ORIGINAL ARTICLE
Responses of soil nitrogen-fixing, ammonia-oxidizing,
and denitrifying bacterial communities to long-term
chlorimuron-ethyl stress in a continuously cropped soybean
field in Northeast China
Xiaoli Zhang&Xu Li&Chenggang Zhang&Xinyu Li&
Huiwen Zhang
Received: 23 August 2012 / Accepted: 1 March 2013 / Published online: 21 April 2013 #Springer-Verlag Berlin Heidelberg and the University of Milan 2013
Abstract Chlorimuron-ethyl is a type of long-residual
her-bicide applied widely to soybean fields in China, but little information is available about the long-term impact of this herbicide on soil nitrogen-transforming microbial commu-nities. Soil samples (0–20 cm) were collected from three treatments (no, 5-year and 10-year application of chlorimuron-ethyl) in a continuously cropped soybean field. Plate count (CFU), most probable number (MPN) count, and clone library analyses were conducted to investigate the abundance and composition of nitrogen-fixing, ammonia-oxidizing, and denitrifying bacterial communities, and a chlorate inhibition method was adopted to measure the soil nitrification potential. Long-term chlorimuron-ethyl ap-plication reduced the abundance of soil culturable nitrogen-fixing, ammonia-oxidizing, and denitrifying bacteria. More-over, chlorimuron-ethyl decreased the diversity of nitrogen-fixing and ammonia-oxidizing bacteria but promoted that of denitrifying bacteria. Chlorimuron-ethyl restrained some uncultured nitrogen-fixing bacteria, ammonia-oxidizing bacteriaNitrosospirasp. cluster 3a and 3d, and some novel
or putative denitrifying bacteria. The nitrogen-fixing bacte-ria were closely related to Bradyrhizobium sp., ammonia-oxidizing bacteria Nitrosospira sp. cluster 3b and 3c, and most denitrifying bacteria were resistant to chlorimuron-ethyl. There was a negative correlation between the nitrifi-cation potential and the residual amount of soil chlorimuron-ethyl (R2=0.88,n=3,P<0.05). Therefore, long-term appli-cation of chlorimuron-ethyl in the continuously cropped soybean field could seriously disturb soil N-transforming communities, and might impact soybean soil biological quality and soybean growth. Further studies should address rational amendment models of this herbicide to reduce the possible ecological risks of long-term application of this herbicide to soybean fields.
Keywords Nitrogen-fixing bacteria . Ammonia-oxidizing
bacteria . Denitrifying bacteria . Nitrification potential . Chlorimuron-ethyl . Continuously cropped soybean field
Introduction
Chlorimuron-ethyl {ethyl 2-[[[[(4-chloro-6-methoxypyrimidin-2-yl) amino] carbonyl] amino] sulfonyl] benzoate} is a member of the sulfonylurea herbicides applied particularly in soybean fields to control weeds. It is characterized by good crop selec-tivity, high herbicidal acselec-tivity, low mammalian toxicity, and low application rate (Reddy et al.1995; Boldt and Jacobsen1998). The herbicide exerts phytotoxic effects on sensitive weeds by X. Zhang
:
X. Li:
C. Zhang:
X. Li:
H. Zhang (*)State Key Laboratory of Forest and Soil Ecology, Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 110016, China
e-mail: hwzhang@iae.ac.cn X. Zhang
Yantai Institute of Costal Zone Research, Chinese Academy of Sciences, Yantai 264003, China
inhibiting acetolactate synthase (ALS), which is also found in microorganisms (Kaur et al. 2009; Jin et al. 2010). Chlorimuron-ethyl has longer persistence in soil (over 3 months); therefore, the likely risk of its toxicity to soil microbial inhabitants has to be considered when using this herbicide long-term.
It has been shown that sulfonylurea herbicides have toxic effects on microorganisms. A major proportion of fluores-cent Pseudomonas strains isolated from agricultural soils were sensitive to metsulfuron-methyl and thifensulfuron-methyl (Boldt and Jacobsen 1998). The sulfonylureas chlorsulfuron and rimsulfuron were also toxic to rhizobacterial Pseudomonas strains (Forlani et al. 1995). Recent studies found that long-term application of chlorimuron-ethyl in soybean fields reduced the antifungal activities of Pseudomonas strains (Wang et al. 2013). In addition, it decreased soil bacterial community diversity and evenness, while stimulating some soybean root rot pathogenic fungi such asFusarium oxysporum,Rhizoctonia solaniandPhytophthora sojae(Zhang et al.2011).
Soil nitrogen transformation and utilization have definite effects on the productivity and sustainable development of agro-ecosystems. Nitrogen-fixing bacteria (NFB), ammonia-oxidizing bacteria (AOB), and denitrifying bacteria (DNB) participate directly in the transfer of the organic nitrogen and inorganic nitrogen in soil, and are related closely to nitrogen utilization. Previous studies have employed cultivation-independent molecular techniques targeting gene markers of these functional groups:nifHgene, 16S rRNA gene, andnosZ gene (Moseman et al.2009; Innerebner et al.2006; Chen et al. 2011). By using these gene markers, many environmental disturbances affecting on N-availability microbial communities in soils, such as fertilization, irrigation, and pesticides, have been studied (Innerebner et al.2006; Chen et al.2011). There is strong evidence that sulfonylurea herbicides can distinctly dis-turb N-transforming microbes in short-term laboratory micro-cosms. Forlani et al. (1995) found that sulfonylurea restrained the growth of the nitrogen-fixing bacteriaAzospirillum sp. under pure culture conditions. He et al. (2006) reported that metsulfuron-methyl inhibited soil DNB under laboratory con-dition (80 days). Bensulfuron decreased soil nitrification activ-ity in 4 weeks, and autotrophic nitrifiers were sensitive to this herbicide in in vitro toxicity tests (Gigliotti and Allievi2001). In order to determine the long-term effects of chlorimuron-ethyl on soil N-transforming microbial communities, in situ investi-gations are essential but have been studied only rarely, espe-cially using molecular methods.
In some state farms of Northeast China (important soy-bean production bases of this country), the increasing use of chlorimuron-ethyl in the continuously cropped soybean field has contributed significantly to weed control over the past decade. In this study, plate count (CFU), most probable number (MPN) count, clone library and chlorate inhibition
methods were using to monitor the responses of NFB, AOB, and DNB communities to long-term chlorimuron-ethyl stress. The objective was to assess the potential ecological risks of long-term (5-year and 10-year) application of chlorimuron-ethyl in the soybean field.
Materials and methods
Site description and sample collection
Surface (0–20 cm) soybean field soil was collected from a state farm (48°12′28″ N, 134°15′56″ E) in Heilongjiang Province, Northeast China in October 2009. Three treat-ments, each containing three replicate plots, were NT (man-ual weeding, no chlorimuron-ethyl), T5 (5-year application of 30 g active component of chlorimuron-ethyl per hectare), and T10 (10-year application of 30 g active component of chlorimuron-ethyl per hectare). All treatment plots have uniform geomorphology, close geographical position, and the same field management measures. The soil was taken randomly from 5 points within each plot after the soybean harvest, combined to form one composite sample, and sieved (2 mm) to remove rocks, roots and coarse fragments. A portion of fresh soil was designated for culture experi-ments and the remaining soil was stored at −80 °C until molecular analysis was performed.
Soil characteristics
Soil pH was measured using a 1:1 soil:water extract. The organic matter content was determined using the potassium dichromate volumetric method. Total nitrogen was estimat-ed using micro-kjeldahl method. A granulometer (Malvern Instruments, Malvern, England) was used for soil texture analysis. The chlorimuron-ethyl residual was determined by HPLC equipped with a Zorbax SB-18 ODS Spherex column and DAD array detector.
Abundance of soil culturable NFB, AOB and DNB
Soil suspensions were prepared by adding 10.0 g fresh soil to 90 mL distilled water, and shaking for 30 min at 30 °C. The plate count method was used to estimate CFU of NFB grown on Ashby’s nitrogen-free culture medium (Bakulin et al.2007). Dilutions (10−3–10−5) of soil sample were used as
inoculums. The CFU were counted after 3 days of incuba-tion at 30 °C. The MPN count method was used to deter-mined the approximate viable counts of AOB and DNB. AOB were determined by using dilutions from 10−2to 10−7.
in the dark at 28 °C for 4 weeks. As a negative control, 1 mL sterile water was used instead of soil suspension. The test tubes were scored as positive or negative based on the pres-ence or abspres-ence of nitrite and/or nitrate, and the MPN values were calculated using the table prepared by Xu and Zheng (1986). DNB were determined using dilutions from 10−3to
10−7. Soil suspension (1 mL) and 10 mL medium composed
of 20.0 g peptone, 1.0 g glucose, 2.0 g Na2HPO4, 1.0 g KNO3, 1.0 g agar, and 1,000 mL distilled water were added to a Durham test tube and incubated statically in dark at 28 °C for 2 weeks. The counting of DNB was based on the produc-tion of gas or ammonia and the consumpproduc-tion of nitrite and nitrate in Durham tubes as described by Xu and Zheng (1986).
DNA extraction and PCR amplification
For each treatment, triplicate soil samples were mixed to-gether for homogenization, and then 1.0 g soil was used for extracting DNA (Zhang et al.2011). Crude extracts were purified using WizardRDNA CleanUp kit (Promega, Mad-ison, WI), according to the manufacturer’s instructions. The DNA concentration and purity were determined by a Nanodrop® 2000c spectrophotometer (Thermo, Madison, WI), and the PCR was performed on a PCR express thermal cycler (HBPX220 ThermoHybaid, Ashford, UK). For the three functional groups, nested PCR amplifications were carried out as previously described (Table1).
Clone library, sequencing, and phylogenetic analysis
PCR products were purified using the TIANgel Midi Puri-fication Kit (Tiangen Biotech, Beijing, China), and cloned into the pTZ57R/T vector (Fermentas, Amherst, NY). Pos-itive clones were screened by RFLP (digested using restric-tive endonucleaseMspI for NFB-nifHgene,MspI andRsaI for AOB-16S rRNA gene, and MspI and RsaI for nosZ gene). Clones with similar banding patterns were grouped together as one OTU (operation taxonomic unit), and then representative clones were selected randomly for sequenc-ing. The nucleotide sequences obtained in this study were deposited with the GenBank database under accession num-bers KC515095-KC515096 and JX514915-JX514941. Phy-logenetic trees were constructed by MEGA 5.0 (Tamura et al.2007) with the neighbor-joining method, and a bootstrap resampling with 1,000 replicates was performed to estimate the confidence values of the tree nodes.
Nitrification potential
nitrite oxidation, sodium chlorate was added to each flask at a final concentration of 7.5 mmol/L. The suspension was incu-bated in the dark at 28 °C for 5 h, and the nitrite was extracted with 5 mL (2 mol/L) KCl and determined by a spectropho-tometer at wavelength 520 nm with N-(1-napthyl) ethylene diamine dihydrochloride. Finally, the NP was calculated by the amount of NO2-N per gram soil per 5 h.
Data analysis
Data of soil parameters and culture experiments were subjected to one-way analysis of variance (ANOVA) and mean comparisons were made using the least sig-nificant difference (LSD) method at P<0.05 level. All statistical analyses were conducted through SPSS 13. 0 program 2009 (SPSS, Chicago IL). The estimated coverage of all constructed clone libraries was calculat-ed as C=[1 − (n1/N)]×100, where n1 is the number of unique (frequency =1) RFLP pattern detected in a library and N is the total number of RFLP patterns in the same library. The useful online diversity calculator at http:// www.changbioscience.com/ was used to generate a di-versity index (Shannon index H) for each clone library.
Results and discussion
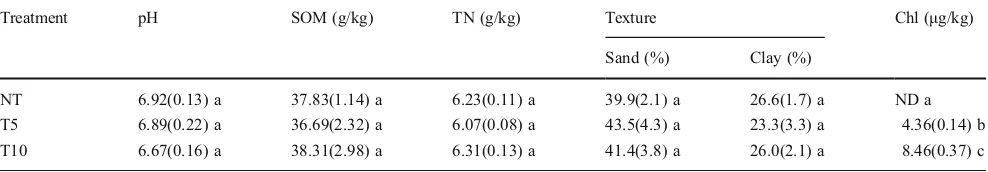
Effects of soil properties
From the results shown in Table2, we can see that soil pH and sand/clay ratio decreased while organic matter and total N content increased from NT to the T10 treatment. However, there was no statistically significant difference between treat-ments in relation to these parameters. In contrast, chlorimuron-ethyl accumulated significantly in soil with increasing years of herbicide application. In this re-search, all treatment plots were from a big field, and had same field management measures. Therefore, any changes in N-transforming bacterial communities should be caused by different chlorimuron-ethyl residuals in the soybean field soil.
Abundance of nitrogen-fixing, ammonia-oxidizing, and denitrifying bacteria
Compared with NT, treatments T5 and T10 had significantly lower abundance of soil culturable NFB, AOB, and DNB (Fig.1). In T10, the CFU of NFB was only 2.0×104, less than one-fifth of that in T5 and one-tenth of that in NT. The viable counts of AOB and DNB in T5 and T10 were roughly just one-half of those in NT. This result indicated that long-term application of chlorimuron-ethyl led to persistent toxicity to N-functional microbes. Certainly, distinct groups responded differently to the herbicide. NFB seemed to be more sensitive to chlorimuron-ethyl; the CFU decreased with extended ap-plication time. Some AOB and DNB might become gradually accustomed to the herbicide and their viable counts seemed to be stable after 5-year application (no significant difference between T5 and T10). This result suggested microbial flexi-bility to environmental changes (Richardson2000).
Nitrification potential
NP decreased significantly when encountering chlorimuron-ethyl, and in NT was about 2–3 times that in T5 and T10 (Fig. 2). Inconsistent with the viable count of culturable AOB (no significant difference between T5 and T10), NP was restrained significantly with the accumulation of ap-plied chlorimuron-ethyl, and had a negative correlation with the residual amount of soil chlorimuron-ethyl (R2=0.88,n= 3, P< 0.05). NP was sharply and lastingly inhibited by chlorimuron-ethyl, suggesting that the herbicide could sup-press the soil active AOB community and bring soil nitrifi-cation activity into decline (Diosma et al. 2003; He et al. 2007). This result was consistent with a previous study showing that sulfonylureas decreased soil nitrification ac-tivity (Gigliotti and Allievi2001).
Community structure of NFB
The coverage values (C) indicated that more than 87 % of NFB-nifH, AOB-16S rRNA and DNB-nosZsequence types were captured in three libraries (Table 3). Therefore, the
Table 2 Soil characteristics of three chlorimuron-ethyl treatments.SOMSoil organic matter,TNtotal nitrogen,Chlchlorimuron-ethyl residue;
Values followed by different letters are significantly different (P<0.05) based on one-way ANOVA
Treatment pH SOM (g/kg) TN (g/kg) Texture Chl (μg/kg)
Sand (%) Clay (%)
dominant and common NFB, AOB and DNB in the soybean field soil were probably detected.
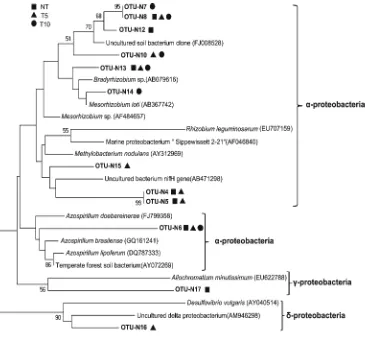
A total of 12 nifH OTUs were obtained from 167 se-quences, with the Shannon index (H) ranging from 2.37 to 2.45 (Table 3). It is worth noting that T10 had a higher diversity value than T5. A possible explanation could be that some NFB groups adapted gradually to chlorimuron-ethyl stress due to their resilience during a longer time, and formed a relatively stable structure. The phylogeny (Fig.3) indicated that all OTUs pertained to proteobacteria, with dominance of α-proteobacteria. The OTUs N6, N8 and N13 were found in all samples, indicating that these clones were tolerant or resistant to chlorimuron-ethyl. The OTU N12 (97 % sequence identity to unculturedα-proteobacteria nifHgene) and N17 (97 % sequence identity to uncultured γ-proteobacterianifHgene) appeared only in the NT treat-ment, suggesting that chlorimuron-ethyl could inhibit these uncultured NFB species. The OTU N7, N10, N14, N15, and N16 were derived only from chlorimuron-ethyl treated soil (T5 and T10), showing 95–97 % sequence identity to Bradyrhizobium, Azospirillum, Methylobacterium and Mesorhizobium, respectively. These NFB genera thrived in treated soil due to domestication of the herbicide.
Some NFB OTUs closely related toBradyrhizobiumand Azospirillum in our research were resistant to chlorimuron-ethyl, which is of significance for the continuous cropping of soybean.Bradyrhizobiumcan infect the soybean root to form root nodules that supply soybean with more than 50 % of its required nitrogen (Franche et al.2009).Azospirillum—known as important plant growth promoting rhizobacteria (PGPR)—
can enhance root growth and nodulation of soybean (Molla et al.2001). AlthoughBradyrhizobiumsp. was not affected by chlorimuron-ethyl even when its application rate was 150 times higher than the recommended field doses in pure culture, the nodulation of soybean treated with chlorimuron-ethyl at standard application rates for 5 days was impaired under laboratory controlled conditions (Zawoznik and Tomaro 2005). This indicated that irrational application of chlorimuron-ethyl could influence biological nitrogen fixation in soybean. Moreover, some uncultured nitrogen-fixing bacte-ria were inhibited by chlorimuron-ethyl, which is likely to influence the soil N cycle.
Community structure of ammonia-oxidizing bacteria
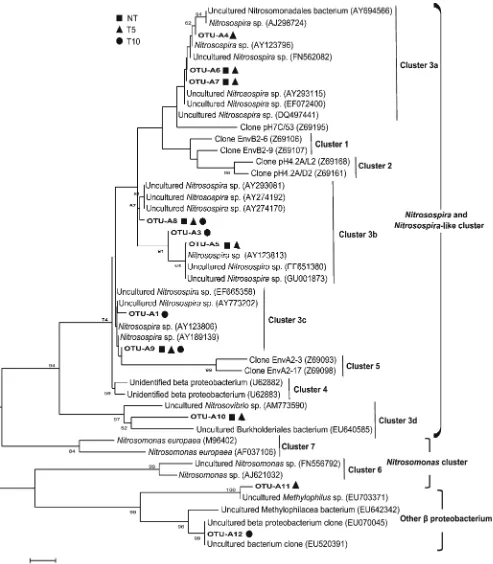
Long-term application of chlorimuron-ethyl greatly reduced AOB diversity, with the Shannon index (H) in NT, T5, and T10 being 2.07, 1.94, and 1.94 respectively (Table3). Sum-marized the abundance and diversity data, we concluded that the toxic effects of chlorimuron-ethyl on AOB was lasting. In spite of the same diversity value, T5 and T10 had distinct AOB bacterial structure according to the phylogeny (Fig.4). Only OTU A8 was found in all treatments. OTUs A4 and A11 were unique to T5, suggesting these two DNB groups might be transitional groups under long-term chlorimuron-ethyl stress. OTUs A5, A6, A7 and A10 disappeared in T10, indicating the herbicide had chronic toxicity to these groups. Nitrosospira cluster 3 (Stephen et al.1996) predominated in our soybean field, being subclustered into a, b, c, and d.Nitrosospiracluster 3a (OTU A5, A6 and A7) and 3d (OTU A10) appeared to be sensitive to the toxicity of 10-year chlorimuron-ethyl applica-tion, but Nitrosospira Cluster 3b (OTU A3 and A8) and
Fig. 1 Abundance of soil
culturableanitrogen-fixing bacteria (NFB), andb ammonia-oxidizing bacteria (AOB) and denitrifying bacteria (DNB).Error barsStandard deviation (n=3). Different
lettersfor the same columns
represent significant differences atP<0.05
Fig. 2 Nitrification potential (NP).Error barsStandard deviation (n=3).
Nitrosospira Cluster 3c (OTU A1 and A9) seemed to be resistant to the herbicide. The OTU A11 and A12 (98 % similarity toMethylophilaceae bacterium clone (EU703371) and (EU642342)) belonging to other β-proteobacteria, just appeared in the chlorimuron-ethyl applied soils.
NitrosospiraorNitrosospira-like AOB dominated in our soybean field, which is in agreement with previous obser-vations (He et al.2007; Mendum et al.1999; Phillips et al. 2000; Olsson et al.2006). However, an interesting observa-tion was that chlorimuron-ethyl had chronic toxic effects on Nitrosospira cluster 3a and 3d groups but had few
side-effects onNitrosospira cluster 3b and 3c. A similar result was reported by a previous study (Li et al.2008) showing that the herbicide acetochlor changed the AOB community structure from Nitrosospira subcluster 2 to subcluster 1. These results suggested that these herbicides might possess selective toxicity to AOBNitrosospira.
Community structure of DNB
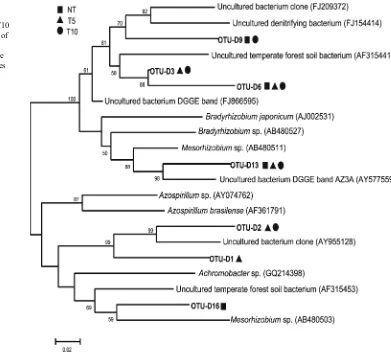
Unlike NFB and AOB, the DNB in T5 and T10 had a much higher Shannon index value (2.27 and 2.05, respectively) than
Table 3 Analyses of NFB-nifH, AOB-16S rRNA and DNB-nosZgene clone libraries in NT, T5 and T10 treatments.CCoverage index,Hdiversity
index,OTUoperation taxonomic unit
Treatment NFB-nifH AOB-16S rRNA DNB-nosZ
No. of clones
No. of OTUs
C H No. of clones
No. of OTUs
C H No. of clones
No. of OTUs
C H
NT 57 7 87.0 2.45 53 6 88.2 2.07 59 4 90.4 1.77 T5 52 8 89.9 2.37 55 8 92.3 1.94 55 5 88.6 2.27 T10 58 6 92.4 2.43 52 5 90.2 1.94 57 5 93.0 2.05
Fig. 3 Phylogenetic
NT (1.77) (Table 3). All OTUs were grouped into α -proteobacteria, closely related to Bradyrhizobium sp., Mesorhizobium sp., and Azospirillum sp. (Fig. 5). Most OTUs were insensitive or accustomed to chlorimuron-ethyl.
Only OTU D16 disappeared in chlorimuron-ethyl treated soils, which might represent novel or putative DNB, because of low similarity (<86 %) to the DNB se-quences from BLAST.
Fig. 4 Phylogenetic relationships of AOB-16S rRNA gene sequences. Bootstrap values of >50 % (obtained with 1,000 resamplings) are shown at
He et al. (2006) found that metsulfuron-methyl inhibited the growth of DNB. In the present study, chlorimuron-ethyl also reduced the viable count of DNB. However, most DNB OTUs were resistant to the herbicide and the DNB diversity increased in chlorimuron-ethyl treatments. One could spec-ulate that the viable count involved only the culturable DNB, but that the OTU and diversity analyses related to both culturable and uncultured DNB. Alternatively, some resistant bacteria could have obtainednosZ genes by hori-zontal gene transfer.
In the same plots, we had previously evaluated the effects on bacterial communities of long-term chlorimuron-ethyl application (Zhang et al. 2011). The results revealed no information about N-transforming bacteria. This might be because N-transforming bacteria are not the dominant group in the soil and did not reach the detection limit of DGGE. The diversity of DNB increased but that of bacteria de-creased under herbicide stress. The herbicide also clearly changed other functional groups in the soil. In our previous research, chlorimuron-ethyl caused the demise of someγ -proteobacteria (Zhang et al.2011). We also found that the herbicide could inhibit NFB related toγ-proteobacteria in
this study. As we know,γ-proteobacteria are an important group in soil, contributing to soil nitrification, antimicrobial activity and many other functions (Bremner1997; Samson et al. 1998). Therefore, long-term chlorimuron-ethyl appli-cation clearly disturbs soil biological functions.
Conclusions
To sum up our results, long-term application of chlorimuron-ethyl in a continuously cropped soybean field could seriously disturb the soil nitrogen-transforming bacterial communities. Chlorimuron-ethyl reduced the N bacterial abundance and altered the bac-terial community structure, which suggested that the herbicide could influence soybean soil N cycling and biological quality, and increase the possibility of an obstacle to soybean’s continuous cropping regime. Fur-ther studies should be focused on changes in soil N-cycling function to explore possible links between mi-crobial diversity and activity, and on developing rational application models for this herbicide to reduce its
Fig. 5 Phylogenetic
impact on the biological quality of soybean soil and soybean growth.
Acknowledgments This work was supported by the National
Natu-ral Science Foundation of China (No. 41071202) and the National High Technology Research and Development Program (863) of China (No. 2012AA101403).
References
Bakulin MK, Grudtsyna AS, Pletneva AY (2007) Biological fixation of nitrogen and growth of bacteria of the genusAzotobacterin liquid media in the presence of perfluorocarbons. Appl Biochem Microbiol 43:399–402
Boldt TS, Jacobsen CS (1998) Different toxic effects of the sulfonyl-urea herbicides metsulfuron methyl, chlorsulfuron and thifensulfuron methyl on fluorescent pseudomonads isolated from an agricultural soil. FEMS Microbiol Lett 161:29–35
Bremner JM (1997) Sources of nitrous oxide in soils. Nutr Cycl Agroecosys 49:7–16
Chen Z, Hou HJ, Zheng Y, Qin HL, Zhu YJ, Wu JS, Wei WX (2011) Influence of fertilisation regimes on a nosZ-containing denitrifying community in a rice paddy soil. J Sci Food Agric 92:1064–1072 Diosma G, Golik SI, Chidichimo HO, Balatti PA (2003) Nitrification
potential, dehydrogenase activity and microbial biomass in an argiudol soil cultivated with wheat under two tillering methods. Span J Agric Res 1:111–119
Enwall K, Philippot L, Hallin S (2005) Activity and composition of the denitrifying bacterial community respond differently to long-term fertilization. Appl Environ Microbiol 71:8335–8343
Forlani G, Mantelli M, Branzoni M, Nielsen E, Favilli F (1995) Differential sensitivity of plant-associated bacteria to sulfonylurea and imidazolinone herbicides. Plant Soil 176:243–253
Franche C, Lindström K, Elmerich C (2009) Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 321:35–59
Gigliotti C, Allievi L (2001) Differential effects of the herbicides bensulfuron and cinosulfuron on soil microorganisms. J Toxicol Environ Health B 36:775–782
He YH, Shen DS, Fang CR, He R, Zhu YM (2006) Effects of metsulfuron-methy on the microbial population and enzyme activities in wheat rhizosphere soil. J Toxicol Environ Health B 41:269–284
He JZ, Shen JP, Zhang LM, Zhu YG, Zheng YM, Xu MG, Di HJ (2007) Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ Microbiol 9:2364–2374
Innerebner G, Knapp B, Vasara T, Romantschuk M, Insam H (2006) Traceability of ammonia oxidizing bacteria in compost-treated soils. Soil Biol Biochem 38:1092–1100
Jin C, Zhou Q, Zhou Q, Fan J (2010) Effects of chlorimuron-ethyl and cadimum on biomass growth and cadimum accumulation of wheat in the phaiozem area, Northeast China. B Environ Contam Tox 84:395–400
Junie P, Kim OS, Hadas O, Imhoff JF, Witzel KP (2008) Evaluation of PCR primer selectivity and phylogenetic specificity by using amplification of 16S rRNA genes from betaproteobacterial ammonia-oxidizing bacteria in environmental samples. Appl En-viron Microbiol 74:5231–5236
Kaur T, Brar LS, Walia US (2009) Residual effect of sulfonylurea herbicides applied to wheat on the following kharif crops as affected by organic matter. Indian J Ecol 36:139–142
Kowalchuk GA, Stephen JR, Boer WD, Prosser JI, Embley TM, Woldendorp JW (1997) Analysis of ammonia-oxidizing bacteria
of the beta subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl Environ Microbiol 63:1489–1497
Li XY, Zhang HW, Wu MN, Su ZC, Zhang CG (2008) Impact of acetochlor on ammonia-oxidizing bacteria in microcosm soils. J Environ Sci 20:1126–1131
Mendum TA, Sockett RE, Hirsch PR (1999) Use of molecular and isotopic techniques to monitor the response of autotrophic ammonia-oxidizing populations of the beta subdivision of the class Proteobacteria in arable soils to nitrogen fertilizer. Appl Environ Microbiol 65:4155–4162
Molla AH, Shamsuddinb ZH, Halimi MS, Morziahc M, Putehd AB (2001) Potential for enhancement of root growth and nodulation of soybean co-inoculated withAzospirillumandBradyrhizobium
in laboratory systems. Soil Biol Biochem 33:457–463
Moseman SM, Zhang R, Quan PY, Levin LA (2009) Diversity and functional responses of nitrogen-fixing microbes to three wetland invasions. Biol Invasions 11:225–239
Olsson PA, Hansson MC, Burleigh SH (2006) Effect of P avail-ability on temporal dynamics of carbon allocation and glo-mus intraradices high-affinity P transporter gene induction in
Arbuscular mycorrhiza. Appl Environ Microbiol 72:4115–
4120
Phillips CJ, Harris D, Dollhopf SL, Gross KL, Prosser JI, Paul EA (2000) Effects of agronomic treatments on structure and function of ammonia-oxidizing communities. Appl Environ Microbiol 66:5410–5418
Reddy KN, Locke MA, Wagner SC, Zablotowicz RM, Gaston LA, Smeda RJ (1995) Chlorimuron-ethyl sorption and desorption kinetics in soils and herbicide-desiccated cover crop residues. J Agric Food Chem 43:2752–2757
Richardson DJ (2000) Bacterial respiration: a flexible process for a changing environment. Microbiology 146:551–571
Rosch C, Mergel A, Bothe H (2002) Biodiversity of denitrifying and dinitrogen-fixing bacteria in an acid forest soil. Appl Environ Microbiol 68:3818–3829
Samson R, Shafik H, Benjamo A, Gardan L (1998) Description of the bacterium causing blight of leek asPseudomonas syringaepv.
porri(pv. nov.). Phytopathology 88:844–850
Skinner FA, Walker N (1961) Growth ofNitrosomonas europaeain batch and continuous culture. Arch Mikrobiol 38:339–349 Stephen JR, McCaig AE, Smith Z, Prosser JI, Embley TM (1996)
Molecular diversity of soil and marine 16S rRNA gene sequences related to beta-subgroup ammonia-oxidizing bacteria. Appl Envi-ron Microbiol 62:4147–4154
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Wang JJ, Zhang HW, Zhang XL, Qin SH, Tan HB, Li XY (2013) Effects of long term chlorimuron-ethyl application on the diver-sity and antifungal activity of soilPseudomonasspp. in a soybean field in Northeast China. Ann Microbiol 63:335–341. doi:10.1007/s13213-012-0479-7
Xu GH, Zheng GY (1986) Handbook of soil microbes analysis. China Agricultural Press, Beijing
Zawoznik MS, Tomaro ML (2005) Effect of chlorimuron-ethyl on
Bradyrhizobium japonicumand its symbiosis with soybean. Pest
Manag Sci 61:1003–1008
Zehr JP, McReynolds LA (1989) Use of degenerate oligonucleotides for amplification of thenifHgene from the marine cyanobacterium
Trichodesmium thiebautii. Appl Environ Microbiol 55:2522–2526