T
he carotenoids are yellow, orange and red pigments, which are common in a variety of living organisms. Although de-novocarotenoid biosynthesis takes place only in plants and some microorganisms, carotenoids are much more widely distributed and occur extensively in animals1as well. The functions of carotenoids in nature are diverse, and include light harvesting, photoprotection and pollinator attraction in plants, and protec-tive and sex-related coloration in animals. They are also vitamin A precursors in verte-brates and protect against oxidative damage within cells. Because of their hydrophobic nature, carotenoids in plant and animal cells are found in association with lipid–protein com-plexes, where they interact with hydrophobic domains2. The unique protein–chromophore interactions in these complexes are responsible for the red, blue and green coloration of cara-paces in many invertebrates, such as lobster, shrimp and crayfish1. In humans, over 80% of the carotenoids absorbed from the intestine accumulate in adipose tissues, transported there by lipoproteins3. Furthermore, the primary structure of, for example, human retinol (a b-carotene derivative) binding protein is homologous to the carotenoprotein from lob-ster carapace4. In plant cells, carotenoids are located mainly in semiautonomous organelles – chloroplasts and chromoplasts. Because of dramatic differences in the amounts of carotenoids accumulated in these plastids, each organelle uses a unique mechanism to sequester carotenoids5. By delineating these mechanisms it might be possible to improve our understand-ing of how plant carotenoids function in vivo.Carotenoid sequestration in chloroplasts Practically all the carotenoids located in photo-synthetic membranes are present in the form
of chlorophyll-carotenoid–protein complexes and are involved in light harvesting and chlorophyll photoprotection. Chlorophyll-carotenoid–protein complexes are also instru-mental in the stabilization of the three-dimensional integrity of bacterial and plant light-harvesting complexes (LHCs) and in the assembly of a functional photosystem II (PSII)6,7. Interestingly, whereas LHCII binds most of the neoxanthin and lutein of PSII and has a chlorophyll:carotenoid ratio of five to six, minor chlorophyll a/bproteins bind mostly violaxanthin and have relatively high carotenoid contents, with a chlorophyll:carotenoid ratio of between three and four (Ref. 8). Because violaxanthin is the precursor of zeaxanthin, which is involved in dissipating excess light energy, a role has been proposed for these pro-teins in the regulation of energy transfer to the reaction center7,8. Here, we focus our attention on proteins that preferentially bind or associate with carotenoids.
In higher plant chloroplasts, exclusive carotenoid–protein interactions are rather uncommon; however, there is evidence of spe-cific carotenoid–protein interactions in photo-synthetic prokaryotes and algae. Moreover, several carotenoid-binding proteins from photo-synthetic cyanobacteria, which differ in their sequences and cell localization, have been shown to play a role in photoprotection. Some of these are hydrophobic and are localized in the cytoplasmic membrane9, whereas others are water-soluble and are located in the cytosol10or on the cell surface11. It is interest-ing to note that independent of their localization pattern and solubility, all of the carotenoid-binding proteins preferentially bind xantho-phylls, thus indicating similar carotenoid-binding specificities.
In eukaryotes, much information on carotenoid–protein interactions has been
culled from the unicellular green alga Dunaliella bardawil, which, under conditions potentially engendering photo-oxidative damage, accumulates massive amounts of carotenoids, not only within chloroplast thylakoids12,13, but also in plastoglobules located in the interthylakoid space14. In both cases, changes in carotenoid level involve changes in the expression of carotenoid-associated genes. For example, in response to light stress, the D. bardawilgene cbr, encoding a 19 kDa thylakoid protein, is activated in parallel to the accelerated accumulation of xanthophylls, preferentially zeaxanthin12. This protein is associated with a minor LHCII component and is thought to be involved in zeaxanthin binding to form photoprotective complexes within the light-harvesting antennae13. In the same species, in addition to thylakoid-located xanthophylls, large amounts of b-carotene accumulate in plastoglobules together with neutral lipids and a 38 kDa protein, as a result of high light exposure14. This protein, termed Cgp, is local-ized in the periphery of the globules, and might stabilize them and prevent their coales-cence. This type of carotenoid–protein inter-action is also very common in nonphotosynthetic plastids, the chromoplasts, which accumulate extremely high levels of carotenoids.
Carotenoid sequestration in the chromoplast
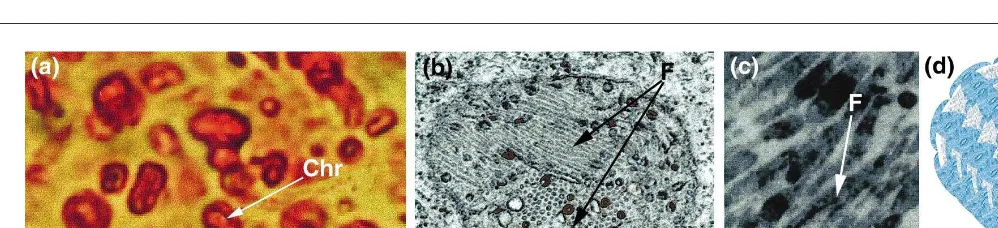
Chromoplasts (chromo 5 color), bring aesthetic value to many flowers and fruits. Although any plastid can be converted into a chromo-plast during fruit maturation and flower mor-phogenesis, they are usually derived from chloroplasts. During this process, the thylakoid membranes disintegrate, chlorophyll and most of the components of the photosynthetic machinery disappear, and there is a massive accumulation of carotenoids in novel struc-tures. In the absence of chlorophyll, the photoprotective and light-harvesting functions of carotenoids are no longer essential, so their main function appears to be in the attraction of pollinators and seed dispersers5. Ultrastructural studies of chromoplasts have revealed differ-ent kinds of carotenoid-accumulating structures, leading to the classification of chromoplasts as globular, crystalline, membranous, fibrillar and tubular15. Variations in the composition of carotenoids, polar lipids and proteins in these structures might be the key to the development of each type of chromoplast.
Most information on carotenoid–protein interactions comes from globular- and fibrillar or tubular-type chromoplasts, whereas mem-branous chromoplasts – containing numerous concentric membranes – have been less studied, and crystalline chromoplasts contain only crystals of pure carotenoids. Globular chromoplasts, which contain lens-shaped or spheroidal plastoglobules, are the most
com-232
trends in plant science
perspectives
June 1999, Vol. 4, No. 6 1360 - 1385/99/$ Ð see front matter © 1999 Elsevier Science. All rights reserved. PII: S1360-1385(99)01414-4
Carotenoid sequestration in
plants: the role of
carotenoid-associated proteins
Michael Vishnevetsky, Marianna Ovadis and
Alexander Vainstein
mon type and are considered to be the oldest and most primitive in evolutionary terms. This view is supported by the observation that in algae, bryophytes, pteridophytes and gymno-sperms, excess carotenoids are dissolved in lipid plastoglobules of different sizes within primitive, chromoplast-like plastids15. A char-acterization of the constituents of chromoplast plastoglobules revealed a very high ratio (10:1) of apolar to polar components, with carotenoids comprising 15–25% of the highly hetero-geneous mixture of apolar compounds16. Based on this and other physicochemical data, plasto-globules have been proposed to consist of a thin monolayer of polar lipids and proteins covering the surface, with apolar components buried in the interior16.
In contrast to plastoglobules, fibrils – often shown to originate from plastoglobules – are characterized by the high homogeneity of the apolar compounds, most of which are esteri-fied xanthophylls15. The low ratio (1:1) of apo-lar to poapo-lar substances, consisting of almost equal parts of proteins and lipids, is another characteristic trait of these structures17. Because ultrastructure and carotenoid–protein inter-actions in fibrillar and tubular chromoplasts are very similar, hereafter we will refer to them as a single ‘fibrillar’ group with respect to the role of carotenoid-associated proteins in the formation and organization of these structures.
Carotenoid-associated proteins as an integral part of the chromoplastÕs carotenoid sequestration machinery Fibrillar chromoplasts accumulate extremely high levels of protein in the fibril’s external half-membrane. Numerous studies in different plant species have shown that the major proteins, of 30–35 kDa, comprise up to ~80% of the total chromoplast proteins17–20. These proteins accumulate in parallel to carotenoid accumulation and chromoplast fibril for-mation during flower morphogenesis and fruit ripening. Recently, the search for additional, less abundant fibrillar proteins led to the iso-lation of a chromoplast-specific 14 kDa protein
(called CHRD), the accumulation pattern of which is identical to that of the major pro-teins21. Collectively, these proteins have been termed carotenoid-associated proteins, because they are components of the carotenoid–protein complexes resolved from chromoplast fibrils. With the aim of explaining the molecular architecture of fibril assembly, two models, based on biochemical, molecular and electron-microscopic analyses, were proposed. Accord-ing to the first of these, the fibrillar surface coat is built up of a monolayer of polar lipids, with integrated globular carotenoid-associated proteins, whereas the central core consists, almost exclusively, of carotenoid molecules with their long chains in strictly parallel orientation17. In the second model, carotenoids occupying the fibrillar core interact with the acyl residues of the polar lipids, which in turn interact, via polar head groups, with carotenoid-associated proteins located at the periphery of the fibril and in contact with the plastid stroma20. Although in both models, accumu-lation of xanthophyll is a prerequisite for fibril formation, the second model does not allow for direct interaction between carotenoid-associated proteins and carotenoids. Instead, it is suggested that carotenoid-associated pro-teins interact directly with only the polar lipids surrounding the carotenoid core, thus acting as a shield for the lipid layer in addition to their role in giving the fibril its shape. The authors of the second model indeed showed that polar lipids are protected against phospholipase by carotenoid-associated proteins; although no data suggesting protein-polar lipid-carotenoid interactions, as opposed to direct protein– carotenoid interactions, were presented. The existence of a hydrophobic hairpin region of 17–19 amino acids in all homologous carotenoid-associated proteins might indicate the direct protein–carotenoid interaction. By integrating all the data available to date on the molecular organization of the fibril, we are tempted to suggest that the monolayer of polar lipids on the fibril surface is protected by the carotenoid-associated proteins, the hydrophobic
regions of which are embedded in the inner carotenoid layer (Fig. 1). A similar organization has been reported for oil bodies, where triacylglycerol core and polar lipids are pro-tected by oleosins22, suggesting that similar biophysical rules apply to lipid and carotenoid sequestration machineries.
Carotenoid-associated genes: properties and comparisons
The cloning and characterization of nuclear-encoded carotenoid-associated genes from different organs has enabled a more detailed understanding of their function and expression patterns. For example, both fibrillin20[FIB, also referred to as CHRB (Ref. 23) or PAP (Ref. 24)], cloned from pepper fruits and CHRC from cucumber corolla25encode proteins with a predicted molecular mass of 35.2 kDa and a transit peptide of ~60 amino acids, and a mature protein of ~29 kDa with a predicted isoelectric point of 4.5–4.9. Both have several highly homologous regions, including the hydrophobic hairpin domain, which might be involved in carotenoid–protein interactions. Based on this domain’s sequence, homologous carotenoid-associated genes, expressed in fi-brillar and even, albeit at low levels, in globular chromoplasts of other plants, have been dis-covered20,25. These findings demonstrate the existence of a group of homologous genes coding for the carotenoid-associated proteins that aid in carotenoid’s sequestration within fibrils, but they also indicate the involvement of carotenoid-associated proteins in plasto-globule formation. Because plastoplasto-globules are common in all kinds of plastids, the homolo-gous carotenoid-associated proteins might be instrumental in the sequestration of not only carotenoids, but also other lipids, in plastids other than chromoplasts. This prompted the question: are the homologs of carotenoid-associated proteins present in plastids other than chromoplasts, and if so, are their func-tions there the same or different from those in chromoplasts? A partial answer was obtained recently, when the presence of FIBtranscript
233
trends in plant science
perspectives
June 1999, Vol. 4, No. 6
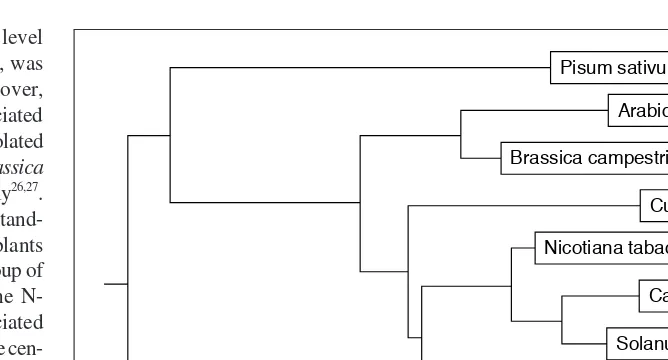
in pepper leaves and roots, albeit at a level 50- to 100-fold lower than that in fruits, was revealed by RT-PCR analyses24. Moreover, proteins homologous to carotenoid-associated proteins – CDSP34 and BCP32 – were isolated from potato chloroplast thylakoids and Brassica napuselaioplast plastoglobules, respectively26,27. From evolutionary and phylogenetic stand-points, carotenoid-associated genes in plants appear to be a very old and conserved group of orthologous genes (Fig. 2). Whereas the N-terminal portions of carotenoid-associated proteins have a low level of similarity, in the cen-tral and C-terminal portions similarity is of the order of 80–90%. Even a putative homologous protein from Synechocystisshares 29% identity with carotenoid-associated proteins from angio-sperms. In spite of this sequence similarity, the expression patterns of carotenoid-associated gene homologs in chromoplastogenic tissues are different from those in non- chromoplastogenic ones, suggesting a tissue-specific functional divergence. From an evolutionary standpoint, the carotenoid-associated proteins’ role in se-questering overaccumulated carotenoids within chromoplasts of angiosperm reproductive organs might be a rather recent development; before this, carotenoid-associated proteins might have been involved in the formation of membrane complexes, where they might also act as lipoproteins.
Regulation of carotenoid-associated genes and carotenoid sequestration The maturation of many fruits and flowers involves the accumulation of carotenoids to extreme levels. Hence it could be expected that genes involved in carotenoid sequestration would be activated in parallel to carotenoid biosynthetic genes, to preserve cell integrity in chromoplastogenic tissues. Indeed, strict spatial and temporal regulation of carotenoid-associated genes has been revealed in both fruit and flower tissues. At both the transcript and protein levels, FIBand CHRCinduction was shown to parallel chromoplast fibril development and carotenoid accumulation, reaching a maximum at peak color develop-ment20,25. At the same time, only very small amounts of FIB and no CHRC were detected in leaves19,24,25,28. Carotenoid-associated gene expression has been recently shown to be tem-porally and spatially regulated, essentially at the transcriptional level28,29. Moreover, the pattern of expression of carotenoid-associated genes during chromoplastogenesis is very similar to that of genes encoding carotenoid biosynthetic enzymes, including capsanthin-capsorubin synthase and geranylgeranyl pyrophosphate synthase in pepper fruits28, and phytoene syn-thase in tomato flowers and fruits30,31.
The expression of carotenoid-associated genes is also strongly affected following modulation of the composition and amount of
carotenoids accumulating in the chromoplast. Treatment of chromoplastogenic tissues with carotenoid biosynthetic inhibitors, such as norflurazon and 2-(4-chlorophenylthio)-triethylamine, caused accumulation of acyclic carotenoids and plastoglobules, and prevented xanthophyll accumulation and fibrillar for-mation, concomitantly leading to a down regulation in carotenoid-associated protein levels20,32. New evidence has revealed that the inhibition of carotenoid biosynthesis affects carotenoid-associated gene expression at the post-transcriptional and translational levels (M. Vishnevetsky, M. Ovadis and A. Vainstein, unpublished), further proving a close relationship between the carotenoid biosynthetic pathway and that generating carotenoid-associated proteins.
In spite of many similar features, the regu-lation of carotenoid-associated genes, as well as that of carotenoid sequestration in different chromoplastogenic tissues, such as flowers and fruits, appears to differ with respect to induction factors. In floral tissue, gibberellin (GA) plays a crucial role in chromoplastogen-esis33,34: it leads to enhanced carotenoid accu-mulation, as well as to the rapid activation in a primary fashion of CHRCgene expression. Ethylene, which is known to promote chloro-plast–chromoplast conversion in climacteric fruits, and abscisic acid (ABA) have the oppo-site effect on CHRC expression and caroteno-genesis21,34. The expression pattern of CHRD is essentially identical to that of CHRC (Ref. 21). Recently, GA, ethylene and ABA have been shown to regulate CHRCexpression at the transcriptional level (M. Vishnevetsky, M.
Ovadis and A. Vainstein, unpublished). In contrast to floral tissue, GA is known to delay chromoplastogenesis in fruits15. Analyses of FIB expression in pepper fruits revealed that GA downregulates, and ABA enhances, albeit only slightly, FIB levels20. Ethylene, shown to exert its effect at the transcriptional level, strongly upregulates FIB::GUS construct expression in transgenic tomato fruits29. FIB expression in fruits is also strongly induced by dehydration and photo-oxidative stress29. Interestingly, these same factors, as well as wounding, also lead to its activation in leaves28. Taken together, the available data suggest that carotenogenesis is regulated by a variety of developmental and environmental factors – the fates of which are spatially con-trolled – via uncharacterized transduction pathways.
Conclusions and future prospects In the past, carotenoid sequestration in plants received little attention relative to studies on the biochemistry and the molecular biology of carotenoid biosynthesis. In recent years, how-ever, based mainly on studies of chromoplasts, genes encoding carotenoid-associated pro-teins participating in carotenoid–lipoprotein complexes have been identified and charac-terized. The carotenoid-associated proteins have been found to play an important struc-tural role in the architecture of fibrillar and globular chromoplasts, acting as a shield for the lipid layer and establishing the fibril’s shape. In evolutionary terms these proteins might be part of a more global program of plastid-membrane stabilization. Nevertheless,
234
trends in plant science
perspectives
June 1999, Vol. 4, No. 6
Fig. 2.Rooted tree constructed from the multiple sequence alignment of carotenoid-associated proteins and their homologs from different species, branch lengths represent the evolutionary distances between sequences. Using the DNAMAN program from Lynnon BioSoft, the tree was generated by the neighbor-joining method, which clusters the sequences in a pairwise fashion. The sequences were obtained from the following references or from the GenBank:
Pisum sativum(AF043905); Arabidopsis thaliana(AL021712);Brassica campestris27 ; Cucumis sativus25
; Nicotiana tabacum(Y15489); Capsicum annuum20
; Solanum tuberosum26 ; Citrus unshiu35
; and Synechocystis sp. (D90904).
Pisum sativum
Arabidopsis thaliana
Brassica campestris
Cucumis sativus
Nicotiana tabacum
Capsicum annuum
Solanum tuberosum
Citrus unshiu
the basis for molecular recognition between carotenoids and carotenoid-associated proteins is poorly understood and the model presented in Fig. 1 is untested.
Delineating the environmental and devel-opmental pathways regulating carotenoid-associated gene homologs in reproductive organs and green tissues should provide important clues as to the role of carotenoid sequestration in the plastid’s developmental program. Furthermore, elucidation of the mol-ecular interactions that govern the assembly of carotenoid–protein complexes and the plas-toglobule–fibril conversion in chromoplasts will undoubtedly also shed light on the mol-ecular structure–function composition of membrane-bound carotenoid biosynthetic enzymes. Detailed knowledge of these issues is in fact one of the major prerequisites for suc-cess in applying molecular approaches to the generation of carotenoid cell factories.
Acknowledgements
We thank Prof. B. Camara for providing part of Fig. 1 and Prof. E.B. Dumbroff for helpful discussions. Our work on carotenoid-associated proteins is supported by a grant from the Israeli Ministry of Science and Fine Arts, the Ministry of Absorption (to M.O.) and the Association of Israeli Flower Growers.
References
1Olsen, J.A. and Krinsky, N.I. (1995) Introduction: the colorful, fascinating world of carotenoids: important physiologic modulators, FASEB J.9, 1547–1550
2Britton, G. (1995) Structure and properties of carotenoids in relation to function, FASEB J.9, 1551–1558
3Parker, R.S. (1996) Absorption, metabolism, and transport of carotenoids, FASEB J.10, 542–551
4Weesie, R.J. et al.(1995) Protein–chromophore interactions in a-crustacyanin, the major blue carotenoprotein from the carapace of the lobster, Homarus gammarus. A study by 13C magic angle spinning NMR, FEBS Lett. 362, 34–38
5Bartley, G. and Scolnik, P. (1995) Plant carotenoids: pigments for photoprotection, visual attraction, and human health, Plant Cell 7, 1027–1038
6Green, B.R. and Durnford, D.G. (1996) The chlorophyll-carotenoid proteins of oxygenic photosynthesis, Annu. Rev. Plant Physiol. Mol. Biol.47, 685–714
7Havaux, M. (1998) Carotenoids as membrane stabilizers in chloroplasts, Trends Plant Sci. 3, 147–151
8Bassi, R. et al.(1993) Carotenoid-binding proteins of photosystem II, Eur. J. Biochem.212, 297–303
9Reddy, K.J. et al. (1989) DNA sequence and regulation of the gene (cbpA) encoding
42-kilodalton cytoplasmic membrane carotenoprotein of the cyanobacterium Synechococcussp. strain PCC 7942, J. Bacteriol. 171, 3486–3493
10 Wu, Y.P. and Krogmann, D.W. (1997) The orange carotenoid protein of Synechocystis PCC 6803, Biochim. Biophys. Acta1322, 1–7 11 Engle, J.M. et al.(1991) Purification and
characterization of a surface-associated carotenoid-binding complex from the photosynthetic prokaryote, Prochlorothrix hollandica, Arch. Microbiol. 155, 453–458 12 Levy, H., Gokhman, I. and Zamir, A. (1992)
Regulation and light-harvesting complex II association of a Dunaliellaprotein homologous to early light-induced proteins in higher plants, J. Biol. Chem. 267, 18831–18836
13 Levy, H. et al.(1993) Cbr, an algal homolog of plant early light-induced proteins, is a putative zeaxanthin binding protein, J. Biol. Chem. 268, 20892–20896
14 Katz, A., Jimenez, C. and Pick, U. (1995) Isolation and characterization of a protein associated with carotene globules in the alga Dunaliella bardawil, Plant Physiol.108, 1657–1664
15 Camara, B. et al. (1995) Biochemistry and molecular biology of chromoplast development, Int. Rev. Cytol.163, 175–247
16 Hansmann, P. and Sitte, P. (1982) Composition and molecular structure of chromoplast globules of Viola tricolor, Plant Cell Rep. 1, 111–114
17 Knoth, R., Hansmann, P. and Sitte, P. (1986) Chromoplasts of Palisotra barteri, and the molecular structure of chromoplast tubules, Planta168, 167–174
18 Emter, O., Falk, H. and Sitte, P. (1990) Specific carotenoids and proteins as prerequisites for chromoplast tubule formation, Protoplasma157, 128–135
19 Smirra, I., Halevy, A. and Vainstein, A. (1993) Isolation and characterization of a chromoplast-specific carotenoid-associated protein from Cucumis sativuscorollas, Plant Physiol. 102, 491–496
20 Deruere, J. et al. (1994) Fibril assembly and carotenoid overaccumulation in chromoplasts: a model for supramolecular lipoprotein structures, Plant Cell6, 119–133
21 Libal-Weksler, Y. et al.(1997) Isolation and regulation of accumulation of a minor chromoplast-specific protein from cucumber corollas, Plant Physiol. 113, 59–63
22 Tzen, J.T.C. and Huang, A.H.C. (1990) Surface structure and properties of plant seed oil bodies, J. Cell Biol. 117, 327–335
23 Newman, L., Hadjeb, N. and Price, C. (1989) Synthesis of two chromoplast-specific proteins during fruit development in Capsicum annuum, Plant Physiol. 91, 455–458
24 Pozueta-Romero, J. et al.(1997) A ubiquitous plant housekeeping gene, PAP, encodes a major protein component of bell pepper chromoplasts,
Plant Physiol.115, 1185–1194
25Vishnevetsky, M. et al.(1996) Molecular cloning of a carotenoid-associated protein from Cucumis sativuscorollas: homologous genes involved in carotenoid sequestration in chromoplasts, Plant J. 10, 1111–1118
26Gillet, B. et al.(1998) Molecular characterization of CDSP 34, a chloroplastic protein induced by water deficit in Solanum tuberosumL. plants, and regulation of CDSP 34expression by ABA and high illumination, Plant J.16, 257–262
27Ting, J.T.L. et al.(1998) Constituents of the tapetosomes and elaioplasts in Brassica campestristapetum and their degradation and retention during microsporogenesis, Plant J.16, 541–551
28Chen, H.C. et al.(1998) Drought- and wound-induced expression in leaves of a gene encoding a chromoplast carotenoid-associated protein, Plant J.14, 317–326
29Kuntz, M. et al.(1998) Upregulation of two ripening-related genes from a non-climacteric plant (pepper) in a transgenic climacteric plant (tomato), Plant J.13, 351–361
30Giuliano, G., Bartley, G.E. and Scolnik, P.A. (1993) Regulation of carotenoid biosynthesis during tomato development, Plant Cell5, 379–387
31Corona, V. et al.(1996) Regulation of a carotenoid biosynthesis gene promoter during plant development, Plant J.9, 505–512
32Libal-Weksler, Y. et al.(1995) Flower-specific carotenoid accumulation in chromoplasts: molecular control of carotenoid-associated proteins, Acta Hortic.420, 32–34 33Vainstein, A. et al.(1994) Chromoplast
biogenesis in Cucumis sativuscorollas, Plant Physiol. 104, 321–326
34Vishnevetsky, M. et al.(1997) CHRC, encoding a chromoplast-specific
carotenoid-associated protein, is an early gibberellic acid-responsive gene, J. Biol. Chem.272, 24747–24750
35Moriguchi, T. et al.(1998) Characterization of a cDNA homologous to carotenoid-associated protein in citrus fruits, Biochim. Biophys. Acta 1442, 334–338
235
trends in plant science
perspectives
June 1999, Vol. 4, No. 6
Michael Vishnevetsky, Marianna Ovadis and Alexander Vainstein*are at The Kennedy Leigh Centre for Horticultural Research and The Otto Warburg Center for Biotechnology in Agriculture, Faculty of Agricultural, Food and Environmental Quality Sciences, The Hebrew University of Jerusalem, Rehovot 76100, Israel.
*Author for correspondence