Now that the genomes of Saccharomyces cerevisiaeand Caenorhabditis eleganshave been sequenced, and the sequencing of the Arabidopsisgenome is well under way, cell biologists are faced with the daunting chal-lenge of establishing the function and mode of action of thousands of gene products. The ulti-mate goal of these studies will be to under-stand how the collective functions of these proteins and other biomolecules are responsible for life itself. Among the new tools that need to be developed to meet these challenges are opti-cal probes and imaging technologies: these
must be capable of identifying and mapping the interactions and activities of specific proteins and measuring their associated kinetic param-eters with high spatial and temporal resolution. Light-directed activation of caged (inactive) compounds should be a valuable technique for such investigations because it can be used to manipulate the activity of specific biomol-ecules in cells (for a comprehensive review of the methodology involved see Ref. 1). This technique involves a biologically inert, caged compound that is specifically and rapidly (ns to ms) perturbed within a cell using a pulse of
near ultraviolet light. The effects of a controlled and local (sub-micron) concentration or activity jump can then be recorded using time-resolved imaging of a probe of the perturbed reaction. Specific modulation of protein activity with light can initiate a specific cell process that might be used to elucidate the function of the protein, the kinetics of its activity and the mechanism of its action (for example, activation of myosin at the cell cortex and motility).
Over the past 20 years several caged ligands and substrates have been used to investigate the molecular basis of intracellular processes, including muscle contraction2, synaptic trans-mission3, intracellular signaling4and motility5. Unfortunately, small ligands often activate multiple cellular processes, or can be metab-olized to other active species. Caged conjugates of peptides and proteins are often better suited for manipulating the activity of a specific pro-tein within a cell6,7. The principle of light-directed activation of caged polypeptides, and the potential applications of this technique for studying the function of specific proteins in cellular processes are illustrated in Fig. 1. 28 Rathcke, B. (1988) Interactions for pollination
among coflowering shrubs, Ecology 69, 446–457 29 Schürch, S. et al.Effects of ants on the
reproductive success of Euphorbia cyparissias and associated pathogenic rust fungi, Oikos (in press)
30 Primack, R.B. (1985) Longevity of individual flowers, Annu. Rev. Ecol. Syst.16, 15–38 31 Ashman, T-L. and Schoen, D.J. (1994) How
long should flowers live? Nature371, 788–791 32 Williamson, G.B. (1982) Plant mimicry:
evolutionary constraints, Biol. J. Linn. Soc.18, 49–58 33 Waser, N.M. (1983) Competition for pollination
and floral character differences among sympatric plant species: a review of evidence, in Handbook of Experimental Pollination Biology(Jones, C.E. and Little, R.J., eds), pp. 277–292, Van Nostrand Reinhold
34 Murcia, C. and Feinsinger, P. (1996) Interspecific pollen loss by hummingbirds visiting flower mixtures: effects of floral architecture, Ecology 77, 550–560
35 Roy, B.A. and Raguso, R.A. (1997) Olfactory versus visual cues in a floral mimicry system, Oecologia109, 414–426
36 Raguso, R.A. and Roy, B.A. (1998) ‘Floral’ scent production by Puccinia rust fungi that mimic flowers, Mol. Ecol.7, 1127–1136
37 Waser, N.M. (1978) Competition for hummingbird pollination and sequential flowering in two Colorado wildflowers, Ecology 59, 934–944
38 Armbruster, W.S. and McGuire, A.D. (1991) Experimental assessment of reproductive
interactions between sympatric Asterand Erigeron(Asteraceae) in interior Alaska, Am. J. Bot.78, 1449–1457
39Kochmer, J.P. and Handel, S.N. (1986) Constraints and competition in the evolution of flowering phenology, Ecol. Monogr.56, 303–325 40Thomson, J.D. (1980) Skewed flowering
distributions and pollinator attraction, Ecology 61, 572–579
41Dafni, A. et al.(1990) Red bowl-shaped flowers: convergence for beetle pollination in the Mediterranean region, Israel J. Bot.39, 81–92 42Smithson, A. and Macnair, M.R. (1997) Negative
frequency-dependent selection by pollinators on artificial flowers without rewards, Evolution51, 715–723
43Dafni, A. and Calder, D.M. (1987) Pollination by deceit and floral mimesis in Thelymitra antennifera (Orchidaceae), Plant Syst. Evol.158, 11–22 44Heinrich, B. (1975) Energetics of pollination,
Annu. Rev. Ecol. Syst.6, 139–170
45Charlesworth, B. (1994) The genetics of adaptation: lessons from mimicry, Am. Nat.144, 839–847 46Feinsinger, P. et al.(1986) Floral neighborhood
and pollination success in four hummingbird-pollinated cloud forest plant species, Ecology67, 449–464
47Dukas, R. (1987) Foraging behavior of three bee species in a natural mimicry system: female flowers which mimic male flowers in Ecballium elaterium(Curcurbitaceae), Oecologia74, 256–263
48Nilsson, L.A. (1992) Orchid pollination ecology, Trends Evol. Ecol.7, 255–259
49 Kevan, P.G. (1983) Floral colors through the insect eye: what they are and what they mean, in Handbook of Experimental Pollination Biology (Jones, C.E. and Little, R.J., eds), pp. 3–30, Van Nostrand Reinhold
50 Chittka, L. et al.(1994) Ultraviolet as a component of flower reflections, and the colour perception of hymenoptera, Vision Res.34, 1489–1508
51 Dafni, A. and Kevan, P.G. (1997) Flower size and shape: implications in pollination, Isr. J. Plant Sci.45, 201–211
52 Dafni, A. (1983) Pollination of Orchis caspia– a nectarless plant which deceives the pollinators of nectariferous species from other plant families, J. Ecol.71, 467–474
53 Kullenberg, B. (1961) Studies in Ophrys pollination, Zool. Bidr. Uppsala34, 1–349 54 Roy, B.A. (1996) A plant pathogen influences
pollinator behavior and may influence
reproduction of non hosts, Ecology77, 2445–2457
Bitty A. Roy*and Alex Widmer are at the Geobotanical Institute, Swiss Federal Institute of Technology (ETH), Zürichberstr. 38, CH-8044 Zürich, Switzerland.
*Author for correspondence (tel 141 01 632 7787; fax 141 01 632 1215;
e-mail [email protected]).
Caged peptides and proteins:
new probes to study polypeptide
Functional and spectroscopic
characterization of caged polypeptides
Caged polypeptides should be subjected to a rigorous biochemical and spectroscopic char-acterization before and after photoactivation. These studies should establish the stability of the caged amino acid residue, the efficiency of the photoactivation reaction and demonstrate that photoactivation proceeds without del-eterious secondary reactions. It is also impor-tant to confirm that the photoproducts of the photoactivation reaction are benign – this con-dition has been demonstrated in several appli-cations of light-directed activation of caged polypeptides in live cells6,8–10.
Caged peptides
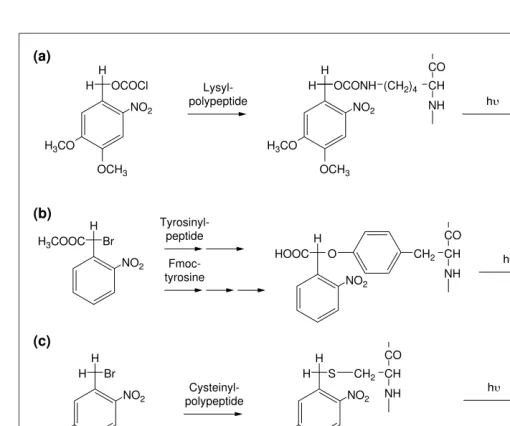
Photolabile reagents were widely used by pep-tide chemists during the 1970s to protect amino, phenolic tyrosine and carboxyl groups of amino acids11. More recently, these reagents have been used to direct the syntheses of surface-based, spatially addressable peptide libraries that can be used to identify novel proteins and drugs by high throughput screening of chemi-cal and biologichemi-cal libraries12. Peptides specifi-cally caged on arginine10 or tyrosine6,13, cysteine or thiophosphate groups14 (Fig. 2) have been described and should prove useful in studying static or kinetic aspects of specific protein activity in vivo. A major challenge is to design a caged peptide that specifically and potently interferes with the protein or process under study only after photoactivation.
Caged peptide inhibitors of calmodulin and myosin light chain kinase
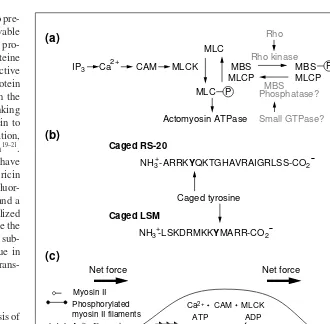
In a comprehensive study involving the devel-opment of novel, caged peptides to probe the function of calmodulin6, the timing and local-ization of calcium-bound calmodulin (CAM) activity was assessed in the context of the motile behavior of eosinophil cells. Several proteins involved in calcium ion signaling pathways are activated as a consequence of CAM binding to specific sequences, including myosin light chain kinase (MLCK), which activates the contraction of actomyosin com-plexes via phosphorylation of myosin light chain (MLC) (Fig. 3). A 20 amino acid pep-tide representing the target sequence in MLCK binds tightly to CAM and inhibits its signaling function in cell motility. A caged peptide is designed to act as an inhibitor of CAM only on removal by light of a protection group from a single tyrosine residue (caged RS-20; Fig. 3). The photolabile group is opti-mized for its rapid photo-release, high quan-tum yield and the low toxicity of its photoproduct (Fig. 2). Caged RS-20 binds CAM 50 times less tightly than the unpro-tected peptide and is effectively inactive when assayed in smooth muscle cells known to be dependent on CAM function. In subsequent
studies, caged RS-20 has been microinjected into eosinophil cells and the majority of micro-injected cells exhibit a normal motile behav-ior before exposure to light, but, within seconds of being irradiated, they round-up and stop moving because of the effects of the increased intracellular level of active RS-20. Irradiated cells resume their motile behavior at least 50 s after triggering the RS-20 concentration jump, indicating that the RS-20-mediated inhibition of motility is reversible. Control experiments show no change in motile behavior. To demonstrate that the cessation of motility had been caused by the inhibition of CAM signaling to MLCK, and not through other proteins,a caged peptide
inhibitor of MLCK was designed, based on a peptide sequence in the auto-inhibitory domain of MLCK (caged LSM-1; Fig. 3).
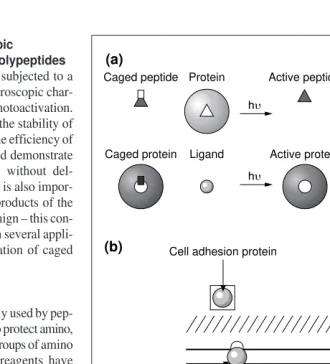
Functional in vitroassays indicate that caged LSM-1 inhibits MLCK activity only after photo-activation. Cells loaded with caged LSM-1 are fully motile, but again, after pho-toactivation they round up and stop moving. Because inhibition of myosin II activity depends on blocking CAM-MLCK activity and also on the removal of phosphates by phosphatase, the timing of the inhibition indi-cates that phosphate turnover on the light chain of myosin II must occur with seconds. These data therefore support the conclusion that CAM-MLCK-mediated phosphorylation of myosin II and de-phosphorylation by pro-tein phosphatases are sufficiently rapid to allow eosinophil cells to respond to the rapid changes in extracellular gradients of chemo-attractant, or fluctuations in intracellular cal-cium ions (Fig. 3). Because several of the Fig. 1.Principle of light-directed activation of caged polypeptides. (a) A peptide that inhibits or activates the activity of a protein is modified with a photolabile group at a specific residue to block its ability to bind to its target protein. Near ultraviolet irradiation removes the protection group and generates an active peptide that binds to the target protein. Similarly, a caged protein can be created by labeling an amino acid in the protein that is essential for its activity (e.g. at a substrate or ligand-binding site). (b) Photoactivation of microinjected caged polypeptides at specific cellular locations can be used to investigate the function and kinetics of specific proteins in processes that might include: membrane receptor signaling, calcium signaling, regulation of cell adhesion at focal contacts, actin polymerization, cellular energetics or metabolism, and Caged peptide
(a)
Active peptide
hu Protein
Caged protein Ligand Active protein
hu
Peptide binding
Ligand binding
Cell wall
Plasma membrane
Cytoplasm
Nucleus
Cell physiology Metabolic enzymes Signal
transduction membrane receptor
Cell adhesion protein
Actin and actin-binding
Trends in Plant Science Gene
regulation transcription factors
(b)
signaling components in the transduction path-way that leads to eosinophil motility are known (Fig. 3), and many of these biomolecules can be caged, it should be possible to use the pho-toactivation technique to completely describe the intracellular kinetics of this pathway.
Caged proteins
A caged protein is defined as a protein whose activity is inhibited following covalent modi-fication of an essential amino acid with a pho-tolabile group – irradiation of a caged protein with near ultraviolet light removes the protec-tion group from the conjugate and restores its
activity7,15. For example, a caged G-actin con-jugate has been described in which lysine residues essential for actin polymerization were modified with 6-nitroveratryloxycar-bonyl chloride7. Ultraviolet irradiation of caged actin triggers a photoactivation reac-tion that liberates native, polymerizareac-tion- polymerization-competent G-actin, carbon dioxide and 3,4-dimethoxy-2-nitrosobenzaldehyde (Fig. 2). The same approach has been used to prepare caged conjugates of profilin16and the tran-scription factor GAL418 (Ref. 9). The same photolabile group attached to lysine residues of a monoclonal antibody has been used to
prepare a caged antibody that could only bind its antigen after photoactivation17. A caged heavy meromyosin (HMM) has also been pre-pared by labeling an essential cysteine residue with the photolabile reagent, 4,5-dimethoxy-2-nitrobenzenzylbromide15(Fig. 2), whereas a related molecule has been used to prepare a caged pore-forming protein18. In general, cys-teine residues are more selectively labeled compared with lysine because of their lower frequency in proteins and the higher reactiv-ity of the thiol group at pH 6–8. In addition, cysteine-mutagenesis can be employed to design a protein for better caging chemistry Fig. 2.Chemical strategies used to prepare caged peptides and caged proteins. (a) The e-amino group of lysine can be derivatized with 6-nitroveratryloxycarbonyl chloride to form the corresponding carbamate between a pH value of 8.0 and 9.5 at room temperature. Irradiation of the carbamate with 365 nm light results in the de-protection of the e-amino group with the concomitant release of carbon dioxide and 3,4-dimethoxy-2-nitrosobenzaldehyde. (b) The phenolic oxygen of tyrosine can be protected by alkylation via 2-bromo-a-carboxyl-2-nitrophenyl methyl ester. Irradiation of the caged peptide rapidly releases the unprotected tyrosine peptide and a relatively non-reactive photoproduct. (c) The thiol group of a cysteine residue can be protected with 4,5-dimethoxy-2-nitrobenzyl bromide or with 2-bromo-2-(2-nitrophenyl) acetic acid. The products of photolysis are the free thiol group of cysteine and a potentially thiol-reactive photoproduct. A series of bifunctional photocleavable reagents have also been used to prepare caged proteins (d and e), and a caged biotin (f).
H
Lysyl-polypeptide OCOCl
(a)
(b)
(c)
(d) (e) (f)
NO2
OCH3
H
H
NH Br
NO2
H3COOC H
O
NO2
HOOC H3CO
H Br
NO2
OCH3
H
H3CO
H
O Cl
O
NO2
OCH3
CH2
H3CO
H S
NO2
OCH3
H
H3CO
OCH3
H3CO
H
OCONH (CH2)4 CH
CO
NH
CH2 CH CO
NH
CH2 CH CO
NH
HS CH2 CH
CO
NH NH2(CH2)4
+
+
+ + CO2
CH CO
NH NO2
OCH3
H
H3CO
NO O
OCH3
H3CO
H
NO
OH
CH2
CH CO O
HOOC
NO O H
Cysteinyl-polypeptide
Tyrosinyl-peptide
Fmoc-tyrosine
hu
hu
hu
CO N N(CH3)2
O
O
H N O C
O Br
H
H3C
O H
O
O N O C
O
NO2 NO2
Biotin
NH-(CH2)5CONH-CH2
Trends in Plant Science
and photoactivation18. Another method to pre-pare caged proteins is to use photocleavable crosslinking reagents (Fig. 2). Here, the pro-tein is first modified at a lysine or cyspro-teine residue using the photocleavable reactive group of a bifunctional reagent. If the protein activity is caged after this reaction, then the second reactive group of the crosslinking reagent is used to target the caged protein to another biomolecule, or to a specific location, via an antibody for later photo-activation19–21. Photocleavable crosslinking reagents have been used in this way to prepare caged ricin for targeting to specific cancer cells19: a fluor-escent caged actin monomer21(Fig. 1) and a caged actin dimer20(Fig. 2). More specialized methods to prepare caged proteins include the use of photocleavable cinnamate ester sub-strates to target a reactive serine residue in thrombin22, or by employing in vitro trans-lation techniques (as discussed here).
Caged myosin
Motor proteins, powered by the hydrolysis of ATP, are present in all cell types, but the mechanisms that control the organization and function of active motor proteins are poorly understood. Caged motor proteins would be extremely useful for unravelling spatial and temporal aspects of motor protein function23. This approach requires the development of caged motor proteins that can be incorporated into such systems, or the development of methods for modifying endogenous motors with a caging reagent. Progress towards these goals has been achieved in studies on light-directed activation of a caged myosin sub-fragment, HMM (Refs 15,24). The function of myosin II, a chemo-mechanical enzyme, is mediated by coupling two different activities in the motor molecule. The first, an F-actin-activated Mg2+ -ATPase, is located in the head region, whereas the second uses free energy derived from ATP hydrolysis to drive a stroke of the long a-helix in the neck that produces a 5 nm translation of the bound actin filament.
Recent investigations aimed at understanding the molecular basis of myosin function have focused on the region around Cys707, which is close to the fulcrum of the long a-helix. Most chemical modifications of myosin at this residue elevate the Mg2+-activated ATPase activity of the conjugate and inhibit its ability to move actin filaments (as measured in an in vitromotility assay sensitive to the chemo-mechanical coupling25). To elaborate on the importance of the hinge region in coupling the two activities of HMM: 4,5-dimethoxy-2-nitrobenzenzylbromide has been employed to specifically modify Cys707. As expected, the solution-based Mg2+ -activated ATPase activity of this conjugate is higher than native HMM. The functional activ-ity of HMM is determined using a motilactiv-ity assay on the following surface-bound preparations:
• Native HMM.
• HMM modified on Cys707 with the caged reagent (caged HMM).
• Caged HMM after irradiation with a pulse of near ultraviolet light.
Native HMM supports the movement of actin filaments at a velocity of 3–4 mm/s, whereas no movement is observed with caged HMM. HMM is therefore caged in the sense that the two activities in the conjugate are uncoupled. However, irradiation with a short pulse of 365 nm light removes the protection group from Cys707, which results in most actin fil-aments sliding in the image field at an average velocity of 2 mm/s. These, and other studies,
suggest that modifying Cys707 with chemical groups physically hinders the progression of an ATP hydrolysis-dependent conformational transition from the head to the neck region that ordinarily leads to the power stroke of the lever arm. Light-directed removal of the pro-tection group from Cys707 relieves this phys-ical constraint and restores chemo-mechanphys-ical coupling within HMM with a time constant of 22 ms (Ref. 15). Preliminary studies have shown that caged myosin can also be prepared in functional muscle fibers treated with 4,5-dimethoxy-2-nitrobenzenzylbromide. Force generation is severely impaired in the caged myosin-containing muscle fibers – yet, as in Fig. 3.Essential biomolecules and their sequence in the calcium-mediated signaling pathway regulating actomyosin contraction during cell motility. (a) Caged biomolecules that could be used to further define this signaling pathway include calcium ions, inositol triphosphate and polypeptide inhibitors or activators of Rho kinase, the myosin-binding subunit (MBS) of myosin light chain phosphatase (MLCP), MLCP and myosin II. (b) Caged RS-20 was pre-pared by introducing a photolabile tyrosine at position 5 in the myosin light chain kinase (MLCK) sequence targeted by calmodulin. Photoactivation of the caged peptide generates the deprotected RS-20, which inhibits calmodulin 50 times more effectively than caged RS-20. Similarly, caged LSM-1 was prepared by introducing a photolabile tyrosine residue at pos-ition nine in a MLCK auto-inhibitory sequence. (c) Schematic of a eukaryotic cell undergo-ing locomotion. One process thought to be important is the phosphorylation of the myosin light chain (MLC) of myosin II by Ca2+-calmodulin (CAM)-dependent MLCK. The timing of this process was examined by rapid, localized photorelease of caged peptides in actively motile cells6. Cessation of motility owing to inhibition of CAM or MLCK occurred within 10 s, demonstrating that turnover of active phosphorylated myosin II occurs in this time domain. The organization of active CAM and MLCK can be established with these caged peptides if peptide diffusion is restricted.
(a)
(b)
(c)
IP3 Ca CAM MLCK
Caged RS-20
NH3 -ARRKYQKTGHAVRAIGRLSS-CO2
Caged LSM
NH3 -LSKDRMKKYMARR-CO2
MLC
Caged tyrosine
Net force
MBS MLCP
Rho
Rho kinase
MBS
Small GTPase? Phosphatase?
MBS MLCP P
MLC
Actomyosin ATPase
P
Net force
Myosin II
MLCP ATP
Pi
ADP Ca2+ CAM MLCK Phosphorylated
myosin II filaments Actin filaments
Net force
Trends in Plant Science
2+
+
+
-the previous study, chemomechanical force production recovers in the fiber about 20 ms after removing protection groups with near ultravio-let light24. Further in vivophotoactivation ex-periments are planned to obtain a better under-standing of how the structural dynamics in the myosin II molecule are related to muscle con-traction. This question could be addressed by recording time-resolved measurements of force in conjunction with time-resolved X-ray struc-tural analysis before and after photoactivation of the caged myosin in the muscle fiber. Light-directed activation of caged non-muscle myosin isoforms microinjected into motile cells is also feasible and these studies should prove useful in defining the kinetics and functional properties of this protein during motility and cytokinesis.
In vitro translation systems
Recently, methods have been described to pre-pare caged proteins by supplementing in vitro translation systems with cRNA containing a rare or nonsense codon and the complimen-tary anti-codon tRNA charged with a caged amino acid. Judicious choice of the site of incorporation of a caged amino acid can lead to the expression of a caged protein whose activity can be triggered with a pulse of near ultraviolet light26. This strategy has been used to replace the active site aspartate residue of lysozyme with caged aspartate26. Caged pro-teins made by in vitrotranslation systems are usually only characterized by activity meas-urements because of the limited amount of caged protein synthesized27. A similar approach has been used to incorporate site-specific photolabile amino acids into ion chan-nels expressed in Xenopusoocytes28, including a caged nicotinic acetylcholine receptor27. Here, oocytes were co-injected with a caged tyrosine-charged tRNA containing the non-sense anticodon (CUA) and a cRNA of the a-subunit of nicotinic acetylcholine receptor, in which a single nonsense codon (UAG) replaces the tyrosine codon. Full inactivation of receptor channel activity requires masking two specific tyrosine residues (one per a-subunit) at a surface loop of the channel opening. The channel activity of the mem-brane-incorporated (caged) receptor complex is recorded with ms time resolution before and after irradiation of the egg with ms pulses of near ultraviolet light. This study demon-strated that a substantial fraction of acetyl-choline receptor activity can be rapidly generated after irradiating the oocyte with several short pulses of near ultraviolet light27. This powerful approach extends the appli-cations of light-directed activation of protein activity to proteins that might be difficult to purify or to specifically label with a caged reagent, or for caged proteins that might be difficult to reconstitute into model or natural membrane systems.
Summary
Light-directed activation of caged compounds has emerged as a powerful and general technique to address the function and mechanisms of pro-tein activity in complex molecular environ-ments. Photoactivation of biomolecules from ions to second messengers and from metabolites to polypeptides can be used to reveal information about the timing and spatial regulation of the distribution and activity of the biomolecule in cells and tissues. More recently, reagents and labeling techniques have been introduced to cage the activity of almost any biologically active polypeptide by in vitroor in vivolabeling of essential amino acids. These include lysine, tyro-sine, cysteine, serine, threonine, aspartic acid, arginine and glutamic acid, as well as phosphate esters of specific amino acids. Indeed, more progress has been made in the chemistry and photochemistry of caged polypeptides than through their application to address important questions in biology. The present challenge will be to redress this imbalance by further exploit-ing the light-directed activation of caged polypeptides to determine the function and mode of action of components in signaling, energy transduction or metabolic processes.
Gerard Marriott and Jeffery W. Walker
Dept of Physiology, University of Wisconsin-Madison Medical School, 1300 University Avenue, Madison, WI 53706-1532, USA
References
1Marriott, G., ed. (1998) Caged compounds, Methods Enzymol.291, 1–500
2Goldman, Y.E., Hibberd, M.G. and Trentham, D.R. (1984) Initiation of active contraction by photogeneration of adenosine-59-triphosphate in rabbit psoas muscle fibres, J. Physiol. 354, 605–613
3Hess, G.P. (1993) Determination of the chemical mechanism of neurotransmitter receptor-mediated reactions by rapid chemical kinetic techniques, Biochemistry32, 989–1000
4Adams, S. and Tsien, R.Y. (1993) Controlling cell chemistry with caged compounds, Annu. Rev. Physiol. 55, 755–784
5Theriot, J.A. and Mitchison, T.J. (1991) Actin microfilament dynamics in locomoting cells, Nature52, 126–131
6Walker, J.W. et al. (1998) Signaling pathways underlying eosinophil cell motility revealed by using caged peptides, Proc. Natl. Acad. Sci. U. S. A. 95, 1568–1573
7Marriott, G. (1994) Caged protein conjugates and light-directed generation of protein activity: preparation, photoactivation and spectroscopic characterization of caged G-actin conjugates, Biochemistry33, 9092–9097
8Ishihara, A. et al. (1997) Photoactivation of caged compounds in single living cells: an application to the study of cell locomotion, BioTechniques23, 268–274
9Cambridge, S., Davis, R.L. and Minden, J. (1997) Drosophilamitotic domain boundaries as cell fate boundaries, Science277, 825–828 10 Wood, J.S. et al. (1998) A caged protein kinase
inhibitor, J. Am. Chem. Soc. 120, 7145–7146 11 Pillai, V.N.R. (1980) Photoremovable protecting
groups in organic synthesis, Synthesis26, 1–27 12 Fodor, S.P.A. et al.(1991) Light-directed,
spatially addressable parallel chemical synthesis, Science251, 767–773
13 Tatsu, Y. et al. (1996) Solid-phase synthesis of caged peptides using tyrosine modified with a photocleavable protecting group: application to the synthesis of caged neuropeptide Y, Biochem. Biophys. Res. Commun.227, 688–693
14 Pan, P. and Bayley, H. (1997) Caged cysteine and thiophosphoryl peptides, FEBS Lett. 405, 81–85 15 Marriott, G. and Heidecker, M. (1996)
Light-directed generation of the actin-activated ATPase activity of caged heavy meromyosin,
Biochemistry35, 3170–3174 16 Marriott, G. et al. (1998) Light-directed
activation of protein activity from caged protein conjugates, Methods Enzymol.291, 129–151 17 Self, C.H. and Thompson, S. (1996) Light
activatable antibodies: models for remotely activatable proteins, Nat. Med.2, 817
18 Chang, C-Y. et al. (1995) A photogenerated pore-forming protein, Chem. Biol.2, 391–400 19 Senter, P.D. et al.(1985) In vivophoto-activation
of an antibody–toxin complex, Photochem. Photobiol. 42, 231–239
20 Marriott, G., Miyata, H. and Kinosita, K. (1992) Photomodulation of the nucleating activity of a caged actin dimer, Biochem. Int. 26, 943–951
21 Ottl, J. and Marriott, G. (1998) Preparation and photoactivation of caged fluorophores and caged proteins using a new class of heterobifunctional, photocleavable cross-linking reagents, Bioconjugate Chem.9, 143–151
22 Arroyo, J.G. et al. (1997) In vivophotoactivation of caged-thrombin, Thromb. Haemost.78, 791–793 23 Gillespie, P.G. and Corey, D.P. (1997) Myosin
and adaptation by hair cells, Neuron19, 955–958
24 Koegler, H., Rueegg, J.C. and Marriott, G. (1998) Flash photodeprotection of caged myosin: a novel method to trigger contraction in skinned muscle fibers, Biophys. J. 74, A335
25 Root, D.D. and Reisler, E. (1992) Cooperativity of thiol-modified myosin filaments. ATPase and motility assays of myosin function, Biophys. J. 63, 730–740
26 Mendel, D., Ellman, J.A. and Shultz, P.G. (1991) Construction of light-activated protein by unnatural amino acid mutagenesis, J. Am. Chem. Soc. 113, 2758–2760
27 Miller, J.C. et al. (1998) Flash decaging of tyrosine sidechains in an ion channel, Neuron20, 619–624 28 England, P.M. et al.(1997) Site-specific,