 [F(1,29)511.01, p5.003] and day 4 [F(1,29)517.25, p5.0003]. Moreover, significant differences between the drug and control groups were evident 7 days [F(1,29)5
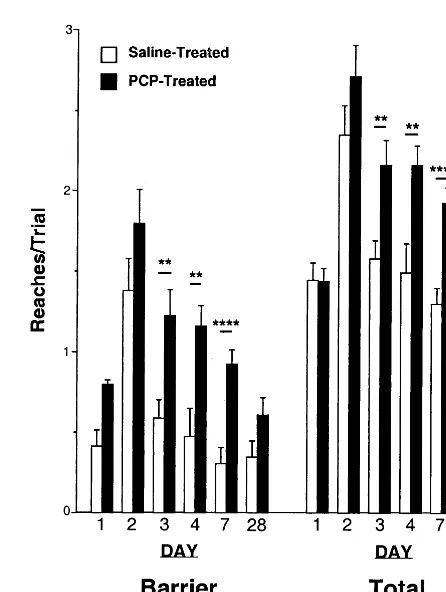
Total Reaches and Barrier Reaches
Both the total number of reaches delivered per trial (total reaches) and the number of reaches into a closed, trans-parent face of the box per trial (barrier reaches) were sensitive to PCP treatment. In particular, PCP-treated monkeys required more reaches per trial to retrieve the reward and delivered more barrier reaches. These data are shown in Figure 2. When analyzing the first 4 days together, a significant effect of group was measured for total reaches [F(1,29)54.29, p5.04], but barrier reaches just failed to reach significance [F(1,29)53.73, p5.06]. Moreover, total reaches but not barrier reaches showed a significant group 3 day interaction [total reaches: F(1,29)53.83, p5.05; barrier reaches: F(1,29)52.76, p5.10].
When each of the days were analyzed separately, the temporal pattern of drug effects were revealed. As with success, no difference between the drug and control groups were detected on day 1 [total reaches: F(1,28)5
0.00, p5.97; barrier reaches: F(1,28)50.20, p5.66] or day 2 [total reaches: F(1,29) 5 1.78, p 5 .19; barrier reaches: F(1,29) 5 2.07, p 5 .16]. Both indices of task
performance showed significant effects of group on day 3, however [total reaches: F(1,29)58.23, p5.008; barrier reaches: F(1,29) 5 9.51, p 5 .005] and day 4 [total reaches: F(1,29) 5 9.85, p 5 .004; barrier reaches: F(1,29)510.69, p5.003]. Furthermore, at 7 days [total reaches: F(1,29) 5 21.26, p , .0001; barrier reaches: F(1,29) 5 19.69, p , .0001], but not at 4 weeks [total reaches: F(1,14) 5 2.78, p 5 .12; barrier reaches: F(1,14) 5 2.82, p 5 .12], after drug treatment, these measures still showed significant differences between PCP- and saline-treated monkeys.
The finding that although success was still reduced at 4 weeks, barrier reaches and total reaches were no longer significantly elevated seems contradictory, but this effect represents a qualitative shift in the type of errors made. Initially, PCP-treated subjects tended to make multiple incorrect reaches on any given trial before retrieving the reward successfully; however, over time, they made fewer reaches before solving the trial (see Figure 2 total reaches). Indeed, Figure 2 shows that PCP-treated monkeys made
Figure 1. Successful performance was impaired in monkeys treated with phencyclidine (PCP) at days 3, 4, 7, and 28 of testing. There were no differences on day 1 of testing, when the trials did not require inhibiting a direct reach (on most of the 11 trials the open side of the box faces the monkeys) and both groups made few errors, or on day 2 of testing, when the animals are initially faced with acquiring the barrier negotiation strategy on the 20 trials and when both groups of subjects make errors). Figures represent mean6SEM. Significantly impaired relative to control subjects: *p , .05, **p , .01, ***p , .001, and ****p,.0001 by one-way analysis of variance with Scheffe’s
F test for post hoc comparisons. n57 for control subjects and
n59 for PCP-treated subjects.
fewer barrier and total reaches over time (sessions), despite making greater number of these responses com-pared with control subjects; however, these measures failed to be significant at 4 weeks, whereas success was still reduced overall.
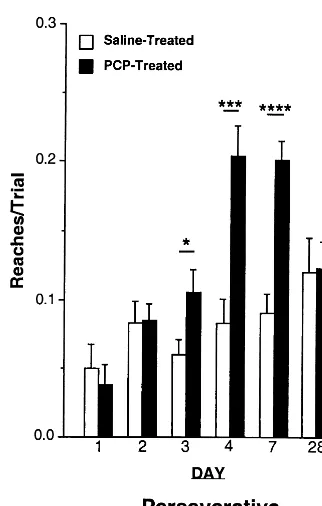
Perseverative Reaches
There were significant overall effects of group across the first 4 days on the propensity of animals to repeat a previously rewarded reach (perseverative reaches). PCP-treated monkeys exhibited more perseverative behavior than did control subjects (Figure 3). Analysis of variance revealed a significant effect of group [F(1,29)57.09, p5
.01] and a significant group3day interaction [F(1,29)5
21.26, p , .0001]. Analysis of each individual day revealed an effect of group on day 3 [F(1,29)54.60, p5
.04] and day 4 [F(1,29)517.15, p5.0003], but not day 1 [F(1,28)50.29, p5.60] or day 2 [F(1,29)50.01, p5
.91]. Perseverative reaches were still elevated in PCP-treated monkeys at 7 days postdrug treatment [F(1,29)5
31.40, p, .0001], but not after 4 weeks [F(1,29)5.01, p5.94].
Response Initiation Latency and Motor Problems
Motivational and motoric problems (nonspecific effects) were specifically assessed by response initiation latency and a motor problems score, respectively. There were no effects of group on either of these two indices when looking at the first 4 days as a whole [response initiation latency: F(1,29) 5 0.91, p 5 .35; motor problems: F(1,29) 5 0.30, p 5 .59]. Interestingly, there was a significant group3day interaction for response initiation latency [F(1,29)54.69, p5.03], but analysis of each of the individual days, separately, showed no significant effects of group on this measure on any one day [day 1: F(1,28)51.61, p5.22; day 2: F(1,29)53.21, p5.08; day 3: F(1,29)5 1.28, p5 .27; day 4: F(1,29) 52.09, p5.16]. Furthermore, there were no significant effects of group on motor problems on any given day [day 1: F(1,28)50.39, p5.54; day 2: F(1,29)50.71, p5.41; day 3: F(1,29)5 0.38, p5 .54; day 4: F(1,29) 50.06, p5.81].
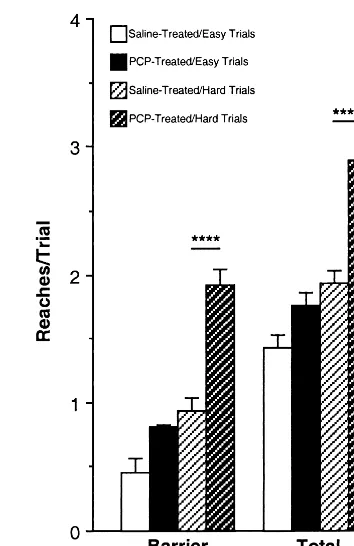
Effect of Trial Difficulty
On day 1, the trials were largely stimulus driven (the box opening faced forward), and as such, performance on this day did not require negotiation of the barrier or inhibition of a prepotent response. As noted above, no group differ-ences were noted on this day because of successful performance by both groups (Figure 1).
Results from days 2 to 7 were compiled, and perfor-mance on trials classified as “easy” versus “hard” was compared. These data are shown in Figures 4 and 5. Overall, there were significant effects of trial difficulty on several behavioral measures for the control group and the PCP-treated group [success: F(1,29)570.98, p,.0001; barrier reaches: F(1,29)549.00, p,.0001; total reaches: F(1,29) 5 55.11, p , .0001; perseverative reaches: F(1,29) 5 127.44, p , .0001], and there were group 3
difficulty interactions for barrier reaches [F(1,29)57.15, p5.008], total reaches [F(1,29)5 7.49, p5 .006], and perseverative reaches [F(1,29) 5 12.64, p 5 .0004]. Subjects treated with PCP were less successful and deliv-ered more barrier reaches than control subjects overall [success: F(1,29) 5 54.27, p , .0001; barrier reaches: F(1,29) 5 43.46, p , .0001]. Although PCP-treated subjects delivered significantly more barrier and perse-verative reaches than did control subjects on “easy” trials [barrier reaches: F(1,29) 5 3.8, p 5 .06; perseverative reaches: F(1,29)52.4, p5 .13], on the “hard” trials the PCP-treated subjects delivered even more barrier reaches [F(1,29) 5 20.1, p , .0001] and perseverative reaches [F(1,29)524.1, p, .0001] than control subjects, hence the interaction.
Discussion
These data provide evidence for deficits on the acquisition, as well as performance, of an object retrieval/detour task by monkeys after subchronic PCP administration. The impairments were greatest during acquisition, but the deficits appeared to be ameliorated over time perhaps due to practice, compensatory changes, time-dependent changes, or a combination thereof, as is the case after frontal lobe lesions (Diamond 1990; Diamond and Gold-man-Rakic 1985; Dias et al 1996b). Critically, the impair-ments were exacerbated by “hard” compared with “easy” trials, supporting the hypothesis that PCP-induced deficits are because of impulsive responding, rather than nonspe-cific factors. These new data extend our previous findings (Jentsch et al 1997) in several significant ways: 1) deficits were shown during the acquisition, as well as perfor-mance, phase, 2) a lack of motor impairment or motiva-tional changes were found, and 3) a selective effect of difficulty of the trial was demonstrated. Together these findings provide direct evidence that subchronic PCP administration produces increases in impulsive behavior, consistent with dysfunction of the corticostriatal system.
The finding that the PCP-induced deficits are
exacer-bated when the trials require response inhibition (hard trials) supports the notion that PCP-treated monkeys ex-hibit corticostriatal-like cognitive deficits, rather than motoric impairments. The effect of trial difficulty on performance can be assessed only when there are suffi-cient numbers of hard trials present to differentiate the cognitive components of the task from nonspecific effects and more stimulus-response components. Our version of the object retrieval task was designed to be difficult enough such that normal subjects perform in the 70 to 80% success range overall (with.80% accuracy on the easy trials and,70% on the hard trials). In our present study, PCP-treated subjects performed on average.60% on the easy trials and ,40% the hard trials. Barrier reaching, a measure of impulsivity, was increased in PCP-treated subjects only on hard trials. We also report that PCP treatment did not affect the overall number of trials completed in a session or the latency to respond, nor did it induce any motor problems. Thus, general motivational and motor functions, respectively, appear to be preserved in PCP-treated monkeys.
Figure 4. Monkeys treated with phencyclidine (PCP) were less successful than control subjects on all trials; however, the PCP-induced impairment was greater on “hard” trials than on “easy” ones. Figures represent mean6SEM. Data were pooled from days 2 through 7 for the analyses. Significantly impaired relative to control subjects: **p ,.01 and ****p,.0001 by one-way analysis of variance with Scheffe’s F test for post hoc comparisons. n 5 7 for control subjects and n 5 9 for PCP-treated subjects.
Figure 5. Monkeys treated with phencyclidine (PCP) made more barrier reaches and delivered more reaches per trial on average than control subjects on “hard” trials, but not on “easy” trials (i.e., there is a significant difficulty3group interaction). Figures represent mean6SEM. Data were pooled from days 2 through 7 for the analyses. Significantly impaired relative to controls: ****p,.0001 by one-way analysis of variance with Scheffe’s
F test for post hoc comparisons. n57 for control subjects and
Corticostriatal Function and Impulsivity
Integrative neuronal activity within the corticostriatal system is important for response selection among multiple competing behavioral tendencies. It has been hypothesized that the domination of behavior by conditioned responses is favored by augmentations in catecholaminergic trans-mission (Lyon and Robbins 1975), particularly dopamine efflux within the shell of the nucleus accumbens (Parkin-son et al 1999; Taylor and Robbins 1984). Nonetheless, prefrontal cortical inputs to the ventral striatum (arising largely from the ventromedial/prelimbic cortex; Haber et al 1995) appear to be a substrate by which cortical structures can modulate or gate these conditioned response tendencies at the level of the striatum (Crofts et al 1999; Jentsch and Taylor 1999; Jentsch et al, in press; Parkinson et al 1999). It thus seems plausible that either a potentia-tion of the impulsive drive to emit condipotentia-tioned responses for appetitive stimuli or the failure of cortical modulation of these impulses (or both) could contribute to the sorts of deficits that are found in monkeys after withdrawal from subchronic PCP treatment.
Dopamine appears to be an important neuromodulatory influence at both the cortical and striatal levels (Jentsch et al, in press), and as such, it might be expected that perturbations in this neurochemical system at either brain site would contribute to performance of tasks that require the cognitive modulation of appetitively motivated re-sponding (such as object retrieval/detour). Previously, we reported that MPTP-treated monkeys with chronic deple-tion of corticostriatal dopamine levels exhibit persistent deficits on the object retrieval/detour task, including both “cognitive-like” and “motor-like” impairments (Schneider and Kovelowski 1990; Taylor et al 1990a, 1990b). This phenomenon, however, is qualitatively quite different than that produced by subchronic PCP-treatment, which pro-duces cognitive, but not motor deficits. Moreover, sub-chronic PCP treatment results in a reduction in cortical dopamine transmission (Jentsch et al 1997, 1999) and a heightened evoked release of dopamine within the stria-tum (Jentsch et al 1998), but no loss of the dopamine innervation per se. Nevertheless, the parallel but opposite changes in cortical and striatal dopaminergic function produced by subchronic PCP treatment may both function-ally contribute to the increases in the emission of impul-sive responses in drug-treated monkeys (Jentsch and Taylor 1999).
Frontal Cortical Function and Object Retrieval/ Detour Performance
Object retrieval/detour tasks have been shown to be sensitive to prefrontal cortical dysfunction. This task was originally used in human infants to chart the development
of cognitive functions across the first 6 to 12 months of life (reviewed in Diamond 1990). Subsequent studies demonstrated that successful performance of this task developed, in human and monkey infants, in rough parallel with the development of the frontal cortex (Diamond 1990; Diamond and Goldman-Rakic 1985, 1986) and that ablation of the dorsolateral prefrontal cortex, but not parietal cortex or hippocampal formation, impaired task performance in adult rhesus monkeys (Diamond 1990; Diamond and Goldman-Rakic 1985; Diamond et al 1989). Moreover, excitotoxic lesions of the frontal cortex in marmosets have been shown to impair performance of an object retrieval/detour task, as well as delayed response and attentional set shifting (Crofts et al 1999; Dias et al 1996a, 1996b; 1997; Wallis et al 1997).
Several studies suggest that some of the cognitive functions of the prefrontal cortex are dependent on the regional dopaminergic innervation of the dorsolateral prefrontal cortex (Goldman-Rakic 1987). Disruption of dopamine transmission in this cortical region by chemo-specific-lesions or local D1 dopamine receptor blockade impairs performance of a variety of tasks that require spatial working memory and flexible responding (Brozo-ski et al 1979; Collins et al 1998; Roberts et al 1994; Sawaguchi and Goldman-Rakic 1991; Seamans et al 1998). Likewise, object retrieval/detour task performance deficits are a consequence of MPTP-induced reductions in corticostriatal dopamine levels (Taylor et al 1990a, 1990b; Schneider and Kovelowski 1990), and PCP-induced re-ductions in prefrontal cortical dopamine transmission in the dorsolateral prefrontal (Walker’s areas 46 and 8) and prelimbic (areas 25, 32, 33) cortex have been recently found to correlate significantly with overall failed task performance (Jentsch et al 1999). These data suggest that reduced dopamine transmission in prefrontal cortex may contribute to the deficits on the object retrieval/detour task produced by subchronic PCP treatment.
Moreover, chronic PCP abuse by humans has been shown to lead to profound reductions in cerebral cortical glucose utilization in the frontal cortices (Hertzman et al 1990; Wu et al 1991), suggestive of frontal cortical hypoactivity. Thus, subchronic PCP exposure may lead to a generalized frontal cortical hypoactivity (possibly medi-ated by reductions in dopaminergic transmission), provid-ing one potential neural substrate for PCP-induced perfor-mance impairments on the object retrieval/detour.
Cortical–Subcortical Interactions and Drug Abuse
and function may lead to impaired cortical gating or modulation of the subcortical dopamine innervation and of reward-motivated behaviors that are dependent on the ventral striatum (Jentsch et al 1998). We therefore have hypothesized that repeated exposure to drugs of abuse such as PCP may produce dysfunction of corticolimbic circuits associated with behavioral inhibition and reward, which may result in “impulsivity” or exaggerated control of behavior by reward-related stimuli (Jentsch and Taylor 1999). This hypothesis of frontal cortical and mesolimbic dysfunction is further supported by the recent findings that repeated exposure to cocaine or heroin can produce augmented responding for conditioned reinforcement in rodents (Cunningham and Kelley 1992; Taylor and Horger 1999) and deficits in frontal-cortical cognitive function, typified by a loss of inhibitory control in drug abusers (McKetin and Mattick 1998; O’Malley et al 1992; Rogers et al 1999). Moreover, we found that PCP-treated rats are also impaired in their ability to modulate behavior based on new or changing information about stimulus-reward associations, possibly due to an inability to inhibit re-sponding toward conditioned stimuli (Jentsch and Taylor, in press). Elucidation of the neurobiological alterations in the neural substrates associated with impulsivity may lead to novel avenues for the development of therapies to ameliorate disorders characterized by abnormal control of behavior by reward-related stimuli, such as drug dependence.
In summary, subchronic PCP treatment in monkeys induces a behavioral deficit on the object retrieval/detour task that may indicate reduced cortical modulation of impulsive tendencies. Elucidation of the behavioral pa-thology and neurobiological substrates underlying PCP-induced cognitive impulsivity should provide insights into the pathophysiology of debilitating human conditions associated with frontal cortical dysfunction, such as drug addiction and schizophrenia, and should aid in the devel-opment of novel therapies for their treatment.
This work was supported in part by U.S. Public Health Service Grants Nos. MH14092 and MH57483 (RHR) and DA11727 (JRT), Neurogen Corporation, and the Theodore and Vada Stanley Foundation.
The authors thank D. Eugene Redmond, Jr. and the staff of the St. Kitts Biomedical Research Foundation for support and for care of the animals.
References
Baddeley A, della Scalla S (1996): Working memory and executive control. Philos Trans R Soc Lond B Biol Sci 351:1397–1404.
Brozoski TJ, Brown RM, Rosvold HE, Goldman PS (1979): Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 205:929 –931. Butter CM (1968): Perseveration in extinction and in
discrimi-nation reversal following selective frontal ablations in
Ma-caca mulatta. Physiol Behav 4:163–171.
Butter N, Butter CM, Rosen J, Stein D (1973): Behavioral effects of sequential and one-stage ablations of orbital prefrontal cortex in the monkey. Exp Neurol 39:204 –214.
Collins P, Roberts AC, Dias R, Everitt BJ, Robbins TW (1998): Perseveration and strategy in a novel spatial self-ordered task for non-human primates: Effect of excitotoxic lesions and dopamine depletions in the prefrontal cortex. J Cogn
Neuro-sci 10:332–354.
Cosgrove J, Newell TG (1991): Recovery of neuropsychological functions during reduction in use of phencyclidine. J Clin
Psychol 47:159 –169.
Crofts HS, Herrero MT, Del Vecchio A, Wallis JD, Collins P, Everitt BJ, et al (1999): Excitotoxic lesions of the caudate nucleus in the marmoset: Comparison with prefrontal lesions on discrimination learning, object retrieval and spatial de-layed response. Soc Neurosci Abstr 25:891.
Cunningham ST, Kelley AE (1992): Evidence for opiate depen-dent cross-sensitization in nucleus accumbens: Studies of conditioned reward. Brain Res Bull 29:675– 680.
Damasio AR (1996): The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc
Lond B Biol Sci 351:1413–1420.
Deutch AY (1992): The regulation of subcortical dopamine systems by the prefrontal cortex: Interactions of central dopamine systems and the pathogenesis of schizophrenia.
J Neural Transm Suppl 36:61– 89.
Deutch AY, Clark WA, Roth RH (1990): Prefrontal cortical dopamine depletion enhances the responsiveness of mesolim-bic dopamine neurons to stress. Brain Res 521:311–315. Diamond A (1990): Developmental time course in human infants
and infant monkeys, and the neural bases of inhibitory control in reaching. Ann N Y Acad Sci 608:637– 676.
Diamond A (1996): Evidence for the importance of dopamine for prefrontal cortex functions early in life. Philos Trans R Soc
Lond B Biol Sci 351:1483–1494.
Diamond A, Ciaramitaro V, Donner E, Djali S, Robinson M (1994): An animal model of early-treated PKU. J Neurosci 14:3072–3082.
Diamond A, Goldman-Rakic PS (1985): Evidence for involve-ment of prefrontal cortex in cognitive changes during the first year of life: Comparison of performance of human infants and rhesus monkeys on a detour task with transparent barrier. Soc
Neurosci Abstr 11:832.
Diamond A, Goldman-Rakic PS (1986): Comparative develop-ment in human infants and infant rhesus monkeys of cogni-tive functions that depend on prefrontal cortex. Soc Neurosci
Abstr 12:274.
Diamond A, Prevor M, Callender G, Druin DP (1997): Prefrontal cortex cognitive deficits in children treated early and contin-uously for PKU. Monogr Soc Res Child Dev 62:1–207. Diamond A, Zola-Morgan S, Squire LR (1989): Successful
performance by monkeys with lesions of the hippocampal formation on AB- and object retrieval, two tasks that mark developmental changes in humans infants. Behav Neurosci 103:526 –537.
Dias R, Robbins TW, Roberts AC (1996b): Primate analogue of the Wisconsin card sorting test: Effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci 110:872– 886.
Dias R, Robbins TW, Roberts AC (1997): Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort test—restriction to novel situations and independence from on-line processing. J Neurosci 17: 9285–9297.
Fuster JM (1980): The Prefrontal Cortex. New York: Raven. Goldman-Rakic PS (1987): Circuitry of the frontal cortex and the
regulation of behavior by representational knowledge. In: Plum F, Mountcastle V, editors. Handbook of Physiology.
Volume V: The Nervous System. Bethesda, MD: American
Physiological Society, 373– 417.
Goldman-Rakic PS (1996): The prefrontal landscape: Implica-tions of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond
B Biol Sci 351:1445–1453.
Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E (1995): The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci 15:4851– 4867.
Hertzman M, Reba RC, Kotlyarove, EV (1990): Single photon emission computerized tomography in phencyclidine and related drug abuse. Am J Psychiatry 147:255–256.
Iversen SD, Mishkin M (1970): Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res 11:376 –386.
Jentsch JD, Redmond DE Jr, Elsworth JD, Taylor JR, Youngren KD, Roth RH (1997): Enduring cognitive dysfunction and cortical dopamine deficits in monkeys after long-term admin-istration of phencyclidine. Science 277:953–955.
Jentsch JD, Roth RH, Taylor JR (in press): Role for dopamine in the behavioral functions of the prefrontal corticostriatal sys-tem: Implications for mental disorders and psychotropic drug action. In: Uylings HBM, van Eden CG, de Bruin JPC, Feenstra MGP, Pennartz CMA, editors. Cognition, Emotion,
and Autonomic Responses: The Integrative Role of the Pre-frontal Cortex and Limbic Structures. Amsterdam: Elsevier.
Jentsch JD, Taylor JR (1999): Impulsivity resulting from fron-tostriatal dysfunction in drug abuse: Implications for the control over behavior by reward-related stimuli.
Psychophar-macology 146:373–390.
Jentsch JD, Taylor JR (in press): Impairments in the ability to modify behavior based upon changing stimulus-reward con-tingencies produced by subchronic phencyclidine administra-tion to rats. Neuropsychopharmacology.
Jentsch JD, Taylor JR, Elsworth JD, Redmond DE Jr, Roth RH (1999): Altered frontal cortical dopaminergic transmission in monkeys after subchronic phencyclidine exposure: Involve-ment of frontostriatal cognitive deficits. Neuroscience 90: 823– 832.
Jentsch JD, Taylor JR, Roth RH (1998): Subchronic phency-clidine administration increases mesolimbic dopamine sys-tem responsivity and augments stress- and amphetamine-induced hyperlocomotion. Neuropsychopharmacology 19: 105–113.
Lyon M, Robbins TW (1975): The action of central nervous system stimulant drugs: A general theory concerning amphet-amine effects. In: Essman WB, Valzelli L, editors. Current
Developments in Psychopharmacology, Vol 2. New York:
Spectrum, 79 –163.
McKetin R, Mattick RP (1998) Attention and memory in illicit amphetamine users: Comparison with non-drug-using con-trols. Drug Alcohol Depend 50:181–184.
Moll L, Kuypers HGJM (1977): Premotor cortical ablations in monkeys: Contralateral changes in visually-guided reaching behavior. Science 198:317–319.
O’Malley S, Adamse M, Heaton RK, Gawin FH (1992): Neuro-psychological impairment in chronic cocaine abusers. Am J
Drug Alcohol Abuse 18:131–144.
Parkinson JA, Buckby TB, Zandi MS, Robbins RW, Everitt BJ (1999): Nucleus accumbens lesions enhance the acquisition of a simple intstrumental visual discrimination. Soc Neurosci
Abstr 25:90.
Ridley RM, Clark BA, Durnford LJ, Baker HF (1993): Stimulus-bound perseveration after frontal ablations in marmosets.
Neuroscience 52:595– 604.
Robbins TW (1996): Dissociating executive functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci 351:1463–1471.
Roberts AC, DeSalvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, Robbins TW (1994): 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analogue of the Wisconsin Card Sort Test: Possible interactions with subcortical dopamine. J Neurosci 14:2531– 2544.
Roberts AC, Robbins TW, Weiskrantz L (1998): The Prefrontal
Cortex: Executive and Cognitive Functions. New York:
Oxford University Press.
Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, et al (1999): Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to the prefrontal cortex, and trytophan-depleted normal volunteers: Evidence for monoaminergic mechanisms. Neuropsychopharmacology 20:322–339.
Sawaguchi T, Goldman-Rakic PS (1991): D1 dopamine recep-tors in prefrontal cortex: Involvement in working memory.
Science 251:947–950.
Schneider JS, Kovelowski CJ (1990): Chronic exposure to low doses of MPTP: I. Cognitive deficits in motor asymptomatic monkeys. Brain Res 519:122–128.
Seamans JK, Floresco SB, Phillips AG (1998): D1 receptor modulation of hippocampal-prefrontal cortical circuits inte-grating spatial memory with executive functions in the rat.
J Neurosci 18:1613–1621.
Taylor JR, Elsworth JD, Roth RH, Sladek JR, Redmond DE Jr (1990a): Cognitive and motor deficits in the acquisition of an object retrieval/detour task in MPTP-treated monkeys. Brain 113:617– 637.
Taylor JR, Horger BA (1999): Enhanced responding for condi-tioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacology 142:31– 40.
Taylor JR, Robbins TW (1986): 6-Hydroxydopamine lesions of the nucleus accumbens, but not the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology 90: 390 –397.
Taylor JR, Roth RH, Sladek JR, Redmond DE Jr (1990b): Cognitive and motor deficits in the performance of an object retrieval task with a barrier-detour in monkeys
(Cercopithe-cus aethiops sabaeus) treated with MPTP: Long-term
perfor-mance and effect of transparency of the barrier. Behav
Neurosci 104:564 –576.
Wallis JD, Collins P, Everitt BJ, Robbins TW, Roberts AC
(1997): Selective lesions of the prefrontal cortex in the marmoset produce dissociable effects on the object retrieval task. Soc Neurosci Abstr 23:1605.
Weissenborn R, Robbins TW, Everitt BJ (1997): Effects of medial prefrontal or anterior cingulate cortex lesions on responding for cocaine under fixed-ratio and second-order schedules of reinforcement in rats. Psychopharmacology 134:242–257.