111Equation Chapter 1 Section 1
EXPERIMENT JOULE
Devi Putriana, Dewi Adyani, Muh.Haliq Ma’ruf L, Nur Mukarramah, Uswatun Hasanah
Basic Physics Laboratory Department Of Biology Science Faculty 2013
State University Of Makassar
Abstrak. Has been done made experiment about “Experiment Joule”. This is experiment purpose to cumprehended principle equivalent heat energy and mechanics energy. Determine value equivalent heat energy and mechanics energy. In experiment only there is one activity and doing to 3x measurement. Procedure work at this experiment that is prepare all tool and material which needful and assemble tool. certainlythat is polarity and limit measure from the resources and tools measure current and voltge correctand thenturn on power supply. After that measure mass calorimetry empty and stirring and then filled water then measure mass. After that measure temperature calorimetry along with content and write as the first temperature.From In this experiment is based on the observations, known that more biggest value voltage and electric current at a matter, then equal heat electric which possessed by matter that more small. Whereas at temperature that is to known that is more highest ascension temperature then more biggest level heat and change time at calorimetry mentioned.
Keywords : Calorimetry, temperature, time, massa, voltage, and electric current
INTRODUCTION
V
Termometer
VS
kalorimeter Joule + Elemen
A + _
is used for heating water, and the calorimeter thus occurring change of form energy of electrical energy to became heat engine, visible to change in the temperature heat engine absorbed by the calorimeter and water in it is: q = m. C. h.k. = price water the calorimeter m = water mass in the calorimeter t1 = temperature early t2 = temperature end so raktor conversion can be calculated if w and q known. For that in this activity we will meakukan experiment experiment joule for the purpose for us to understand the principle of equivalence heat energy and mechanical energy and can determine the value of equality energy heat and energy mechanical.
THEORY
The first law of thermodynamics is a statement of conservation of energy. This law describes the results of many experiments which connects the work done on the system, heat is added or subtracted from the system, and internal energy of the system. The initial definition of calories, we know that it takes 1 calorie of energy to raise the temperature of 1 g of water to 10 c. But we also can raise the temperature of the water or any other system of doing business with him without adding even the slightest heat from outside.
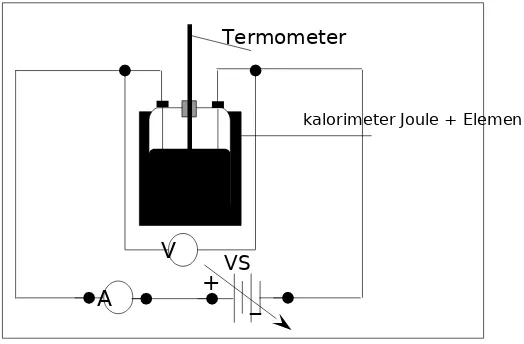
Gambar 7.1. Perangkat percobaan Joule
Images. 7.1 above shows a schematic diagram of the modification of Joule device which can be used to determine the amount of effort that is equivalent to a number of specific heat, that is, the amount of effort it takes to raise the temperature of one gram of water by one degree Celsius. The water in the kalorimeter on the image enclosed in the wall insulation so that the temperature of the system cannot be influenced by the heat into or out of it. With the granting of potential difference VS. on the ends of the coil in the kalorimeter, an electric current will flow through the ammeter and the potential difference occurs at the ends of the coil which will generate electricity on business system to heat water. This effort was known as the joule heat, which can be expressed as,
Where v is a potential difference of the ends of the elements, i is an electrical current in circuit, and t is time pengaliran current to system. Heat energy released by element electric will be accepted by water and the calorimeter so temperature system become inflated ( exception occurring a change of phase ). Large heat energy q required by water to raise temperaturnya comparable to temperature changesTand massm, that is:
Q
=
m
×
c
×
ΔT
[7.2]Where c is hot varieties of water.
The experiment joule experiments and afterward is that it takes 4,18 satuan mechanical work or electricity ( joule ) to increase temperature 1 g water by 1 0c, or 4,18 j energy mechanical or electrical is equivalent to 1 kal heat energy.
METHODOLOGY EXPERIMENT
At this is experiment we are doing activity measurement experiment joule. Before started the activity, more than we are prepare tool and material, that is Power Supplay DC, Basic Meter (ammeter 0-5 A DC and Voltmeter 0-5 V DC), Calorimetry joule complete, Thermometer, Stop Watch, Balance ohaus 311 gram, and some cable connect.
After that we are the started up procedure work. At the first that is prepare tool and material which needful and assemble tool.Don’t turn on power supplybefore you certainly that is polaryty and limit measure from the resource and tools measure current and voltage correct.Measure mass calorimetry empty + stirring. Filled calorimetry with water until half then measure mass.Set calorimetry complete at series, furthermore turn on ration power and arrange in order thatcurrent which flow as big as 2 A, write this current and potential differenceat tips spool. Turn off back Power Supply with not changes regulator voltage. Measure temperature calorimetry with content and write as the first temperature. Turn on power supply be equal to with drive stopwatch for measurement long flow current until temperature system rise to be 100C, 200C and 300C from temperature first and cut it out stopwatch be equal to with turn off power supply. Write indication stop watch. Repeat this is activity until 3 times measurement.
RESULT EXPERIMENT AND ANALYSIS DATA
NST Balance Ohaus 311 g = 0,01 gram NST Voltmeter = 0,2 V
NST Ammeter = 0,1 A
NST Thermometer = 1
℃
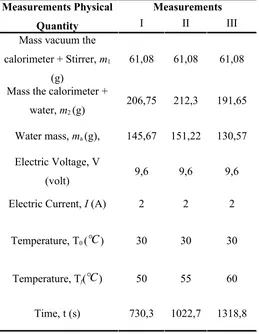
NST Stopwatch = 0,1 sTable 7.1.Table Result Measurument Measurements Physical
Quantity
Measurements
I II III
Mass vacuum the calorimeter + Stirrer, m1
(g)
61,08 61,08 61,08
Mass the calorimeter +
water, m2 (g) 206,75 212,3 191,65
Water mass, ma (g), 145,67 151,22 130,57
Electric Voltage, V
(volt) 9,6 9,6 9,6
Electric Current, I (A) 2 2 2
Temperature, T0 (
℃
) 30 30 30Temperature, Tf(
℃
) 50 55 60Data Analysis Measurement 1
Q = mair
×Cair
×ΔT + malu
×Calu
×ΔT + mtermo
×Ctermo
×ΔT
= 145,67 × 1 kal/g
℃
× 20+
¿
61,08×
0,22 kal/g℃
× 20 + 20,7× 0,2 kal/g℃
× 20 = 3264,952 caloriΔQ =
|
Δm air
m air
+
Δm alu
m alu
+
Δm termo
m termo
+
2
ΔΔT
ΔT
|
Q
=
|
0,01
145,67
+
0,005
61,08
+
0,005
20,7
+
2
(
0,5
)
20
|
3264,95
2 = 164,48 caloriΔm air = NST Ohaus 311 gram = 0,01 gram
Δm alu = Δm termo = ½ NST Ohaus 311 gram = 0,005 gram
ΔΔT = ½ NST Thermometer = 0,5 gram
w = V.I.t
= 9,6 x 2 x 730,3 = 14021,76 joule
Δw =
|
ΔV
V
+
ΔI
I
+
Δt
t
|
w=
|
0,1
9,6
+
0,05
2
+
730,3
0,1
|
14021,76 = |0,01
+
0,025
+
0,00013|
14021,76 = 492,58 jouleΔV
= ½ NST Voltmeter = 0,1 VΔI
= ½ NST Ammeter = 0,05 Iγ =
w
Q
=
14021,76
3264,95
= 4,29 J/kalΔ∂=
|
Δw
w
+
ΔQ
Q
|
∂
=
|
14021,76
492,58
+
3264,94
164,68
|
4,29
= 0,36 J/kal
KR =
Δ∂
∂
˟ 100%=
0,36
4,29
× 100%= 8,39% DK = 100% - KR = 100% - 8,39% = 91,61% PR =
¿
∂ ± ∆ ∂
∨
¿
= | 4,29 ± 0,36 | J/kal
Measurement 2
Q = mair
×Cair
×ΔT + malu
×Calu
×ΔT + mtermo
×Ctermo
×ΔT
= 4219,94 caloriΔQ =
|
Δm air
m air
+
Δm alu
m alu
+
Δm termo
m termo
+
2
ΔΔT
ΔT
|
Q
= 170,43 calorie w = V.I.t
= 9,6 x 2 x 1022,7 = 19635,84 joule
Δw =
|
ΔV
V
+
ΔI
I
+
Δt
t
|
w = 688,72 joule∆ ∂
=
|
∆ W
W
+
∆ Q
Q
|
∂
= 0,34 J/kal
KR =
∆ ∂
∂ ×
100 %
= 7,5 % DK = 100% - KR = 92,5 %
PR
=
¿
∂± ∆ ∂
∨
¿
= | 4,65 ± 0,34 | J/kal
Measurement 3
Q = mair
×Cair
×ΔT + malu
×Calu
×ΔT + mtermo
×Ctermo
×ΔT
= 5925,9 calorieΔQ =
|
Δm air
m air
+
Δm alu
m alu
+
Δm termo
m termo
+
2
ΔΔT
ΔT
|
Q
= 149,07 calorie
w = V.I.t
= 9,6 x 2 x 1318,8 = 25320,96 joule
Δw =
|
ΔV
V
+
ΔI
I
+
Δt
t
|
w = 689,02 joule∂=
W
Q
= 4,27 J/kal∆ ∂
=
|
∆ W
W
+
∆ Q
Q
|
∂
= 0,22 J/kal
KR =
∆ ∂
∂ ×
100 %
= 5,2 % DK = 100% - KR = 94,8 %
PR
=
¿
∂± ∆ ∂
∨
¿
After making the measurements, the following is an analysis and discussion of the experiment of joule.
Table7.2 analysis
No measurement Jpractice Jtheory
1 I | 4,29 ± 0,36 | J/kal
2 II | 4,65 ± 0,34 |
J/kal 4,18 J
3 III | 4,27 ± 0,22 | J/kal
DISCUSSION
On experiment experiment joule that has been done first is measuring the mass of the calorimeter empty + stirrer then measure it again after filled with water by half. After that, we have set the calorimeter complete in the sequence and ignite catu power was later set to a current which flows as much as 2 a and noted arusnya as well as the potential difference of opposite ends of a coil. After that we measure the calorimeter and in it and noted as temperatures the beginning. Flipped its own power supply and simultaneously run stopwatch for measuring time passed by the current until temperature rose to10
℃
, 20℃
, dan 30℃
Then repeating event to 3 times until obtained measurement result. In this experiment there was only one activities which were determine the value of equality energy heat and energy mechanical.In this experiment is based on the observations, known that more biggest value voltage and electric current at a matter, then equal heat electric which possessed by matter that more small. Whereas at temperature that is to known that is more highest ascension temperature then more biggest level heat and change time at calorimetry mentioned.
known that is more highest increase temperature then more biggest level heat and change time at calorimetry mentioned.
CONCLUSSION
In this experiment is based on the observations, known that more biggest value voltage and electric current at a matter, then equal heat electric which possessed by matter that more small. Whereas at temperature that is to known that is more highest ascension temperature then more biggest level heat and change time at calorimetry mentioned.
REFERENCE
Tim Dosen Fisika Dasar 1 Jurusan Fisika FMIPA UNM. 2013. Penuntun Praktikum Fisika Dasar 1.