The journal homepage www.jpacr.ub.ac.id p-ISSN : 2302 – 4690 | e-ISSN : 2541 – 0733
Synthesis of Patchouli Biochar Cr
2O
3Composite Using Double
Acid Oxidators for Paracetamol Adsorption
Tutik Setianingsih1, Masruri1, Bambang Ismuyanto2
1
Department of Chemistry, Brawijaya University, Jl. Veteran 169, Malang, Indonesia
2
Department of Chemical Engineering, Brawijaya University, Jl. Veteran 169 Malang, Indonesia
*
Corresponding email: [email protected]
Received 18 September 2017; Revised 14 December 2017; Accepted 5 January 2018
ABSTRACT
Composite built by patchouli biochar and metal oxide, Cr2O3, is a potential material for remediation of contaminated wasterwater. Oxidation of biochar using acid or salt oxidators can improve its surface polar functional groups. This treatment may be able to increase impregnation of metal cation (as salt) before calcination to form its oxide. In this research, 3 types of oxidators were used to oxidize the biochar before impregnation with purpose to study its influence toward physichochemistry and adsorption performance of the composite. Preparation of the composite included 3 steps, namely preparation of biochar by pyrolisis of patchouli biomass using ZnCl2 activator at 450oC, oxidation of the biochar using 3 different oxidators (H2SO4-HNO3, H3PO4-HNO3, H2O2–HNO3) at 60oC, impregnation of the oxidized biochar using CrCl3 followed by calcination process to form biochar–Cr2O3 composite at 600oC. Characterization using X-ray diffraction indicated that the composite contains the Cr2O3 structure. FTIR spectrophotometry characterization indicates the different content of C=O, C–O, and –OH on the composite surface. SEM images shows irregular micro ball shapes. EDX characterization indicates the different Cr content in the composite with same sequence with FTIR absorbance of both C–O and –OH. Adsorption of paracetamol indicates effect of Cr2O3 showing the same sequence of both.
Key word: composite, biochar, Cr2O3, patchouli biomass, adsorption,
INTRODUCTION
Biochar is one of porous material adsorbents which has potential for adsorption of metal cations such as Pb, Cd, Ni, and Cu [1], Zn [1, 2], anion such as phosphate [3] or arsenic (V) [2], and organic substances such as humic and tannic acid [4], trimethyltin [2], various antibiotics [5]. Combination of biochar using various metal oxides can improve performance of biochar in adsorption [6,7,8].
The journal homepage www.jpacr.ub.ac.id 61 C=O, and COOH [9]. The other researcher reported that oxidation by HNO3 improves carboxylic [10]. Peroxide acid oxidation created C=O, C–O, and OH functional groups of biochar [11]. HNO3, H2SO4, H2O2 gives the micropore and mesopore sizes [12].
Some research also reported double acids as the oxidator of carbon materials, including mixture of HNO3–H2SO4 which creates functional groups of –NO(OH) [9]. Combination HNO3–H2O2 improved the carboxyl on CNT [13].
However, calcination treatment can reduce acidity of biochar surface. Acidity of activated carbon surface increases almost 60 times after oxidation, but decreases again after calcination by increasing of temperature and there is no acidity after calcination at 950oC. Decreasing of activated carbon acidity is caused by decreasing of carboxyl and phenolic content [14].
Functionalization by oxidation reaction can improve adsorption of biochar toward metal cations, such as Zn2+ [15] or Cu2+ [10] and anion such as Cr[VI] [16]. It indicates that oxidation of biochar can improve impregnation of biochar by metal salts. In our previous research, biochar product has been prepared from patchouli biomass using CoCl2 activator and impregnated with CrCl3 which then calcined to form biochar–Cr2O3 composite [6].
In this research, biochar was prepared using ZnCl2 activator by consideration that based on our previous research, ZnCl2 gave highest porosity of biochar after CoCl2 activator [6]. The biochar was oxidized using 2 different acids with purpose to study their effect on the composite’s surface functional groups and its performance in adsorption of paracetamol, as contaminant model.
EXPERIMENT
Chemicals and instrumentation
This research used some chemicals, including nitric acid (Merck), sulfuric acid 96% (Merck), peroxide acid (Merck), phosphoric acid (Merck), chromium chloride hexahydrate (Merck), zinc chloride anhydrate (Merck), paracetamol (pharmacy), distilled water.
Some instrumentations were used in this research for characterization of the products, including FTIR spectrophotometer (Shimadzu FTIR QP89500) by using KBr pellet, X-ray diffractometer (PANalytical type XPert PRO), SEM (FEI type Inspect S50), EDX (EDAX, AMETEK). UV-Vis spectrophotometer (Shimadzu) is used for analysis of paracetamol.
Procedure reaction
Preparation of biochar using ZnCl2 activator
A mixture was prepared by mixing patchouli biomass (60 g), ZnCl2 (180 g) and distilled water (360 mL). The mixture was evaporated at 100oC, then pyrolized it to 450oC for 0.5 h in a closed porcelain cup. Due to the size of the cup, the ZnCl impregnated biomass was divided into 3 parts. The calcined composite product was washed using HCl solution (1 M) and distilled water to remove the activator. The product was dried at 130oC for 6 hrs.
Oxidation of biochar surface
Impregnation of the oxidized biochar
The oxidized biochar was mixed with CrCl3 solution (0.9 M) and shaked at 175 rpm for 24 hrs. After decantation process, the impregnated biochar then was calcinated to 600oC for 1 h to produce biochar–Cr2O3 composite.
Adsorption test
The paracetamol solution (100 ppm, 25mL) was mixed with each 0.05 g of the composites and shaked at 175 rpm for 24 h. The mixtures were filtered and the filtrate was analyzed using UV–Vis spectrophotometry at maximum wavelength (243 nm). Standart curve of paracetamol was prepared based on measurement of a series of paracetamol solution of 10–50 ppm.
RESULT AND DISCUSSION
Characterization of the composites using FTIR spectrophotometry. FTIR spectrophotometry has been used to identify surface functional groups of the composites which may change due to oxidation treatment. FTIR spectra of the composites are reported in
Figure 1 to Figure 2. Figure 1 shows different pattern of spectra, especially correspond to
–OH, C=O, aromatic C=C and C–O at 3400, 1720, (1620 and 1560), and 1240 cm-1, respectively. Those peaks are fit with interpretation given in previous researches, i.e. the band at 1700 cm-1 is related to C=O vibration, 1000 -1300 cm-1 indicates the stretching of C–O bond, 3200–3500 cm-1 is OH stretching band, and 1590 is correspond to oxonium due to breakdown of aromatic carbon after oxidation [16].
The journal homepage www.jpacr.ub.ac.id 63 Figure 2. FTIR spectra of composites prepared using 3 different double oxidators
(HP–HN=H3PO4–HNO3; HS–HN=H2SO4–HNO3; HO–HN=H2O2–HNO3)
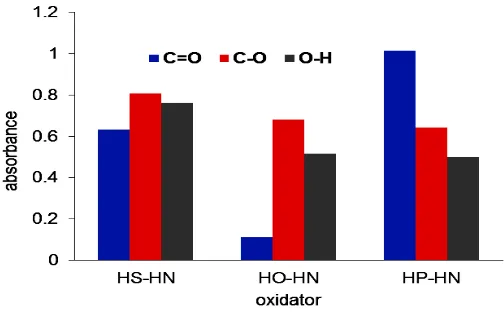
FTIR absorbance was calculated and reported in Figure 3. The absorbance of –OH and C–O bands increases in sequence of H3PO4–HNO3 < H2O2–HNO3 < H2SO4–HNO3. On the other side, the C=O band also increases by using oxidators in sequence of H2O2–HNO3 < H2SO4–HNO3 < H3PO4–HNO3. The presence of OH (at about 3250 cm-1), C=O, and C–O groups indicates that the composites contain carboxyl group. Morover, –OH functional group can includes hydroxyl or hydrate.
After impregnation with CrCl3 and calcination, the bands were reduced which indicate that the degradation reaction occured. However, all composite spectra show sharp bands between 500 and 600 cm-1. These bands are connected correspond to Cr–O of Cr2O3 as interpreted in previous research [17].
Figure 3. FTIR absorbance of C=O, C–O, and O–H functional groups of biochar after
Characterization of the composites using X-ray diffraction
In this research, the active biochar was modified with Cr(III) oxide by adsorption of salt, i.e. CrCl3, in the first step, then was continued by calcination of biochar–CrCl3 composite to form biochar–Cr2O3 composite. Some previous researchs have called this way of modification by term of impregnation [18,19,20].
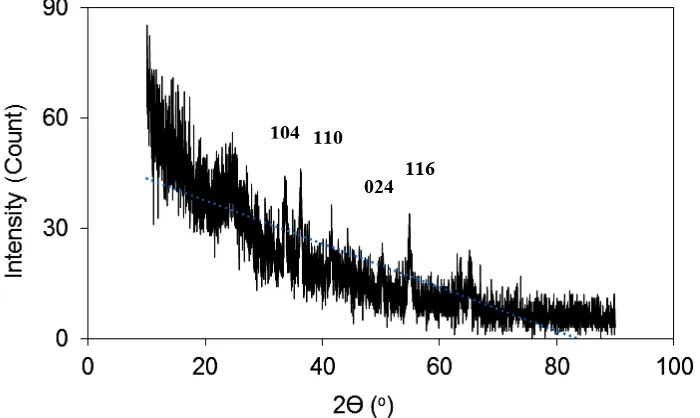
X-ray diffraction method was conducted to identify crystal structure of the impregnant (Cr2O3) in the composite. The X-ray diffractogram is presented in Figure 4 and the interpretation data are listed in Table 1. Figure 4 shows some peaks which indicated that the composite crystalline materials. By comparing the diffractogram data of Cr2O3 in previous researchs [17, 21, 22], the composite indicates that it also contains Cr2O3 structure. By characterization the synthesis Cr2O3 using X-ray diffraction method, another previous research gave more specific term of structure, i.e α–Cr2O3 [23].
Figure 4. X-ray diffractogram of biochar–Cr2O3 composite which was prepared using H2SO4–HNO3
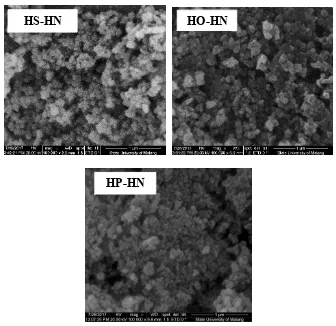
Morphology of the composites were characterized using SEM. The SEM images of all composites in Figure 5 shows micro ball shape. The smallest microball shape was obtained by using H3PO4–HNO3. There are some holes among microball particles shown by SEM
– –
104 110
The journal homepage www.jpacr.ub.ac.id 65 oxidators show more holes than using H3PO4–HNO3. This may be connected to stronger oxidizing ability of the former than the later.
Figure 5. SEM images of the composites prepared using 3 different double oxidizing agents
(HP–HN=H3PO4–HNO3; HS–HN=H2SO4–HNO3; HO–HN=H2O2–HNO3)
Characterization of composition by EDX
EDX characterization is reported in Figure 6 and 7. It indicates that content of Cr in the composite is increasing by sequence of HP–HN < HO–HN < HS–HN. This sequence is relatively fit with sequence of absorbance of C–O and O–H groups in Figure 3. Those functional groups contain Lewis base oxygen atom, which are rich of lone pairs, hence they have better affinity to Lewis acid Cr(III) CrCl3 impregnation process.
On the other side, the content of O on the surface of biochar is increasing by sequence of HS–HN < HO–HN < HP–HN. This sequence is relatively fit with the total C=O, C–O, and OH groups in Figure 3.
HS-HN HO-HN
Figure 6. EDX spectra, content table, and SEM images where the content of the composites prepared using 3 different double oxidizing agents: (HP–HN=H3PO4–HNO3; HS–
HN=H2SO4–HNO3; HO–HN=H2O2–HNO3)
(a)
(b)
The journal homepage www.jpacr.ub.ac.id 67 Figure 7. Content of Cr and O in composites prepared using 3 different double oxidizing
agents (HP–HN=H3PO4–HNO3; HS–HN=H2SO4–HNO3; HO–HN=H2O2–HNO3)
Adsorption test
Adsorption test was performed using paracetamol adsorbate. The adsorption data was presented in Figure 8. The adsorption values show that there is an increasing of adsorption value in sequence of HP–HN < HO–HN < HS–HN. This sequence is fit with the sequence of Cr content in Figure 7. It indicates that Lewis acid of Cr(III) in Cr2O3 influence the adsorption. The other side, the order of adsorption is fit with order of both C-O and –OH content in Figure 3. Hence, it indicates that –COOH group also gives effect on adsorption.
Adsorption of carbon material lasts through 3 steps, including mass transfer of adsorbate molecules onto exterior of the carbon, diffusion of molecules into the pores of carbon, and physical adsorption, i.e through the weak interactive forces [24]. This weak interaction can be hydrogen bond or Van der Waals force [25]. Based on its chemical structure, paracetamol molecule has functional groups of C=O, -OH, and –NHR [26]. Thus, it can be predicted that those functional groups of paracetamol can make hydrogen bonds with –COOH groups of the composite. The Cr(III) cations is in Cr2O3 structure is also potential to make Van der Waals force (ion – dipole) with them.
CONCLUSION
Synthesis and application of composite based on patchouli biomass has been conducted. Based on characterization of the composites it can be concluded that type of oxidizing agents affects the content of Cr2O3 in the composite, surface functional groups of biochar before impregnation, and paracetamol adsorption. Sequence of adsorption value is in line with sequence of Cr2O3 content in the composite, i.e H3PO4–HNO3 < H2O2–HNO3 < H2SO4–HNO3. Sequence of Cr2O3 content is also fit with the sequence of both C–O and –OH infrared absorbance in the same sequence of the oxidizing agents.
ACKNOWLEDGMENT
We thank DIKTI for research funding and acknowledge Brawijaya University for supports for this research.
REFERENCES
[1] Heckley, E., The Structural Changes of Hydrothermally Treated Biochar Caused by
Ball-Milling, Juan Mauricio Venegas, 2014
[2] Klinsmann Ng Weng Sum, Adsorption of Trimethyltin, Arsenic (v), Zinc and Copper
by Palm Oil Mill Sludge Biochar Prepared by Microwave, Faculty of Engineering
and Green Technology, University Tunku Abdul Rahman, 2016
[3] Fang, C., Zhang, T., Li, P., Jiang, R., and Wang, Y., Int. J. Environ. Res. Public
[6] Setianingsih, T., Ismuyanto, B., Masruri, Int J Chemtech Res., 2016, l9(12), 610-621. [7] Moosavi, E., Dastgheib, S., and Karimzadeh, R., Energies., 2012, 5, 4233-4250 [8] Han, Z., Sani, B., Mrozik, W., Obst, M. Beckingham, B., Karapanagioti, HK.,
Werner, D., Water Res.,2015, 70(1), 394-403
[9] Shen, W., Li, Z., and Liu, Y., Recent Patents on Chemical Engineering, 2008, 1, 27-40
[10] Kulkarni, S., JCCS, 2015, 5(8), 443-447
[11] Olivier, C.F., An investigation into the degradation of biochar and its interactions
with plants and soil microbial community, Faculty of AgriSciences, Stellenbosch
University, 2011
The journal homepage www.jpacr.ub.ac.id 69 [21] Hassen, A, El Sayed, M, Morsi, W.M. and El-Sayed, S., J. Appl. Phys., 2012, 112,
093525-1-8.
[22] Cellard, A., Zenati, R., Garnier, V., Fantozzi, G., Baret, G., J. Eur. Ceram. Soc., 2007, 27, 1017-1021.
[23] Sonea, B.T., Manikandana, E., Gurib-Fakima, A., and Maazaa, M., Green Chem. Lett. Rev., 2016, 9(2), 85–90.
[24] Wu, J., 2004, Modeling Adsorption of Organic Compounds on Activated Carbon A multivariate approach, Université de Neuchâtel, Neuchâtel, Schweiz, 2004.
[25] Oscik, J., Cooper, I.L (editor), Adsorption, 1982, John Wiley & Sons Inc., Queensland.