www.elsevier.com / locate / bres
Research report
Cholinergic modulation of neocortical long-term potentiation in the
awake, freely moving rat
1
*
Tiffany E. Boyd, Christopher Trepel , Ronald J. Racine
Department of Psychology, McMaster University, 1280 Main Street West, Hamilton, Ontario, Canada L8S-4K1
Accepted 18 July 2000
Abstract
The neocortex has proven resistant to LTP induction using standard in vitro and acute, in vivo preparations. Because the neocortex is widely thought to be involved in long-term information storage, this resistance raises questions about the validity of LTP as a memory model. Recently, we have shown that the neocortex of freely moving rats reliably supports LTP, provided that the stimulation is spaced and repeated over days. The following experiments were designed to evaluate the neuromodulatory role played by cholinergic systems in the induction of LTP in this preparation. Chronically implanted rats received either low- or high-intensity LTP-inducing tetani in combination with the administration of either a cholinergic agonist or antagonist injected systemically. Potentiation was evidenced as amplitude changes in both early and late components of the evoked field potential, the former including population spikes. The cholinergic agonist facilitated LTP induction in the late component of both high- and low-intensity groups. The cholinergic antagonist blocked LTP induction in the early component of the high-intensity group. The possibility that there are component-specific modulatory effects of cholinergic agents on the induction of neocortical LTP is discussed. 2000 Elsevier Science B.V. All rights reserved.
Theme: Excitable membranes and synaptic transmission
Topic: Long-term potentiation: pharmacology
Keywords: LTP; Acetylcholine; Freely moving; Rat; Neocortex
1. Introduction The model that is most widely used to study the
physiology of memory is long-term synaptic potentiation There is considerable evidence that the cholinergic (LTP) (see [4] for review). Properties such as longevity system plays an important role in learning and memory and associativity make it an attractive memory model, and processes (for review, see [17]). The cognitive deficits it has also been shown in structures, such as the hippocam-associated with Alzheimer’s disease [12,16,34] and normal pus, that are known to be involved in learning and aging [15], for example, may be mediated by a reduction memory.
in cholinergic functioning. Also, it has been established in Recently, we have successfully demonstrated an rodents that cholinergic antagonism [14,25,43] and agon- NMDA-dependent LTP in the neocortex of awake, freely ism [18,35,41] attenuate and facilitate, respectively, learn- moving rats using induction parameters that mimic con-ing in a variety of tasks. Studies uscon-ing human and non- ditions considered optimal for the construction of non-human primates have produced similar results [1,11,13,21]. declarative memories (i.e. spaced and repeated stimulation sessions) [37,42]. This form of LTP does not reach asymptotic levels for 7–12 days [37,42]. These results are consistent with those from memory experiments, where the *Corresponding author. Tel.:11-905-525-9140, ext. 23022; fax:11- acquisition of nondeclarative information into presumed
905-529-6225. cortical stores has been found to be a slow process [31].
E-mail address: [email protected] (R.J. Racine).
1 These properties of LTP raise questions about the
suitabili-Current address: W.M. Keck Foundation Center for Integrative
Neuro-ty of the cortical slice model for studying neocortical LTP. science and Department of Physiology, Box 0444, University of
Califor-nia, 513 Parnassus Avenue, San Francisco, CA, 94143. It is not yet clear that the LTP induced in the slice (e.g. 0006-8993 / 00 / $ – see front matter 2000 Elsevier Science B.V. All rights reserved.
[23]) is the same phenomenon as that monitored over surgery to provide optimal response amplitudes. The weeks and months in the awake animal. resulting mean depths for the white matter stimulating and LTP, like memory, has been shown to be affected by cortical recording electrodes were 3.0 mm and 1.8 mm cholinergic manipulations, but these experiments were ventral to dura, respectively. The electrodes were con-done in in vitro preparations. Cholinergic agonists, for nected to gold-plated male pins that were then inserted into example, enhance LTP induction in both the CAl [5] and a nine-pin miniature connector plug that was mounted onto dentate gyrus [7] regions of the hippocampus as well as in the skull with dental cement and anchored with stainless the visual cortex [6]. The facilitatory action of acetyl- steel screws. One of the screws served as the ground choline on the induction of LTP is most likely the result of electrode. Data acquisition began 10–14 days following enhanced postsynaptic depolarization, increasing the prob- surgery. All experiments were carried out following proper ability, or extent, of NMDA receptor activation [9,32]. guidelines as stipulated by the Canadian Council of Even cholinergic agents by themselves have been shown to Animal Care and approved by the university veterinarian. produce a long-term enhancement of responses in the
hippocampus [3] or sensorimotor cortex [27]. In the
hippocampus, this cholinergically-induced LTP has been 2.2. Baseline measures and induction of LTP shown to occlude subsequent LTP induction by electrical
stimulation [3], suggesting that these two forms of LTP Electrical stimuli were produced by a Grass S88 share a common substrate. stimulator, coupled to photoelectric stimulus isolation Previously in our hands, cholinergic stimulation has units. Recorded signals were fed into Grass Model 12 EEG been shown to promote a long-term depression effect in amplifiers, high pass filtered at 0.3 Hz, low pass filtered at the neocortex of freely moving animals following single- 3 Hz, and digitized (10 KHz) with a 12-bit A / D converter. session stimulation protocols [37,38]. The following ex- Three sets of field potential input / output (I / O) mea-periments were undertaken to characterize the role of sures, spaced at 48 h, were taken to establish baseline. cholinergic neuromodulation in the induction of neocorti- Pulses of increasing intensity were delivered to white cal LTP induced by the spaced and repeated stimulation matter at a frequency of 0.1 Hz. Six 50 ms responses were protocol. Based on the LTP slice literature and behavioural evoked, amplified, digitized and averaged at each of 10 data, we predicted that the cholinergic agonist pilocarpine intensities (16, 32, 64, 100, 160, 250, 500, 795, 1000, 1260 would enhance LTP induction, while the antagonist mA). The evoked field potentials comprised two main scopolamine would attenuate LTP induction. Portions of components: an early, monosynaptic, surface-negative this research have been presented previously in abstract response, and a larger, polysynaptic late response. The
form [39]. polysynaptic components were often most evident
follow-ing LTP induction. Also evident at latencies coincidfollow-ing with the early component were at least one, and usually several, population spikes that increased in amplitude and
2. Materials and methods number following potentiation. Further characterization of these response components can be found in [8,42]. 2.1. Animals and surgery Beginning 24 h after the third baseline I / O test, 60
2.3. Cholinergic agonism and antagonism
The cholinergic agonist pilocarpine (10–20 mg / kg i.p. in saline, Sigma Chemical Co., USA) and antagonist scopolamine (15 mg / kg i.p. in saline, Sigma Chemical Co., USA) were administered in conjunction with high-frequency trains. These drugs were chosen specifically because they are widely used in behavioural tests of memory (e.g. [10]), and because they have broad-spectrum effects on ACh receptors. Pilocarpine binds to all mus-carinic receptors, but with more affinity to M1. Scopolamine binds to all five muscarinic receptors with equal affinity.
On the first day of experimentation, animals were injected with pilocarpine (low-intensity group, n55; high-intensity group, n57), scopolamine (low-intensity group, n56; high-intensity group, n510) or saline (low-intensity
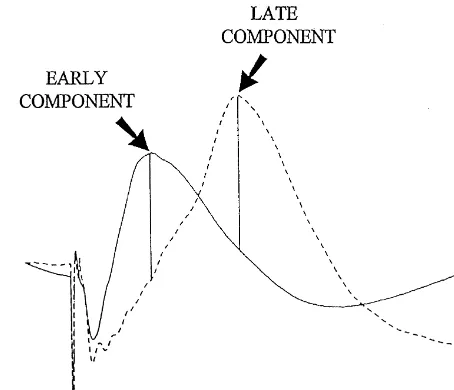
group, n56; high-intensity group, n58), followed 15 min Fig. 1. Representative examples of neocortical field potentials evoked by white matter stimulation. The solid line represents an unpotentiated later by the delivery of 60 high-frequency trains. On
evoked field potential, while the dashed line represents an evoked field subsequent days, the injection was preceded by an I / O test
potential following spaced and repeated tetani. Peaks of early and late and followed 15 min later by 60 high-frequency trains.
components are identified with arrows. As well, examples of the cursor Additionally, a seventh (n57) and eighth (n56) group positions used to set the latencies for the peak amplitude measurements received daily pilocarpine or scopolamine injections, re- are drawn. Vertical calibration 1.0 mV; horizontal calibration 10 ms. spectively, preceded immediately by an I / O test, but did
not receive trains (these groups provided drug-only con-trols). These daily LTP induction regimens were continued
for 10 and 15 days for the high- and low-intensity groups, field potentials recorded during the last baseline I / O test respectively. Following completion of the LTP induction and the I / O test following completion of trains. One phase, 2-weekly I / O measures were collected to monitor animal each from the SAL / High, PILO / High and SCOP/
decay. High groups was discarded from the population spike
measures because of artifactual contamination. Once all
2.4. Analyses recordings were complete, animals were perfused and the
brains were sliced and stained with Cresyl violet to verify Changes in the field potentials over LTP-induction and electrode placements. In all animals, stimulating and decay sessions were measured by subtracting the final recording electrodes were positioned within their target baseline responses from all other baseline and potentiated structures [36].
responses at a single mid-range I / O test intensity that best reflected potentiation. Therefore, all responses, including
the first two baseline responses, were standardized to the 2.5. Behavior third baseline. The dominant early and late components
3. Results are shown in Fig. 2. While the early component showed a significant effect of session overall (F(19,475)55.24, P, 3.1. Response morphology 0.001), there were no significant group differences in the pattern or magnitude of the early-component changes. The The responses were similar to those previously reported groups receiving trains all showed a small surface-positive (Fig. 1) [8,42]. There was a very short-latency surface shift that was indistinguishable from the change displayed positive spike-like response, which previous experiments by animals receiving pilocarpine or scopolamine alone. have shown to be a mix of antidromic and orthodromic This apparent lack of an effect was probably due to a effects [8]. This is followed by an early response com- confounding of early-component EPSP depression in some ponent with a mean latency-to-peak of 7.8 ms (range: groups with population spike enhancement (which can 5.0–12.0 ms) and a mean amplitude of 1.560.09 mV. have a similar effect on field potential morphology) in There is usually one strong late peak in the post-LTP others.
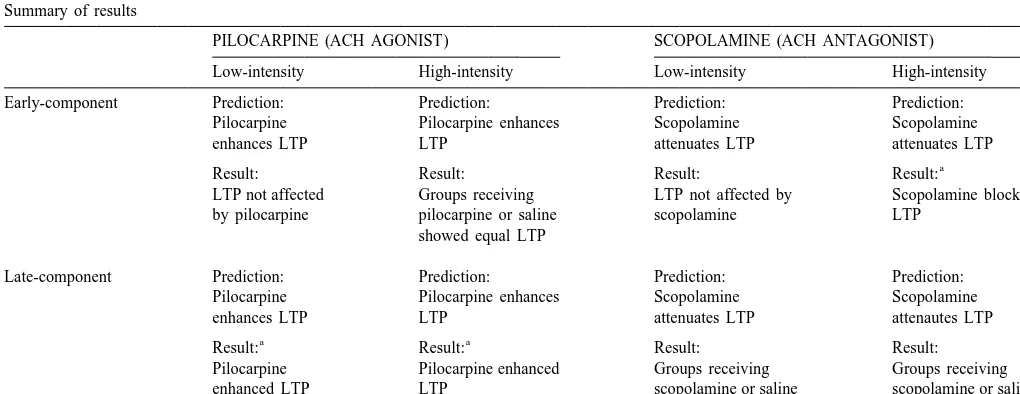
record representing polysynaptic activity. At the latency at Fig. 3 illustrates this measurement problem using repre-which these peaks emerge (mean latency-to-peak: 19.3 sentative examples from the groups treated with pilocar-ms), the baseline amplitude was 0.660.1 mV. pine. There are clear population spike enhancements evidenced by the PILO / Low group and an absence of 3.2. Cholinergic manipulations combined with low- population spike potentiation in the PILO group. In our intensity trains preferentially modulate late-component bipolar recordings, the population spikes have a polarity potentiation opposite to that of the population EPSP. As a result, population spike potentiation often results in an initial Table 1 reflects a summary of the data by component, apparent decrease in amplitude followed by a reversal in drug and intensity. In each case, we predicted that pilocar- the polarity of the early component [8,42]. By contrast, the pine would enhance LTP in each component and changes shown by the PILO group more closely resemble scopolamine would attenuate LTP in each component. Our the depression resulting from the application of the NMDA predictions came to fruition in the late component data antagonist CPP [42], a similarity further supported by the from rats given pilocarpine in conjunction with low- or small amplitude decreases at the late-component latencies. high-intensity LTP-inducing tetani. Additionally, early Therefore, although both the PILO and PILO / Low groups component data from rats receiving scopolamine in con- displayed surface-positive shifts of similar magnitude, the junction with high-intensity tetani, confirmed our predic- mechanisms mediating these shifts are presumably differ-tion that scopolamine would attenuate LTP. Changes in the ent.
response amplitude of the early and late components for The population spike measures confirm the small, and animals receiving pilocarpine (PILO) or scopolamine seemingly contradictory, early-component changes that (SCOP) alone, or in conjunction with low-intensity trains, followed the low-intensity train regimen. There was
con-Table 1
Summary of results
PILOCARPINE (ACH AGONIST) SCOPOLAMINE (ACH ANTAGONIST) Low-intensity High-intensity Low-intensity High-intensity
Early-component Prediction: Prediction: Prediction: Prediction:
Pilocarpine Pilocarpine enhances Scopolamine Scopolamine
enhances LTP LTP attenuates LTP attenuates LTP
a
Result: Result: Result: Result:
LTP not affected Groups receiving LTP not affected by Scopolamine blocked by pilocarpine pilocarpine or saline scopolamine LTP
showed equal LTP
Late-component Prediction: Prediction: Prediction: Prediction:
Pilocarpine Pilocarpine enhances Scopolamine Scopolamine
enhances LTP LTP attenuates LTP attenautes LTP
a a
Result: Result: Result: Result:
Pilocarpine Pilocarpine enhanced Groups receiving Groups receiving enhanced LTP LTP scopolamine or saline scopolamine or saline
showed equal LTP showed equal LTP
a
Fig. 3. Representative sweeps taken pre- and post-LTP induction for groups that received pilocarpine and low-intensity trains (Top) or pilocarpine alone (Bottom). It is clear that while the early component of both groups showed an amplitude shift in the same direction, the mechanisms mediating these effects were different (as indicated by the lack of population spike enhancement displayed by the pilocarpine-alone animal). Moreover, pilocarpine administration alone had no effect on the late component.
Low groups (P,0.001 in each case), which did not differ from each other.
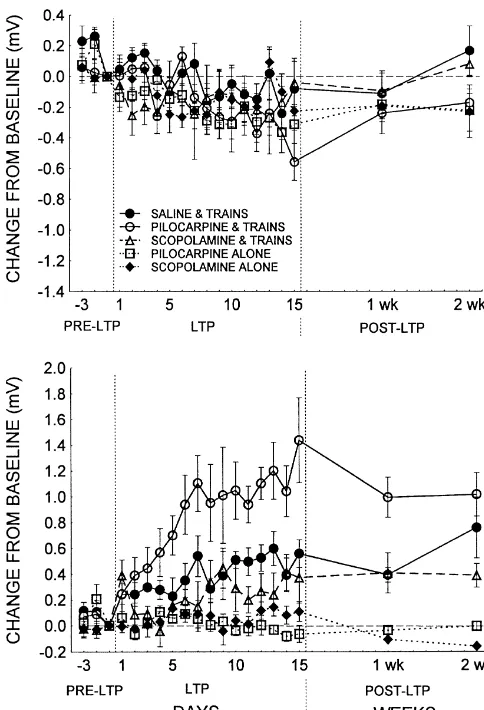
3.3. Cholinergic agents combined with high-intensity Fig. 2. The effects of cholinergic agonism and antagonism on the trains preferentially modulate early-component induction of long-term potentiation by application of low-intensity potentiation
stimulation. The mV differences between the last baseline and all other sweeps are plotted in this figure for the baseline (PRE-LTP), LTP
In contrast to the effects of low-intensity stimulation, the induction (LTP) and 2 week decay (POST-LTP) periods. Top: Changes
application of high-intensity trains in combination with the in the early component for groups receiving either saline, pilocarpine or
scopolamine and trains, or pilocarpine or scopolamine alone. Regardless administration of cholinergic agents produced robust of the treatment conditions, all animals showed a similar small surface- changes in the early component of the evoked responses. positive shift (indicated as a negative mV shift from baseline). Bottom:
Fig. 4 shows changes in the early and late components of Changes in the late component over days. While the groups that received
the neocortical evoked potentials for animals receiving either pilocarpine or scopolamine alone did not show any late-component
high-intensity trains in conjunction with saline (SAL / changes, the groups that received trains all showed statistically significant
late-component potentiation. The PILO / Low group showed the greatest High), pilocarpine (PILO / High) or scopolamine (SCOP/ amount of late-component potentiation, followed by the SAL / Low and High), or pilocarpine (PILO) or scopolamine (SCOP) SCOP/ Low groups, respectively (Tukey HSD test, P,0.001 in all cases).
alone. The early component showed a significant group-by-session interaction (F(56,462)52.52, P,0.001). Post-hoc tests further indicated that the SAL / High and PILO / High groups both showed substantial (and equivalent) siderable variability in the population spike measures from early-component potentiation, while the SCOP/ High group animal to animal within the various groups, and as a result did not appear to undergo early-component potentiation there were no significant differences between any of the (P,0.001 in all cases).
groups (data not shown). Despite this depression of early-component LTP, there
The late-component data showed a significant inter- was some residual enhancement of the population spikes in action between group and session (F(76,475)53.91, P, the SCOP/ High group (Fig. 6). This point is made clearly 0.001). All three groups receiving trains showed late- by a comparison of representative sweeps from a SCOP component potentiation (Fig. 2), though post-hoc analysis and SCOP/ High animal (Fig. 5).
Fig. 5. Representative sweeps taken pre- and post-LTP induction for groups that received scopolamine and high-intensity trains (Top) or scopolamine alone (Bottom). Similar to the effect shown in Fig. 3, both the SCOP/ High and SCOP animals showed similar surface-positive shifts, but the animal that received trains also showed population spike enhancements. This suggests that different processes are responsible for the changes shown by these groups. In fact, the SCOP group change is more indicative of a depression effect than potentiation.
Fig. 4. The effects of cholinergic agonism and antagonism on the induction of long-term potentiation by application of high-intensity stimulation. The mV differences between the last baseline and all other sweeps are plotted in this figure for the baseline (PRE-LTP), LTP induction (LTP) and 2 week decay (POST-LTP) periods. Top: Changes in the early component for groups receiving either saline, pilocarpine or scopolamine and trains, or pilocarpine or scopolamine alone. Animals that received pilocarpine or saline in conjunction with high-intensity stimula-tion showed a similar and large deep negative shift indicative of population spike potentiation, while animals that received trains in the presence of scopolamine did not differ from control animals. Bottom: Changes in the late component over days. The effects of cholinergic agonism and antagonism on LTP induced by high-intensity stimulation are very similar to that following low-intensity trains. All groups that received trains showed substantial late-component potentiation relative to controls, with the PILO / High group showing the greatest amount of LTP, followed by the SAL / High and SCOP/ High groups, which showed
similar levels of potentiation (Tukey HSD test, P,0.001 in all cases). Fig. 6. Total population spike height measures are shown following the high-intensity LTP induction regimens for time-points prior to LTP induction (PRE-LTP), 24 h following the induction protocols (POST-LTP) and after a 2 week decay period (DECAY). All groups that received high-intensity trains showed strong population spike enhancements, with 2.30, P,0.03). All groups that received trains in conjunc- the SAL / High group achieving the largest amplitude response by the end tion with a drug or saline injection showed population of the LTP induction protocol. Interestingly, while the PILO / High group average is larger than that of the SCOP/ High group, both groups spike enhancements, while the animals that received
ultimately achieve equivalent population spike amplitudes despite the fact pilocarpine or scopolamine alone showed no significant
0.001). As Fig. 4 reveals, all three groups that received late-component potentiation effects than either their saline high-intensity stimulation showed robust late-component or scopolamine counterparts in both the low- and high-enhancements, with the PILO / High group showing the intensity groups. Moreover, following the low-intensity greatest amount of late-component potentiation, and the induction regimen, the SAL / Low group showed a stronger SAL / High and SCOP/ High groups showing equivalent effect than the SCOP/ Low group. These data indicate that potentiation. Neither the PILO nor SCOP groups showed while cholinergic agonism enhances the induction of late-any late-component potentiation effects. component LTP, antagonism slows the development of potentiation. These data are consistent with demonstrations
3.4. Decay that scopolamine blocks LTP in the hippocampus [20,28]
and that cholinergic agonists facilitate neocortical LTP All groups that showed potentiation effects remained at induction in vitro [6,27].
least partially potentiated by the end of the 2-week decay
period. The groups that showed the most potentiation 4.3. Contrasting the high- and low-intensity induction generally showed the greatest decay (e.g.: PILO / High regimens
early component, SAL / High and PILO / High late
com-ponents). We did not find a reliable LTP effect in the early
component of the low-intensity groups, precluding an assessment of cholinergic manipulation. The late
com-4. Discussion ponent, however, showed a strong potentiation effect at the low intensity. As discussed in [42], such early- and late-Previous attempts to induce neocortical long-term poten- component dissociations may be due to a non-optimal tiation by pairing tetanic stimulation with cholinergic placement of the recording electrode. The polysynaptic activation in freely moving rats have produced depression field of activation might be expected to cover more area effects that lasted several weeks [37,38]. These studies, than the monosynaptic field of activation. Despite this, however, did not use multiple, spaced stimulation sessions both and early and late components were well represented to induce LTP. The present data are the first to describe the in our sweeps, so the dissociation may be due to an modulatory influence of cholinergic agonists and antago- independent LTP induction within horizontal pathways nists on the induction parameters of neocortical LTP using (which presumably carry much of the polysynaptic input) our multiple-session paradigm in the awake, freely moving [8].
rat. Except for scopolamine administration, which depressed
In contrast to the depressed field potentials we obtained early-component LTP, the effects of cholinergic manipula-previously, these experiments yielded clear, but compo- tions were shown most clearly in the late-component nent-specific, LTP effects. measures. By day 7 of the induction regimen the average late-component response amplitudes of the stimulated 4.1. Early-component changes experimental groups are arranged as follows: SCOP/ Low,SAL / Low,PILO / Low,SCOP/ High,SAL / High-Our prediction that cholinergic antagonist administration ,PILO / High. This is exactly what one would predict, would attenuate LTP was realized in the early-component assuming that (1) low-intensity trains result in less activa-response changes occurring after repeated application of tion than high-intensity trains [24] and; (2) cholinergic high-intensity tetani. The application of high-intensity neurotransmission can act to increase post-synaptic depo-trains resulted in strong potentiation effects in both the larization [7,22,28,29,33]. It must be kept in mind, how-SAL / High and PILO / High groups. Scopolamine, on the ever, that our results are based upon systemic drug other hand, suppressed LTP in this condition. These data administration. Further work is required to confirm that are consistent with reports that cholinergic antagonists these results are due to the modulation of cholinergic block hippocampal LTP [28] and behavioral tasks requir- function at the cortical site itself
ing memory. No significant differences were found in our
early component or population spike measures following 4.4. Monosynaptic vs. polysynaptic effects the application of low-intensity trains.
potential in hippocampal pyramidal cells, Science 221 (1983) 1299– LTP of the late component, but had no effect on the early
1301. component.
[10] M.W. Decker, J.L. McGaugh, The role of interactions between The fact that the monosynaptic and polysynaptic LTP cholinergic systems and other modulatory systems in learning and effects are showing somewhat independent responses to memory, Synapse 7 (1991) 151–168.
these cholinergic agents indicates that the LTP effects may [11] D.A. Drachman, J. Leavitt, Human memory and the cholinergic system, Arch. Neurol. 30 (1974) 113–121.
be induced within separate targets (as opposed to the
[12] H.C. Fibiger, Cholinergic mechanisms in learning, memory and polysynaptic targets simply responding passively to an
dementia: A review of recent evidence, Trends Neurosci. 14 (1991) increased volley from the monosynaptic source). Likely 220–223.
potentiation sites for the polysynaptic responses are the [13] C. Flicker, M. Serby, S.H. Ferris, Scopolamine effects on memory, synaptic connections of horizontal fibers [2,19,26,30,40], a language, visuospatial praxis and psychomotor speed,
Psycho-pharmacology 100 (1990) 243–250. possibility that could be tested by stimulating these fibers
[14] J.F. Flood, D.W. Landry, M.E. Jarvik, Cholinergic receptor interac-directly. In addition, likely targets could be determined by
tions and their effects on long-term memory processing, Brain Res. the local application of drugs specific to the Ml
acetyl-215 (1981) 177–185.
choline muscarinic receptor (most dense in layers II / III) or [15] E. Giacobini, Cholinergic receptors in human brain: Effects of aging the M2 receptor (most dense in layer V) (see [44] for and Alzheimer disease, J. Neurosci. Res. 27 (1990) 548–560.
[16] J.T. Greenamyre, W.F. Maragos, Neurotransmitter receptors in review).
Alzheimer disease, Cerebrovasc Brain Metabol. Rev. 5 (1993) 61–94.
[17] J.J. Hagan, R.G.M. Morris, The cholinergic hypothesis of memory: a review of animal experiments, in: L.L. Iversen, S.D. Iversen, S.H.
Acknowledgements
Snyder (Eds.), Psychopharmacology of the Aging Nervous System, Plenum Press, New York, NY, 1989.
We thank Mathew W. Loveless for assistance with data [18] V. Haroutunian, E. Barnes, K.L. Davis, Cholinergic modulation of memory in rats, Psychopharmacology 87 (1985) 266–271. collection. This work was supported by a grant from the
[19] G. Hess, C.D. Aizenman, J.P. Donoghue, Conditions for the Natural Sciences and Engineering Research Council of
induction of long-term potentiation in layer II / III horizontal con-Canada (NSERC) to RJR, an NSERC Postgraduate
Scho-nections in the rat motor cortex, J. Neurophysiol. 75 (1996) 1765– larship to CT and a Clifton W. Sherman Graduate Scholar- 1778.
ship for Doctoral Study in Science and Engineering to [20] I. Hirotsu, N. Hon, N. Katsuda, T. Ishihara, Effect of anticholinergic drug on long-term potentiation in rat hippocampal slices, Brain Res. TEB.
482 (1989) 194–197.
[21] T.J. Hudzik, G.R. Wenger, Effects of drugs of abuse and cholinergic agents on delayed matching-to-sample responding in the squirrel monkey, J. Pharmacol. Exp. Ther. 265 (1993) 120–127.
References
[22] A. Keller, E. Miyashita, H. Asanuma, Minimal stimulus parameters and the effects of hyperpolarization on the induction of long-term [1] T.G. Aigner, D.L. Walker, M. Mishkin, Comparison of the effects of potentiation in cat motor cortex, Exp. Brain Res. 87 (1991) 295–
scopolamine administered before and after acquisition in a test of 302.
visual recognition memory in monkeys, Behav. Neural. Biol. 55 [23] A. Kirkwood, M.F. Bear, Hebbian synapses in visual cortex, J.
(1991) 61–67. Neurosci. 14 (1994) 1634–1645.
[2] V.A. Aroniadou, A. Keller, Mechanisms of LTP induction in rat [24] M. Kobayashi, M. Ohno, S. Shibata, T. Yamamato, S. Watanabe, motor cortex in vitro, Cereb. Cortex 5 (1995) 353–362. Concurrent blockade of beta-adrenergic and muscarinic receptors [3] J.M. Auerbach, M. Segal, A novel cholinergic induction of long- suppresses synergistically long-term potentiation of population term potentiation in rat hippocampus, J. Neurophysiol. 72 (1994) spikes in the rat hippocampal CAl region, Brain Res. 777 (1997)
2034–2040. 242–246.
[4] T.V.P. Bliss, G.L. Collingridge, A synaptic model of memory: [25] Y. Lamberty, A.J. Gower, Cholinergic modulation of spatial learning Long-term potentiation in the hippocampus, Science 361 (1993) in mice in a Morris-type water maze, Arch. Int. de Pharmacol. Ther.
31–39. 309 (1991) 5–19.
[5] R.D. Blitzer, O. Gil, E.M. Landau, Cholinergic stimulation enhances [26] S.M. Lee, M.G. Weisskopf, F.F. Ebner, Horizontal long-term long-term potentiation in the CA1 region of rat hippocampus, potentiation of responses in rat somatosensory cortex, Brain Res. Neurosci. Lett. 119 (1990) 207–210. 544 (1991) 303–310.
[6] S. Brocher, A. Artola, W. Singer, Agonists of cholinergic and [27] Y. Lin, J.W. Phillis, Muscarinic agonist-mediated induction of long-noradrenergic receptors facilitate synergistically the induction of term potentiation in rat cerebral cortex, Brain Res. 551 (1991) long-term potentiation in slices of rat visual cortex, Brain Res. 573 342–345.
(1992) 27–36. [28] V. Markevich, A.M. Scorsa, G.S. Dawe, J.D. Stephenson, Choliner-[7] E.C. Burgard, J.M. Sarvey, Muscarinic receptor activation facilitates gic facilitation and inhibition of long-term potentiation of CAl in the
the induction of long-term potentiation (LTP) in the rat dentate urethane-anaesthetized rats, Brain Res. 754 (1997) 95–102. gyrus, Neurosci. Lett. 116 (1990) 34–39. [29] H. Markram, M. Segal, Acetylcholine potentiates responses to N-[8] C.A. Chapman, C. Trepel, T.L. Ivanco, D.J. Froc, K. Wilson, R.J. methyl-D-aspartate in the rat hippocampus, Neurosci. Lett. 113
Racine, Characterization of field potentials and membrane currents (1990) 62–65.
in adult rat sensorimotor cortex, in vivo, before and after repeated [30] H. Markram, M. Segal, Long-lasting facilitation of excitatory tetanization of the corpus callosum, Cereb. Cortex 8 (1998) 730– postsynaptic potentials in the rat hippocampus by acetylcholine, J.
742. Physiol. 427 (1990) 381–393.
complimentary learning systems in the hippocampus and neocortex: [38] R.J. Racine, G.C. Teskey, D. Wilson, E. Seidlitz, N.W. Milgram, insights from the successes and failures of connectionist models of Post-activation potentiation and depression in the neocortex of the learning and memory, Psychol. Rev. 102 (1995) 419–457. rat: II. Chronic preparations, Brain Res. 637 (1994) 83–96. [32] D.A. McCormick, D.A. Prince, Mechanisms of action of acetyl- [39] R.J. Racine, C. Trepel, Cholinergic modulation of neocortical
long-choline in the guinea-pig cerebral cortex in vitro, J. Physiol. (Lond). term potentiation in the freely moving rat, Soc. Neurosci. Abstr. 23
375 (1986) 169–194. (1997) 1734.
[33] R. Metherate, N. Tremblay, R.W. Dykes, Acetylcholine permits [40] M.S. Rioult-Pedotti, D. Friedman, G. Hess, J.P. Donoghue, long-term enhancement of neuronal responsiveness in cat primary Strengthening of horizontal cortical connections following skill somatosensory cortex, Neuroscience 22 (1987) 75–81. learning, Nat. Neurosci. 1 (1998) 230–234.
[34] J.L. Muir, Acetylcholine, aging, and Alzheimer’s disease, Phar- [41] R.D. Smith, M.K. Kistler, M. Cohen-Williams, V.L. Coffin, macol. Biochem. Behav. 56 (1997) 687–696. Cholinergic improvement of a naturally occurring memory deficit in [35] A.H. Nagahara, R.J. Handa, Fetal alcohol-exposed rats exhibit the young rat, Brain Res. 707 (1996) 13–21.
differential response to cholinergic drugs in a delay-dependent [42] C. Trepel, R.J. Racine, Long-term potentiation in the neocortex of memory task, Neurobiol. Learn Mem. 72 (1999) 230–243. the adult, freely moving rat, Cereb. Cortex 8 (1998) 719–729. [36] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates,, [43] J.M. Whitehouse, The effects of physostigmine on discrimination
3rd Edition, Academic Press, San Diego, CA, 1997. learning, Psychopharmacologia 9 (1966) 183–188.
[37] R.J. Racine, C.A. Chapman, C. Trepel, G.C. Teskey, N.W. Milgram, [44] K. Zilles, A. Wree, Cortex: areal and laminar structure, in: G. Post-activation potentiation in the neocortex: IV. Multiple sessions Paxinos (Ed.), The Rat Nervous System, 2nd Edition, Academic required for induction of longterm potentiation in the chronic Press, San Diego, (1995).