Overview of Nicotinic Receptors and Their Roles in the
Central Nervous System
John A. Dani
Alzheimer’s disease is a complex disorder affecting mul-tiple neurotransmitters. In particular, the degenerative progression is associated with loss within the cholinergic systems. It should be anticipated that both muscarinic and nicotinic mechanisms are affected as cholinergic neurons are lost. This review focuses on the basic roles of neuronal nicotinic receptors, some subtypes of which decrease during Alzheimer’s disease. Nicotinic acetylcholine recep-tors belong to a superfamily of ligand-gated ion channels that play key roles in synaptic transmission throughout the central nervous system. Neuronal nicotinic receptors, however, are not a single entity, but rather there are many different subtypes constructed from a variety of nicotinic subunit combinations. This structural diversity and the presynaptic, axonal, and postsynaptic locations of nico-tinic receptors contribute to the varied roles these recep-tors play in the central nervous system. Presynaptic and preterminal nicotinic receptors enhance neurotransmitter release, and postsynaptic nicotinic receptors mediate a small minority of fast excitatory transmission. In addition, some nicotinic receptor subtypes have roles in synaptic plasticity and development. Nicotinic receptors are dis-tributed to influence many neurotransmitter systems at more than one location, and the broad, but sparse, cholinergic innervation throughout the brain ensures that nicotinic acetylcholine receptors are important modula-tors of neuronal excitability. Biol Psychiatry 2001;49:
166 –174 © 2001 Society of Biological Psychiatry
Key Words: Acetylcholine, nicotine, Alzheimer’s dis-ease, tobacco, addiction, plasticity
Introduction
N
icotinic acetylcholine receptors (nAChRs) belong to the superfamily of ligand-gated ion channels that includes g-aminobutyric acid A (GABAA), glycine, and serotonin 3 (5-HT3) receptors (Albuquerque et al 1997; Dani 2000; Dani and Heinemann 1996; Dani and Mayer 1995; Jones et al 1999; Lena and Changeux 1998; Lind-strom 1997; LindLind-strom et al 1996; Luetje et al 1990; McGehee and Role 1995; Role and Berg 1996; Sargent1993; Wonnacott 1997). Agonists, such as endogenous acetylcholine or exogenous nicotine, stabilize the open conformation of the nAChR channel, which transiently permeates cations before closing back to a resting state or to a desensitized state that is unresponsive to agonists.
Multiple Subunits Produce Nicotinic
Receptor Diversity
The structure of the nicotinic receptor– channel complex arises from five polypeptide subunits assembled like staves of a barrel around a central water-filled pore (Cooper et al 1991). Although various subunit combina-tions can produce many different nAChR subtypes, the nicotinic receptor/channel family can be separated into three general functional classes that are consistent with their evolutionary development and their pharmacologic and physiologic properties: muscle subunits (a1,b1,d,e, g), which are not discussed here; standard neuronal subunits (a2–a6 and b2–b4) that form nAChRs in ab combinations; and subunits (a7–a9) capable of forming homomeric nAChRs that are inhibited bya-bungarotoxin (Colquhoun and Patrick 1997; Le Nove`re and Changeux 1995; McGehee and Role 1995). In the third classification, only thea7 subunit (nota8 ora9) is widely distributed in the mammalian central nervous system (CNS). There is evidence that subunits from the separate classes may combine to form nAChRs, possibly making these group-ings less than perfectly distinct (Girod et al 1999; Yu and Role 1998; Zoli et al 1998).
Heterologous expression systems have been used to determine possible combinations of nAChR subunits ca-pable of forming active ion channels (McGehee and Role 1995; Patrick et al 1993). Those studies indicated group-ings of nAChRs that are easily formed in the Xenopus oocyte expression system:a7 homomeric receptors, het-erodimeric ab nAChRs formed by a combination of a subunits (a2,a3, ora4) andbsubunits (eitherb2 orb4), and complex receptors that include more than one type of a or b subunit (e.g., a4b2b4). This third group of complex combinations also includes nAChRs containing subunits such as a5 and b3 that do not form channels when they are expressed alone or in combination with any other single a or b subunit (e.g., a4a5b2) (Conroy and Berg 1995; Conroy et al 1992; Ramirez-Latorre et al
From the Division of Neuroscience, Baylor College of Medicine, Houston, Texas. Address reprint requests to John A. Dani, Division of Neuroscience, Baylor College
of Medicine, Houston TX 77030-3498. Received May 25, 2000; accepted July 11, 2000.
1996). a6 can rarely form an ab receptor, and it also participates in more complex combinations (Gerzanich et al 1997). Evidence indicates that thea7 subunit can also form more complex combinations (Girod et al 1999; Yu and Role 1998; Zoli et al 1998). The basic conclusion drawn from expression systems is that a limited number of subunit combinations are favored, but more rare combina-tions are possible. There is tremendous potential for nAChR diversity within the CNS.
There are some general rules, and these can be applied when trying to simplify the influence of particular sub-units. For example, a7 homomeric nAChRs have high calcium permeability and rapid activation and desensitiza-tion kinetics, and they are specifically inhibited bya -bun-garotoxin and methyllycaconitine (Alkondon et al 1992; Castro and Albuquerque 1995; Gray et al 1996). Nicotinic AChRs with similar properties are found in the hippocam-pus and other neuronal regions, consistent with the hy-pothesis thata-bungarotoxin binding sites represent a 7-containing nAChRs (Albuquerque et al 1997; Alkondon and Albuquerque 1993; Lindstrom 1997; Lindstrom et al 1996; Samuel et al 1997; Zarei et al 1999). Supporting that hypothesis, a-bungarotoxin binding sites were not de-tected ina7-knockout mice (Orr-Urtreger et al 1997). The high-affinity [3H]nicotine binding sites, however, were still present. Most high-affinity [3H]nicotine sites appear to contain a4 and b2 subunits, and possibly some also contain additional subunits. These high-affinity sites are lost after knockout of theb2 subunit (Zoli et al 1998). In mice lacking theb2 subunit, other lower affinity nicotine binding sites are revealed, and these sites are likely to contain thea3 subunit.
Expression of nAChRs in the CNS
Most nAChRs in the mammalian brain contain either a4b2 or a7 (Charpantier et al 1998; Cimino et al 1992; Clarke et al 1985; Schoepfer et al 1990; Se´gue´la et al 1993; Wada et al 1989, 1990). Although many of the nAChRs containa4 andb2 subunits, agonist and antago-nist profiles differ for cells derived from different nuclei. For example, both medial habenula and locus coeruleus neurons express functional nAChRs with a pharmacologic profile that is consistent witha3 andb4 subunits in those regions (Mulle et al 1991). Studies indicate that there are fewb2 subunits contributing to medial habenular nAChRs (Quick et al 1999). Another example is thata7-containing nAChRs mediate the predominant nicotinic current in hippocampal neurons, but other nAChR responses may be attributable to a4b2-containing, a3b4-containing, and other nAChRs (Albuquerque et al 1997; Alkondon and Albuquerque 1993; Zarei et al 1999; Zoli et al 1998). These electrophysiology results are consistent with in situ
hybridization studies that indicated high expression ofa7 and b2 throughout the rat hippocampus and weaker expression of a3, a4, a5, and b4 (Se´gue´la et al 1993; Wada et al 1989, 1990; Zoli et al 1998).
Radiolabeled nicotinic ligands and in situ hybridization for nAChR messenger RNA indicate a wide, nonuniform distribution of various subunits, and nuclei often contain subgroups of nAChR subunits (Charpantier et al 1998; Cimino et al 1992; Clarke et al 1985; Lena et al 1999; Le Nove`re et al 1996; Schoepfer et al 1990; Se´gue´la et al 1993; Sihver et al 1998; Wada et al 1989, 1990). Although one class of nAChRs often predominates within a region, more than one class or subclass of nAChRs is often present (e.g., Pidoplichko et al 1997). In addition, individ-ual neurons often express multiple classes of nAChRs, and even related neighboring neurons can express nicotinic responses that are significantly different (Dani et al 2000).
Basic Functions of Nicotinic Receptors
Upon binding ACh, the nAChR ion channel is stabilized in the open conformation for several milliseconds. Then the open pore of the receptor/channel closes to a resting state or closes to a desensitized state that is unresponsive to ACh or other agonists for many milliseconds or more. While open, nAChRs conduct cations, which can cause a local depolarization of the membrane and produce an intracellular ionic signal.
Although sodium and potassium carry most of the nAChR current, calcium can also make a significant contribution (Castro and Albuquerque 1995; Decker and Dani 1990; Dani and Mayer 1995; Se´gue´la et al 1993; Vernino et al 1992, 1994). Calcium entry through nAChRs can be biologically important and is different from cal-cium influx mediated by voltage-gated calcal-cium channels or by the N-methyl-D-aspartate (NMDA) subtype of
glu-tamate receptors. Both voltage-gated calcium channels and NMDA receptors require membrane depolarization to pass current freely. At hyperpolarized (negative) potentials, voltage-gated calcium channels generally do not open and NMDA receptors are blocked by extracellular magnesium ions. Nicotinic nAChRs do open and pass current freely at negative potentials that provide a strong voltage force driving cations into the cell. Thus, calcium currents mediated by nAChRs can have a different voltage depen-dence than is seen for other calcium-permeable ion chan-nels. Also, incoming calcium has a different spatial distri-bution that depends on the location of nAChRs on the cell surface.
selectivity with which it conducts cations in the open state depend on many factors, including the subunit composi-tion. Therefore, the extensive nAChR diversity has the potential to produce many different responses to endoge-nous or exogeendoge-nous agonists. The speed of activation, the intensity of the membrane depolarization, the size of the ionic signal, the rates of desensitization and recovery from desensitization, the pharmacology, and the regulatory controls of the ACh response will all depend on the subunit composition of nAChRs as well as other local factors. To add further complexity, the three basic confor-mational states (rest, open, and desensitized) do not account for the actual kinetic properties of nicotinic receptors. Rather, there are multiple conformations in-volved in the gating. Desensitization, in particular, can encompass many time constants (Dani et al 2000; Fenster et al 1999b). Thus, there may be short- and long-lived
states of desensitization. Long exposures to low concen-trations of agonist will favor deeper levels of desensitiza-tion, and this situation is often the case for smokers, who maintain low concentrations of nicotine throughout the day (Benowitz et al 1989; Benwell et al 1995; Clarke 1990, 1991; Dani and Heinemann 1996; Ochoa et al 1990; Reitstetter et al 1999; Russell 1989; Wonnacott 1990).
In progressing through this section, our view of the nAChR has grown from an on-off switch regulating cationic conductance to a sophisticated allosteric macro-molecule (Lena and Changeux 1993, 1998). Figure 1 represents sites on a nAChR that can alter function. Competitive antagonists and partial agonists can occupy the agonist binding sites. Open channel blockers, such as phencyclidine, can bind within the pore, and there are multiple sites for other noncompetitive inhibitors and modulators. In addition, nAChRs are regulated by several Figure 1. A representation of the allosteric sites on a nicotinic
acetylcholine receptor (nAChR), a cut-away view showing three of the five subunits that form the pentameric receptor– channel complex.
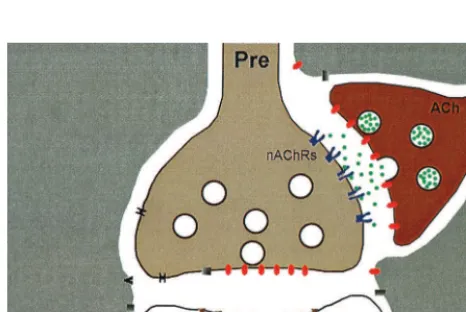
Figure 2. A representation of presynaptic nicotinic acetylcholine receptors (nAChRs). The nAChRs are positioned so they can influence the release of another neurotransmitter, which is released by the presynaptic terminal (Pre) onto the postsynaptic bouton (Postsynaptic). Evidence indicates that nAChRs initiate an increase of calcium in the presynaptic terminal. The calcium then enhances the release of the neurotransmitter.
Figure 3. A representation of preterminal or axonal nicotinic acetylcholine receptors (nAChRs). The nAChRs are located in a position along the axon arbor to indicate they could more easily influence some presynaptic terminals while not affecting others.
other factors, including peptide transmitters, various pro-tein kinases, the cytoskeleton, and calcium. Although calcium modulation can act intracellularly (as is usually the case), nAChRs can also be allosterically modulated by extracellular calcium, leading to dramatic changes in the channel opening probability (Adams and Nutter 1992; Amador and Dani 1995; Mulle et al 1992; Vernino et al 1992). This modulation occurs over the physiologic con-centration range of external calcium. Therefore, high levels of neuronal activity that can diminish extracellular calcium (Heinemann et al 1990; Wiest et al 2000) could cause a negative feedback that lowers the opening proba-bility of nAChRs.
Calcium modulation is well documented, but there is great potential for nAChR modulation by other allosteric effectors. This potential offers points of entry for thera-peutic drugs, such as those that can enhance nAChR activity to assist Alzheimer’s disease (AD) patients. Pres-ently, cholinergic activity can be enhanced in patients with AD by antiacetylcholinesterase drugs that prolong the time that ACh is present before being broken down. There is evidence, however, that some antiacetylcholinesterase drugs (such as physostigmine and galantamine) can allo-sterically potentiate nAChRs (Maelicke and Albuquerque 2000). Drugs that could act specifically to enhance the activity of the required nAChR subtype, while also pro-longing the action of ACh, could be vital in combating the debilitating effects of AD.
In addition to loss of nicotinic mechanisms, as seen in AD (Nordberg 1994; Paterson and Nordberg 2000; Perry et al 1995), there are other physiologic forms of regulation that may become disrupted during pathologic conditions (Dani, in press; Dani and Heinemann 1996; Lena and Changeux 1998; Lindstrom 1997; Perry et al 1995; Stein-lein et al 1995). For example, during chronic tobacco use, low concentrations of nicotine cause certain neuronal nAChRs to increase in number and to accumulate in deep states of desensitization (Fenster et al 1999c; Peng et al 1994, 1997). Chronic desensitization may uncouple regu-latory mechanisms important for proper nAChR function-ing and promote incorrect recyclfunction-ing of receptors, thereby leading to an increase in the number of receptors. Inap-propriate changes in the expression of nAChRs may be important in the process of tobacco addiction.
Competing Processes of nAChR Activation
and Desensitization
At a cholinergic synapse, approximately 1 mmol/L ACh is rapidly released into the cleft, immediately activating the nicotinic receptors. In a few milliseconds, the ACh is hydrolyzed by acetylcholinesterase and/or diffuses away. The delivery and removal of ACh is very rapid, and
therefore desensitization is usually not thought to be important even though the desensitization process is com-plex. As neuronal nicotinic receptors are diverse and neuronal synapses are anatomically and compositionally varied, the role of desensitization in the brain is not well understood and remains a difficult problem. Repeatedly exposing a synapse to about 1 mmol/L ACh might normally produce little desensitization. If the synaptic stimulation is extremely high, however, even though the rates of recovery from desensitization are fast they may not allow complete recovery (Dilger and Liu 1992; Fenster et al 1999b; Franke et al 1992). Furthermore, the break-down of ACh releases choline, which could reach locally high concentrations. Higher concentrations of choline could desensitize a7-containing nicotinic receptors (Alkondon et al 1997b; Papke et al 1996).
The physiologic role of desensitization may become especially important when considering the desensitizing levels of nicotine that bathe the brains of smokers (Clarke 1991; Dani and Heinemann 1996; Ochoa et al 1990) or when considering the effects of drugs that inhibit acetyl-cholinesterase (e.g., to treat patients with AD). Under those two conditions, some nAChRs are likely to desen-sitize, but not in a uniform manner. At extremely active cholinergic synapses, nAChRs are more susceptible to the desensitizing influence of the endogenous agonist (ACh). When high rates of cholinergic activity are occurring in conjunction with antiacetylcholinesterase or long expo-sures to nicotine, then the synaptic nAChRs are more susceptible to desensitization. Evidence indicates that longer exposures to agonist allow slower rates of desen-sitization to come into play, such that some nicotinic receptors can enter longer lasting desensitization (Lester and Dani 1994; Reitstetter et al 1999).
If desensitization comes into play (normally, pathologi-cally, or owing to drugs), then the rate of synaptic firing will be an important determinant of how much current enters the synapse via nicotinic receptors per unit time. The highest synaptic firing rates would not necessarily produce the most effective nicotinic signal because the nAChR would desen-sitize more at the highest rates (Dani et al 2000). Kinetic parameters including desensitization depend on the subunit composition, and modulatory processes, such as protein kinases, influence the nAChRs subtypes differently and selectively (Fenster et al 1999a; Paradiso and Brehm 1998). Depending on dynamic modulatory influences, different nAChR subtypes might become particularly susceptible to desensitization, and the modulatory processes can vary from synapse to synapse. Thus, modulatory processes acting upon nAChRs could provide a continually varying, powerful, computational mechanism for manipulating how information is processed at synapses.
important parameters of the nicotinic cholinergic systems. Creating drugs that discriminate nAChRs based on their subtype or location would be important for combating specific problems—for example, AD (Wang et al 2000) or forms of epilepsy (Dani 2000; Steinlein et al 1995).
Main Cholinergic Projections
Before looking further into the roles of nAChRs, we need to consider some of the basic properties of the cholinergic innervation. Cholinergic systems provide diffuse innerva-tion to practically all of the brain, but a relatively small number of cholinergic neurons innervate each neural area (Kasa 1986; Woolf 1991). Despite the sparse innervation, cholinergic activity drives or modulates a wide variety of behaviors. By initially acting on nAChRs, nicotine or nicotinic innervation can increase arousal, heighten atten-tion, influence rapid eye movement sleep, produce states of euphoria, decrease fatigue, decrease anxiety, act cen-trally as an analgesic, transiently normalize sensory gating in schizophrenic patients, and influence a number of cognitive functions (Adler et al 1999; Everitt and Robbins 1997; Levin 1992; Marubio et al 1999; Rose and Levin 1991). It is thought that cholinergic systems particularly affect discriminatory processes by increasing the signal-to-noise ratio and by helping to evaluate the significance and relevance of stimuli.
Although cholinergic neurons are distributed along the axis from the spinal cord and brain stem to the basal telencephalon, there are two major cholinergic project subsystems that can be identified. One cholinergic system arises from neurons in the pedunculopontine tegmentum and the laterodorsal pontine tegmentum, providing wide-spread innervation mainly to the thalamus and midbrain areas and also descending innervation that reaches to the brain stem. The second major cholinergic system arises in the basal forebrain and makes broad projections mainly throughout the cortex and hippocampus. In general, a relatively few cholinergic neurons make spare projections that reach broad areas. Thus, the activity of a rather small number of cholinergic neurons can influence relatively large neuronal structures.
Nicotinic Mechanisms in the CNS
The most widely observed synaptic role of nAChRs in the CNS is to influence neurotransmitter release. Figure 2 depicts presynaptic nAChRs, which have been found to increase the release of nearly every neurotransmitter that has been examined (Albuquerque et al 1997; Alkondon et al 1997a; Gray et al 1996; Guo et al 1998; Jones et al 1999; Li et al 1998; McGehee et al 1995; McGehee and Role 1995; Radcliffe and Dani 1998; Radcliffe et al 1999;
Role and Berg 1996; Wonnacott 1997). Exogenous appli-cation of nicotinic agonists can enhance and nicotinic antagonists can often diminish the release of ACh, dopa-mine, norepinephrine, and serotonin as well as glutamate and GABA. In many cases, the a7-containing nAChRs, which are highly calcium permeable, mediate the in-creased release of neurotransmitter, but in other cases different nAChR subtypes are involved. In rat hippocam-pal slices and cultures, presynaptica7-containing nAChRs were shown to initiate a calcium influx that, consequently, enhances glutamate release from presynaptic terminals (Albuquerque et al 1997; Gray et al 1996). Intense nicotinic stimulation was able to enhance glutamate re-lease on multiple time scales, extending from seconds to a few minutes (Radcliffe and Dani 1998). Similar enhance-ments of release were obtained with a higher probability at GABAergic presynaptic terminals (Radcliffe et al 1999). The forms of enhancement lasting several minutes or more require that the incoming calcium acts as a second mes-senger to modify glutamatergic synaptic transmission indirectly. Properly localized calcium influx mediated by nAChRs initiates enzymatic activity (such as protein kinases and phosphatases) that is known to modify gluta-matergic synapses.
Figure 3 depicts nAChRs at a preterminal location, where they can also alter the release of various neurotrans-mitters. Particularly at GABAergic synapses, activation of preterminal nAChRs has been found to depolarize the membrane locally, leading to activation of voltage-depen-dent channels that directly mediate the synaptic calcium influx underlying enhanced GABA release (Alkondon et al 1997a; Lena et al 1993). The agonist-induced effect mediated by preterminal nAChRs was inhibited by tetro-dotoxin, which blocks sodium channels and, thereby, prevents the regenerative voltage activation of calcium channels in the presynaptic terminal. Axonal nAChRs may also modulate transmitter release and local excitability in another way. As represented in Figure 3, strategically located nAChRs might enable an action potential to invade only a portion of the axonal arbor. By directly exciting or by shunting the progress of an action potential at a bifurcation, axonal nAChRs could initiate or alter the spread of neuronal excitation.
brain slice preparations. Where it has been reported, fast nicotinic transmission is a minor component of the exci-tatory input, which is overwhelmingly glutamatergic. It is likely that nicotinic transmission is present at low densities in more neuronal areas than the few that have been presently reported. Although direct nicotinic excitation of a neuron usually does not predominate, it could influence the excitability of a group of neurons owing to the broad cholinergic projections into an area. Thus, beyond their specific roles at discrete synapses, nAChRs also modulate neuronal circuits in a broader sense.
An example of nicotinic modulation of circuit excitabil-ity was seen in the hippocampal slice (Ji and Dani 2000). While recording from CA1 pyramidal neurons, local application of nicotinic agonist onto interneurons caused both inhibition and disinhibition. Activating nAChRs on interneurons that directly innervated pyramidal neurons caused inhibition of the pyramidal neuron, and activating interneurons that innervated mainly other interneurons caused disinhibition. The disinhibition probably occurred because the nicotinic agonist activated interneurons that then inhibited other interneurons (Alkondon et al 1999). Consequently, GABAergic inhibitory activity decreased in the area; thus, some pyramidal neurons were temporarily released from their inhibitory inputs (i.e., disinhibition).
The results in the hippocampus indicate that, owing to the broad projections by cholinergic neurons, nicotinic activity can influence not just synaptic events but also the excitability of neuronal areas (Dani 2000; Ji and Dani 2000). By influencing circuits, nAChRs may modulate the rhythmic activity in the hippocampus or in other regions. A property of hippocampal circuits is that they enter into different rhythmic oscillations. For example, theta rhythms and gamma rhythms often occur during paradox-ical sleep or while awake rats explore their environments (Vanderwolf 1969). The periods of circuit activity that accompany these rhythms provide opportunities for syn-aptic plasticity that underlies learning and memory (Huerta and Lisman 1993). Because interneurons are important determinants of the rhythmic oscillations and they receive cholinergic afferents that can modulate the rhythms (Csicsvari et al 1999; Dragoi et al 1999), it seems likely that nAChRs influence the patterns of activity in the hippocampus and elsewhere.
Nicotinic AChRs also have roles during development and neuronal plasticity (Broide and Leslie 1999; Role and Berg 1996). The density of nAChRs varies during the course of development, and nAChRs can contribute to activity-dependent calcium signals. For example, presyn-aptic a7-containing nAChRs increased the release of glutamate preferentially onto postsynaptic NMDA recep-tors in the developing rat auditory cortex, but not in the mature cortex (Aramakis and Metherate 1998). By
en-hancing glutamate release particularly at the locations of NMDA receptors, nAChRs might help to modulate activ-ity-dependent synaptic plasticity that is often initiated by postsynaptic NMDA receptors (Malenka and Nicoll 1999). Nicotinic regulatory, plasticity, and developmental influ-ences are particularly important when considering the etiology of disease. Biological changes that inappropri-ately alter nicotinic mechanisms could immediinappropri-ately influ-ence the release of many neurotransmitters and alter circuit excitability. However, nicotinic dysfunction could have long-term developmental consequences that are ex-pressed later in life.
In summary, the tremendous diversity of nAChRs provides the flexibility necessary for them to play multi-ple, varied roles. Broad, sparse cholinergic projects ensure that nicotinic mechanisms modulate the neuronal excit-ability of relatively wide circuits. Although fast nicotinic transmission is not the predominant driving force, it can contribute excitatory input to many synapses at one time. Presynaptic and preterminal nAChRs modulate the release of all the major neurotransmitters that have been tested. During the progression of AD, cholinergic inputs degen-erate and the number of nAChRs in some areas decreases (Nordberg 1994; Perry et al 1995). Loss of nicotinic mechanisms, which modulate the gain and fidelity of synapses and modulate the excitability of circuits, are likely to contribute to the overall cognitive deficits asso-ciated with AD.
Work from this laboratory is supported by National Institutes of Health grants from the National Institute on Drug Abuse (Nos. DA09411 and DA12661) and from the National Institute of Neurological Disorders and Stroke (No. NS21229).
Aspects of the work were presented at the symposium “Nicotine Mechanisms in Alzheimer’s Disease,” March 16 –18, 2000, Fajarko, Puerto Rico. The conference was sponsored by the Society of Biological Psychiatry through an unrestricted educational grant provided by Janssen Pharmaceutica LP.
References
Adams DJ, Nutter TJ (1992): Calcium permeability and modu-lation of nicotinic acetylcholine receptor-channels in rat parasympathetic neurons. J Physiol Paris 86:67–76. Adler LE, Freedman R, Ross RG, Olincy A, Waldo MC (1999):
Elementary phenotypes in the neurobiological and genetic study of schizophrenia. Biol Psychiatry 46:8 –18.
Albuquerque EX, Alkondon M, Pereira EF, Castro NG, Schrat-tenholz A, Barbosa CT, et al (1997): Properties of neuronal nicotinic acetylcholine receptors: Pharmacological character-ization and modulation of synaptic function. J Pharmacol Exp Ther 280:1117–1136.
Phar-macological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther 265:1455–1473.
Alkondon M, Pereira EF, Albuquerque EX (1998): Alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic re-ceptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res 810:257–263.
Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX (1997a): Neuronal nicotinic acetylcholine receptor activation modu-lates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther 283:1396 – 1411.
Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX (1997b): Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neu-rosci 9:2734 –2742.
Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX (1999): Choline and selective antagonists identify two sub-types of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci 19:2693–2705.
Alkondon M, Pereira EF, Wonnacott S, Albuquerque EX (1992): Blockade of nicotinic currents in hippocampal neurons de-fines methyllycaconitine as a potent and specific receptor antagonist. Mol Pharmacol 41:802– 808.
Amador M, Dani JA (1995): Mechanism for modulation of nicotinic acetylcholine receptors that can influence synaptic transmission. J Neurosci 15:4525– 4532.
Aramakis VB, Metherate R (1998): Nicotine selectively en-hances NMDA receptor-mediated synaptic transmission dur-ing postnatal development in sensory neocortex. J Neurosci 18:8485– 8495.
Benowitz NL, Porchet H, Jacob P III (1989): Nicotine depen-dence and tolerance in man: Pharmacokinetic and pharmaco-dynamic investigations. Prog Brain Res 79:279 –287. Benwell ME, Balfour DJ, Birrell CE (1995): Desensitization of
the nicotine-induced mesolimbic dopamine responses during constant infusion with nicotine. Br J Pharmacol 114:454 – 460.
Broide RS, Leslie FM (1999): The alpha7 nicotinic acetylcholine receptor in neuronal plasticity. Mol Neurobiol 20:1–16. Castro NG, Albuquerque EX (1995):
alpha-Bungarotoxin-sensi-tive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys J 68:516 –524.
Charpantier E, Barne´oud P, Moser P, Besnard F, Sgard F (1998): Nicotinic acetylcholine subunit mRNA expression in dopa-minergic neurons of the rat substantia nigra and ventral tegmental area. Neuroreport 9:3097–3101.
Cimino M, Marini P, Fornasari D, Cattabeni F, Clementi F (1992): Distribution of nicotinic receptors in cynomolgus monkey brain and ganglia: Localization of alpha 3 subunit mRNA, alpha-bungarotoxin and nicotine binding sites. Neu-roscience 51:77– 86.
Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A (1985): Nicotinic binding in rat brain: Autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bunga-rotoxin. J Neurosci 5:1307–1315.
Clarke PBS (1990): Mesolimbic dopamine activation—the key to nicotine reinforcement? In: Bock G, Marsh J, editors. The Biology of Nicotine Dependence. New York: Wiley, 153–168.
Clarke PBS (1991): The mesolimbic dopamine system as a target for nicotine. In: Adlkofer F, Thurau K, editors. Effects of Nicotine on Biological Systems. Basel: Birkha¨user Verlag, 285–294.
Colquhoun LM, Patrick JW (1997): Pharmacology of neuronal nicotinic acetylcholine receptor subtypes. Adv Pharmacol 39:191–220.
Conroy WG, Berg DK (1995): Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. J Biol Chem 9:4424 – 4431. Conroy WG, Vernallis AB, Berg DK (1992): The alpha 5 gene
product assembles with multiple acetylcholine receptor sub-units to form distinctive receptor subtypes in brain. Neuron 9:679 – 691.
Cooper E, Couturier S, Ballivet M (1991): Pentameric structure and subunit stoichiometry of a neuronal acetylcholine recep-tor. Nature 350:235–238.
Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G (1999): Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J Neurosci 19:1274 –1287. Dani JA (2000): Properties underlying the influence of nicotinic receptors on neuronal excitability and epilepsy. Epilepsia 41:1063–1065.
Dani JA, Heinemann S (1996): Molecular and cellular aspects of nicotine abuse. Neuron 16:905–908.
Dani JA, Mayer ML (1995): Structure and function of glutamate and nicotinic acetylcholine receptors. Curr Opin Neurobiol 5:310 –317.
Dani JA, Radcliffe KA, Pidoplichko V (2000): Variations in desensitization of nicotinic acetylcholine receptors from hip-pocampus and midbrain dopamine areas. Eur J Pharmacol 393:31–38.
Decker ER, Dani JA (1990): Calcium permeability of the nicotinic acetylcholine receptor: the single-channel calcium influx is significant. J Neurosci 10:3413–3420.
Dilger JP, Liu Y (1992): Desensitization of acetylcholine recep-tors in BC3H-1 cells. Pflugers Arch 420:479 – 485.
Dragoi G, Carpi D, Recce M, Csicsvari J, Buzsaki G (1999): Interactions between hippocampus and medial septum during sharp waves and theta oscillation in the behaving rat. J Neu-rosci 19:6191– 6199.
Everitt BJ, Robbins TW (1997): Central cholinergic systems and cognition. Annu Rev Psychol 48:649 – 684.
Fenster CP, Beckman ML, Parker JC, Sheffield EB, Whitworth TL, Quick MW, et al (1999a): Regulation of alpha4beta2 nicotinic receptor desensitization by calcium and protein kinase C. Mol Pharmacol 55:432– 443.
Fenster CP, Hicks JH, Beckman ML, Covernton PJ, Quick MW, Lester RA (1999b): Desensitization of nicotinic receptors in the central nervous system. Ann N Y Acad Sci 868:620 – 623. Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RA (1999c): Upregulation of surface alpha4beta2 nicotinic recep-tors is initiated by receptor desensitization after chronic exposure to nicotine. J Neurosci 19:4804 – 4814.
Synaptic potentials mediated via a-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneu-rons. J Neurosci 18:8228 – 8235.
Gerzanich V, Kuryatov A, Anand R, Lindstrom J (1997): “Orphan” alpha6 nicotinic AChR subunit can form a func-tional heteromeric acetylcholine receptor. Mol Pharmacol 51:320 –327.
Girod R, Crabtree G, Ernstrom G, Ramirez-Latorre J, McGehee D, Turner J, et al (1999): Heteromeric complexes of alpha 5 and/or alpha 7 subunits. Effects of calcium and potential role in nicotine-induced presynaptic facilitation. Ann N Y Acad Sci 868:578 –590.
Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA (1996): Hippocampal synaptic transmission enhanced by low concen-trations of nicotine. Nature 383:713–716.
Guo J-Z, Tredway TL, Vincent A, Chiappinelli VA (1998): Glutamate and GABA release are enhanced by different subtypes of presynaptic nicotinic receptors in the lateral geniculate nucleus. J Neurosci 18:1963–1969.
Hefft S, Hulo S, Bertrand D, Muller D (1999): Synaptic transmission at nicotinic acetylcholine receptors in rat hip-pocampal organotypic cultures and slices. J Physiol (Lond) 515:769 –776.
Heinemann U, Stabel J, Rausche G (1990): Activity-dependent ionic changes and neuronal plasticity in rat hippocampus. Prog Brain Res 83:197–214.
Huerta PT, Lisman JE (1993): Heightened synaptic plasticity of CA1 hippocampal neurons during a cholinergically induced rhythmic state. Nature 364:723–725.
Ji D, Dani JA (2000): Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J Neurophysiol 83:2682–2690.
Jones S, Sudweeks S, Yakel JL (1999): Nicotinic receptors in the brain: correlating physiology with function. Trends Neurosci 22:555–561.
Kasa P (1986): The cholinergic systems in brain and spinal cord. Prog Neurobiol 26:211–272.
Lena C, Changeux JP (1993): Allosteric modulations of the nicotinic acetylcholine receptor. Trends Neurosci 16:181– 186.
Lena C, Changeux JP (1998): Allosteric nicotinic receptors, human pathologies. J Physiol Paris 92:63–74.
Lena C, Changeux JP, Mulle C (1993): Evidence for “pretermi-nal” nicotinic receptors on GABAergic axons in the rat interpeduncular nucleus. J Neurosci 13:2680 –2688. Lena C, de Kerchove D’Exaerde A, Cordero-Erausquin M, Le
Novere N, del Mar Arroyo-Jimenez M, Changeux JP (1999): Diversity and distribution of nicotinic acetylcholine receptors in the locus ceruleus neurons. Proc Natl Acad Sci U S A 96:12126 –12131.
Le Nove`re N, Changeux J-P (1995): Molecular evolution of the nicotinic acetylcholine receptor: an example of multigene family in excitable cells. J Mol Evol 40:155–172.
Le Nove`re N, Zoli M, Changeux J-P (1996): Neuronal nicotinic receptor alpha 6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of rat brain. Eur J Neurosci 8:2428 –2439.
Lester RA, Dani JA (1994): Time-dependent changes in central nicotinic acetylcholine channel kinetics in excised patches. Neuropharmacology 33:27–34.
Levin ED (1992): Nicotinic systems and cognitive function. Psychopharmacology (Berl) 108:417– 431.
Li X, Rainnie DG, McCarley RW, Greene RW (1998): Presyn-aptic nicotinic receptors facilitate monoaminergic transmis-sion. J Neurosci 18:1904 –1912.
Lindstrom J (1997): Nicotinic acetylcholine receptors in health and disease. Mol Neurobiol 15:193–222.
Lindstrom J, Anand R, Gerzanich V, Peng X, Wang F, Wells G (1996): Structure and function of neuronal nicotinic acetyl-choline receptors. Prog Brain Res 109:125–137.
Luetje CW, Patrick J, Se´gue´la P (1990): Nicotine receptors in the mammalian brain. FASEB J 4:2753–2760.
Maelicke A, Albuquerque EX (2000): Allosteric modulation of nicotinic acetylcholine receptors as a treatment strategy for Alzheimer’s disease. Eur J Pharmacol 393:165–170. Malenka RC, Nicoll RA (1993): NMDA-receptor-dependent
synaptic plasticity: multiple forms and mechanisms. Trends Neurosci 16:521–527.
Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Lena C, Le Novere N, de Kerchove d’Exaerde A, et al (1999): Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature 398:805– 810.
McGehee DS, Heath MJ, Gelber S, Devay P, Role LW (1995): Nicotine enhancement of fast excitatory synaptic transmis-sion in CNS by presynaptic receptors. Science 269:1692– 1696.
McGehee DS, Role LW (1995): Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol 57:521–546.
Mulle C, Le´na C, Changeux JP (1992): Potentiation of nicotinic receptor response by external calcium in rat central neurons. Neuron 8:937–945.
Mulle C, Vidal C, Benoit P, Changeux JP (1991): Existence of different subtypes of nicotinic acetylcholine receptors in the rat habenulo-interpeduncular system. J Neurosci 11:2588 – 2597.
Nordberg A (1994): Human nicotinic receptors—their role in aging and dementia. Neurochem Int 25:93–97.
Ochoa EL, Li L, McNamee MG (1990): Desensitization of central cholinergic mechanisms and neuroadaptation to nico-tine. Mol Neurobiol 4:251–287.
Orr-Urtreger A, Goldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, et al (1997): Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha-bungaro-toxin binding sites and hippocampal fast nicotinic currents. J Neurosci 17:9165–9171.
Papke RL, Bencherif M, Lippiello P (1996): An evaluation of neuronal nicotinic acetylcholine receptor activation by qua-ternary nitrogen compounds indicates that choline is selective for the alpha 7 subtype. Neurosci Lett 213:201–204. Paradiso K, Brehm P (1998): Long-term desensitization of
nicotinic acetylcholine receptors is regulated via protein kinase A-mediated phosphorylation. J Neurosci 18:9227– 9237.
Paterson D, Nordberg A (2000): Neuronal nicotinic receptors in the human brain. Prog Neurobiol 61:75–111.
Peng X, Gerzanich V, Anand R, Wang F, Lindstrom J (1997): Chronic nicotine treatment up-regulates alpha3 and alpha7 acetylcholine receptor subtypes expressed by the human neuroblastoma cell line SH-SY5Y. Mol Pharmacol 51:776 – 784.
Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J (1994); Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Mol Pharmacol 46:523–530.
Perry EK, Morris CM, Court JA, Cheng A, Fairbairn AF, McKeith IG, et al (1995): Alteration in nicotine binding sites in Parkinson’s disease, Lewy body dementia and Alzheimer’s disease: possible index of early neuropathology. Neuro-science 64:385–395.
Pidoplichko V, DeBiasi M, Williams JT, Dani JA (1997): Nicotine activates and desensitizes midbrain dopamine neu-rons. Nature 390:401– 404.
Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RA (1999): Alpha3beta4 subunit containing nicotinic receptors dominate function in rat medial habenula neurons. Neuro-pharmacology 38:769 –783.
Radcliffe KA, Dani JA (1998): Nicotinic stimulation produces multiple forms of increased glutamatergic synaptic transmis-sion. J Neurosci 18:7075–7083.
Radcliffe KA, Fisher J, Gray R, Dani JA (1999): Nicotine potentiates glutamate and GABA synaptic transmission. Ann N Y Acad Sci 868:591– 610.
Ramirez-Latorre J, Yu C, Qu X, Perin F, Karlin A, Role L (1996): Functional contributions of a5 subunit to neuronal acetylcholine receptor channels. Nature 380:347–351. Reitstetter R, Lukas RJ, Gruener R (1999): Dependence of
nicotinic acetylcholine receptor recovery from desensitization on the duration of agonist exposure. J Pharmacol Exp Ther 289:656 – 660.
Roerig B, Nelson DA, Katz LC (1997): Fast synaptic signaling by nicotinic acetylcholine and serotonin 5-HT3receptors in
developing visual cortex. J Neurosci 17:8353– 8362. Role LW, Berg DK (1996): Nicotinic receptors in the
develop-ment and modulation of CNS synapses. Neuron 16:1077– 1085.
Rose JE, Levin ED (1991): Inter-relationships between condi-tioned and primary reinforcement in the maintenance of cigarette smoking. Br J Addict 86:605– 609.
Russell MAH (1989): Subjective and behavioral effects of nicotine in humans: Some sources of individual variation. Prog Brain Res 79:289 –302.
Samuel N, Wonnacott S, Lindstrom J, Futerman AH (1997): Parallel increases in [alpha-125I]bungarotoxin binding and alpha 7 nicotinic subunit immunoreactivity during the devel-opment of rat hippocampal neurons in culture. Neurosci Lett 222:179 –182.
Sargent P (1993): The diversity of neuronal nicotinic acetylcho-line receptors. Annu Rev Neurosci 16:403– 443.
Schoepfer R, Conroy W, Whiting P, Gore M, Lindstrom J (1990): Brain a-bungarotoxin binding protein cDNAs and mAbs reveal subtypes of this branch of the ligand-gated ion channel gene superfamily. Neuron 5:35– 48.
Se´gue´la P, Wadiche J, Miller K, Dani JA, Patrick J (1993): Molecular cloning, functional properties and distribution of rat braina7: A nicotinic cation channel highly permeable to calcium. J Neurosci 13:596 – 604.
Sihver W, Gillberg PG, Nordberg A (1998): Laminar distribution of nicotinic receptor subtypes in human cerebral cortex as determined by [3H](2)nicotine, [3H]cytisine and [3H]epiba-tidine in vitro autoradiography. Neuroscience 85:1121–1133. Steinlein OK, Mulley JC, Propping P, Wallace RH, Phillips HA, Sutherland GR, et al (1995): A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet 11:201–203.
Vanderwolf CH (1969): Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol 26:407– 418.
Vernino S, Amador M, Luetje CW, Patrick J, Dani JA (1992): Calcium modulation and high calcium permeability of neu-ronal nicotinic acetylcholine receptors. Neuron 8:127–134. Vernino S, Rogers M, Radcliffe KA, Dani JA (1994):
Quantita-tive measurement of calcium flux through muscle and neu-ronal nicotinic acetylcholine receptors. J Neurosci 14:5514 – 5524.
Wada E, McKinnon D, Heinemann S, Patrick J, Swanson LW (1990): The distribution of mRNA encoded by a new member of the neuronal nicotinic acetylcholine receptor gene family (a5) in the rat central nervous system. Brain Res 526:45–53. Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, et al (1989): Distribution of alpha2, alpha3, alpha4, and beta2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: A hybridization histochemical study in the rat. J Comp Neurol 284:314 –335.
Wang HY, Lee DH, D’Andrea MR, Peterson PA, Shank RP, Reitz AB (2000): Beta-amyloid(1-42) binds to alpha 7 nico-tinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology. J Biol Chem 275:5626 – 5632.
Wiest MC, Eagleman DM, King RD, Montague PR (2000): Dendritic spikes and their influence on extracellular calcium signaling. J Neurophysiol 83:1329 –1337.
Wonnacott S (1990): The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Neurosci 11:216 – 218.
Wonnacott S (1997): Presynaptic nicotinic ACh receptors. Trends Neurosci 20:92–98.
Woolf NJ (1991): Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol 37:475–524.
Yu CR, Role LW (1998): Functional contribution of the alpha7 subunit to multiple subtypes of nicotinic receptors in embry-onic chick sympathetic neurones. J Physiol (Lond) 509:651– 665.
Zarei MM, Radcliffe KA, Chen D, Patrick JW, Dani JA (1999): Distributions of nicotinic acetylcholine receptor alpha7 and beta2 subunits on cultured hippocampal neurons. Neuro-science 88:755–764.