www.elsevier.com / locate / bres
Research report
1 1
Inhibition of Na / H
exchange by 5-(N-ethyl-N-isopropyl)-amiloride
reduces free fatty acid efflux from the ischemic reperfused rat cerebral
cortex
a ,
*
a bJohn W. Phillis
, J. Ren , M.H. O’Regan
a
Department of Physiology, Wayne State University School of Medicine, 540 E. Canfield, Detroit, MI 48201-1928, USA
b
Biomedical Sciences, School of Dentistry, University of Detroit Mercy, 8200 W. Outer Drive, Detroit, MI 48219, USA Accepted 29 August 2000
Abstract
Brain tissue acidosis is considered to be a contributor to ischemic brain injury. The deleterious effects of marked acidosis may be
1 1 1
associated with reperfusion and an excessive entry of Na into cerebral neurons and glia as intracellular pH is restored by Na / H exchange. Normalization of pH, with activation of many calcium-dependent and other phospholipases and proteases with pH optima in the neutral or alkaline range, could account for the pronounced elevation in extracellular levels of free fatty acids which occurs during
1 1
reperfusion following cerebral ischemia. In the present investigation we evaluated the effects of inhibition of Na / H exchange with
N-(N-ethyl-N-isopropyl)-amiloride (EIPA; 25mM) applied topically onto the rat cerebral cortex prior to and during ischemia. Free fatty acid levels in cortical superfusates, withdrawn at 10-min intervals from bilateral cortical windows, were analyzed by high pressure liquid chromatography. EIPA application effectively inhibited the increases in arachidonic and linoleic acid release observed in the control rats during reperfusion, and non-significantly depressed that of palmitic and oleic acids. Superfusate levels of glucose, which decline to near zero levels during ischemia and then rebound during reperfusion, were not affected by EIPA administration. Lactate levels in cortical superfusates from EIPA-treated animals rose more rapidly during reperfusion than did those in the control rats and then significantly
1 1
declined towards basal levels. The data indicate that inhibition of Na / H exchange prevented the activation of phospholipases that usually occurs during reperfusion following a cerebral ischemic episode. These results are the first demonstration of such an effect and may provide an explanation for the cerebroprotective effects that have been observed in stroked animals following administration of
1 1
Na / H exchange inhibitors. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Ischemia; Neurotoxicity
1 1
Keywords: Cerebral ischemia; Cerebroprotection; Free fatty acid; Phospholipase; Na / H exchange; Amiloride derivative; N-(N-ethyl-N-isopropyl)-amiloride
1. Introduction protons released in the hydrolysis of ATP and those
resulting from bioenergetic failure with the production of 1
The contributions of acidosis to ischemic brain damage lactate and H [6,9,13,20]. The acidotic hypothesis of have been controversial. Although severe acidosis has been ischemic injury initially received strong support from the linked to aggravation of ischemic injury, there is also demonstration that acidosis, equivalent to that occurring evidence that milder acidosis can have cerebroprotective during ischemia, could damage brain tissue [19]. Explana-effects. Contributors to ischemic acidosis include the tions to account for the injury include dysfunction of enzymes operating at a pH removed from their pK values
1 1
[16]; cellular swelling due to Na / H counter transport
*Corresponding author. Tel.: 11-313-577-6745; fax: 11-313-577- 21 1 21
[7,18] and Ca accumulation due to the Na / Ca 5494.
E-mail address: [email protected] (J.W. Phillis). exchanger operating in a reversed mode [17,22]. ATP 0006-8993 / 00 / $ – see front matter 2000 Elsevier Science B.V. All rights reserved.
21
depletion can also lead to an increase in intracellular Ca (Penthrane). Body temperature was controlled at 378C with with activation of calcium-dependent enzymes, including a rectal probe and an abdominal heating pad. One femoral phospholipases and proteases. Endogenous inhibitors and artery was cannulated for measurement of arterial blood activators of proteases, which are phosphoproteins, are pressure and to obtain arterial blood samples for pH and present in cells and a change in their phosphorylation blood gas measurements. Cerebral ischemia was induced status during ATP depletion could enhance the activity of by coagulating the vertebral arteries via the alar foramina nonlysosomal proteases. and traction on loops placed around the carotid arteries.
A challenge to the acidotic hypothesis of ischemic injury The dorsal surfaces of both cerebral hemispheres were arose from the observation that at the pH typically exposed and, after reflection of the dura mater, oval generated in ischemic brain, NMDA receptor-mediated cortical windows suspended in flexible mounting brackets currents were almost entirely abolished [38,39]. In that were gently placed on both cortices. The dorsal surface of NMDA-activated receptors are considered to be amongst the head around the windows was covered with a stabiliz-the primary mediators of excitotoxic ischemic injury [8], ing gel of 3% agar in artificial cerebrospinal fluid (aCSF).
1 1
these findings suggested that acidosis could actually limit The aCSF contained: Na , 155.8 mEq / l; K , 2.95 mEq / l;
21 21 2
the extent of excitotoxic injury. Mild acidosis was sub- Ca , 2.5 mEq / l; Mg , 1.85 mEq / l; Cl , 141.13 mEq / l; 2
sequently shown to protect hippocampal and cortical HCO , 22 mEq / l; urea, 40.2 mg / dl which had been3
cultures from ischemic-like conditions [12,38] and cortical equilibrated with 95% nitrogen, 5% carbon dioxide (pH and cerebellar cultures against glutamate toxicity [2,15]. 7.3). Monopolar EEG electrodes were placed on the Furthermore, brain acidosis induced by hypercarbic venti- cortical surface within each window. EEGs and arterial lation attenuated focal ischemic injury in vivo [36] and blood pressure were recorded on a Grass polygraph. Two hypoxic-ischemic damage to the immature rat brain [42]. hundred ml of warmed (378C) aCSF were pipetted into Sapolsky et al. [35] subsequently referred to the potential each window at 10-min intervals, after removal of the for acidosis to be neuroprotective under certain circum- previous superfusate sample with Pasteur pipettes. The stances as a ‘paradoxical wrinkle’. temperature of fluids within the windows was maintained The term ‘pH paradox’ was used by Bond et al. [4] to at 378C using a heat lamp, and the windows were then account for the protective action of intracellular acidosis closed with black plastic covers to protect the superfusate on cultured cardiac myocytes exposed to simulated is- contents from light degradation.
chemia, proposing instead that the injury actually occurs Cerebral ischemia was elicited by occluding the carotid during the return to normal pH. These investigators arteries for 20 min. Induction of cerebral ischemia was suggest that although favorable conditions for the activa- manifested by the rapid development of an isoelectric EEG tion of degradative enzymes such as phospholipases and trace from both cortices. After 20 min the carotid snares proteases exist during ischemia, they are inhibited by the were withdrawn and reperfusion verified by both the initial acidotic conditions [5,10]. During reperfusion, the inhibi- sharp decline in arterial blood pressure and visual inspec-tion is released as intracellular pH recovers, with damage tion of the pial vasculature within the windows. Most to mitochondrial and plasma membranes initiating necrotic animals ceased to respire spontaneously at some point or apoptotic processes [21]. Delays in the recovery of during the period of ischemia and were ventilated me-intracellular pH can be instigated in myocytes using chanically.
1 1
inhibitors of Na / H exchangers, including di- Results from three groups of rats are presented in this methylamiloride and HOE 694 [21] or, in the case of report. One group (n59) comprised the control ischemic neuronal tissue cultures, dimethylamiloride or harmaline animals in which the superfusates were aCSF10.05% [44]. In both instances, powerful protective effects of dimethylsulfoxide (vehicle for EIPA). The cerebral cortices maintaining intracellular acidosis were observed. of the second group (n56) were exposed to EIPA (25mM) The present experiments were designed to evaluate in 0.05% dimethylsulfoxide aCSF for 15 min after the
1 1
whether the potent and selective inhibitor of Na / H completion of superfusate collection 2, and 35 min prior to
1 1
exchange, 5-(N-ethyl-N-isopropyl)-amiloride (EIPA) [43], the onset of ischemia. The Na / H exchange inhibitor would block phospholipase activation, measured by the was then present in all subsequent superfusate samples. efflux of free fatty acids (FFAs), in the ischemic / re- For both groups, a standard protocol of superfusate sample perfused rat cerebral cortex as a consequence of its collections was followed. After two basal (10 min) aCSF stabilizing action on the acidotic intracellular pH. collections the animals were exposed to either aCSF or aCSF plus EIPA for 15 min during which the window contents were replaced three times. Two more
followed by 40 min of reperfusion. Superfusate samples EIPA-treated animals and 1 / 6 of the 30-min ischemia rats, were ejected into chilled microvials, centrifuged at 12003 respectively.
g and stored at 2208C. HPLC assays of perfusate-free
fatty acid contents were initiated within a few hours using 3.2. Superfusate glucose and lactate levels previously published procedures [34]. Superfusate glucose
and lactate levels were measured with a YSI Select Mean basal glucose (4.4 mg / dl;|240mM) and lactate Biochemistry Analyzer Version 2.50D (Yellow Springs, (2.0 mg / dl; |225 mM) levels in cerebral cortical
super-OH). fusates were comparable in the three groups of animals
Statistical differences for free fatty acid, glucose and (Figs. 1 and 2). Superfusion of EIPA did not significantly lactate levels between control and EIPA-treated animals affect basal levels of either compound (Fig. 1; collections were determined by a two-tailed Student’s t-test for each 3 and 4). During ischemia, superfusate glucose levels collection period. A P,0.05 was accepted as denoting a declined rapidly to values approaching detection limits and statistically significant difference. All animal procedures reperfusion was associated with marked increases in were approved by the University Animal Care Committee
and were in full accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
3. Results
3.1. Physiological parameters
Although there were significant declines in pH and PaO2
levels, and increases in PaCO levels post-ischemia there2
were no statistically significant differences between the three groups of animals in the physiological parameters of mean arterial blood pressure, blood gases and pH values in arterial blood samples recorded either prior to EIPA administration and ischemia or after 40 min of reperfusion (Table 1). In all groups, the EEG became isoelectric within a few seconds of the onset of ischemia. Some recovery of EEG activity became apparent in 4 / 9 control animals, 3 / 6
Table 1
Mean arterial blood pressure (MABP) and arterial blood gases in control and EIPA (25mM)-treated rats prior to 20 min or 30 min of ischemia and
a,b
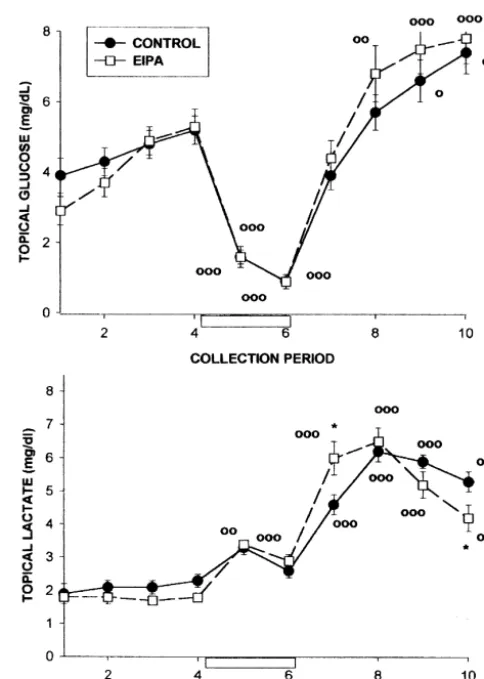
Fig. 1. Effect of 5-(N-ethyl-N-isopropyl)-amiloride (EIPA, 25mM), an
30 min ischemia 7.4260.01 7.3060.03* 1 1
inhibitor of Na / H exchange, on basal and ischemia-evoked four-vessel occlusion levels of glucose and lactate in cerebral cortical superfusates. PaCO2 20 min control 35.661.2 42.262.0* The line plots show the time course of changes in cortical superfusate
EIPA 37.060.9 46.062.8*
levels of these compounds before, during and after a 20-min period of 30 min ischemia 35.861.6 46.262.2*
four-vessel cerebral ischemia (collections 5 and 6, open box). Effluxes of glucose and lactate are compared in control (0.05% DMSO in aCSF;d) PaO2 20 min control 94.963.7 76.163.8** and EIPA (25 mM, applied topically; h) treated animals. EIPA was
EIPA 89.863.4 74.761.3**
administered for 15 min prior to the start of collection 3 and was present 30 min ischemia 92.862.8 73.866.2*
in all subsequent collections. Statistically significant differences between
a
All values are means6S.E.M. n59 for control rats (20 min ischemia); 6 superfusate glucose and lactate levels in control and EIPA-treated animals for the EIPA group and 6 for the 30-min ischemia group. were determined by a two-tailed Student’s t-test; *P,0.05. Significant
b o
Significance values in comparison with pre-ischemic levels: *P,0.05, increases in glucose or lactate levels above basal values; P,0.05,
oo ooo
3.3. Free fatty acid efflux
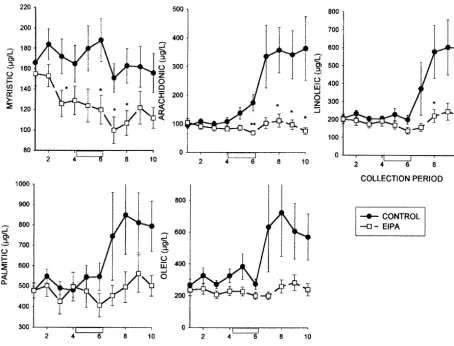
Basal extracellular levels of FFA in cortical superfusates of the control group were: arachidonic acid, 103611mg / l (329635 nM); myristic acid, 172616mg / l (755670 nM); linoleic acid, 213618 mg / l (760664 nM); palmitic acid, 499635 mg / l (19516137 nM) and oleic acid, 297639 mg / l (10526138 nM). During a 20-min ischemia, the levels of arachidonic acid rose slightly, whilst those of the other FFAs were not affected (Fig. 3). Following reperfu-sion there were significant increases in the superfusate levels of all of the FFAs, with the exception of myristic acid. Application of EIPA (25mM) reduced basal levels of myristic acid, but not those of the other FFAs, and there were significant reductions in the ischemia / reperfusion evoked effluxes of arachidonic and linoleic acids and non-significant reductions in superfusate levels of palmitic and oleic acids in comparison with their control ischemia / reperfusion evoked efflux (Fig. 3). The small increases in arachidonic, linoleic, palmitic and oleic acids during reperfusion were not significant.
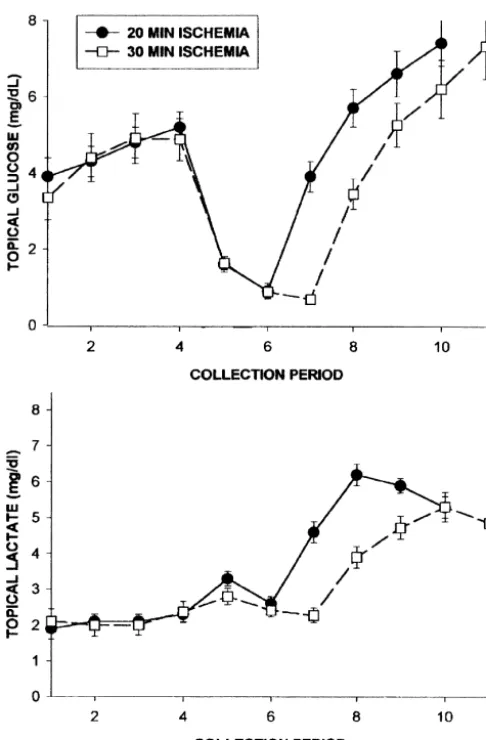
Extending the duration of the cerebral ischemia of rats in the third group to 30 min resulted in decreases in the efflux of linoleic, palmitic and oleic acids during ischemia with a marked increase in the efflux of arachidonic, linoleic, palmitic and oleic acids again being clearly coincident with reperfusion (Fig. 4).
4. Discussion
Fig. 2. Comparison of the effects of 20-min versus 30-min duration of The finding that FFA efflux primarily occurred after the four-vessel occlusion cerebral ischemias on glucose and lactate levels in
onset of reperfusion implies that lipase activity is subject cerebral cortical superfusates.
to inhibition during the preceding ischemia. This conclu-sion received further support from our observation that effluxes of linoleic, palmitic and oleic acids were de-pressed during a 30-min period of ischemia (Fig. 4). glucose which significantly (P,0.01) exceeded those in Calcium-dependent PLA activity is recognized as being2
the pre-ischemic superfusates. Application of EIPA did not very sensitive to pH, with optimal activation occurring affect the pattern of changes in glucose efflux during between pH values of 7.4 and 9.0 for different forms of the ischemia / reperfusion. Extending the period of ischemia to enzyme [10,23]. The development of acidosis during 30 min, in the absence of EIPA, did not affect the pattern anaerobic glycolysis would, therefore, be expected to of topical glucose levels (Fig. 2). severely curtail the activity of this enzyme. Other pro-Lactate levels in the control group exhibited a charac- teases, including calpain-like proteases, are also pH depen-teristic pattern of responses to ischemia with an initial dent, and it has been suggested that mammalian cells increase followed by a decrease during the two collection appear to use the acidosis of ATP depletion as a defense periods. Levels rose rapidly during reperfusion to values mechanism to suppress pH-dependent processes such as exceeding 6 mg / dl and remained elevated during the degradative proteolysis and phospholipid hydrolysis [5]. remaining two collection periods (Fig. 1). Application of The pattern of FFA acid release into the interstitial EIPA resulted in a similar pattern of release during environment observed in the present study differs sig-ischemia. Lactate levels during the initial reperfusion nificantly from that of changes in FFA tissue levels in the collections (7) were significantly higher than those in the ischemic / reperfused brain. With the exception of ara-control animals, and then declined to be significantly lower chidonic acid, which increased slightly during ischemia, in collection 10. After 30 min of ischemia, peak lactate increases in FFA levels in the cortical superfusates only levels were significantly depressed during reperfusion (Fig. became apparent during the initial 10-min reperfusion
Fig. 3. Ischemia-evoked release of myristic, arachidonic, linoleic, palmitic and oleic acids into rat cerebral cortical superfusates. The line plots show the time course of changes in superfusate concentrations (mg / l) of these free fatty acids before, during and after a 20-min period of four-vessel cerebral ischemia (collections 5 and 6, open box). 5-(N-Ethyl-N-isopropyl)-amiloride (EIPA; 25mM) was added to the artificial cerebrospinal 15 min prior to the start of superfusate collection 3 and was present during all subsequent collections (line plots with open squares). The EIPA data are compared with those from control ischemic animals (closed circles). Data represent means6S.E.M. Statistically significant differences between data at each time point were determined by the Student’s t-test. *P,0.05.
experiment. In contrast, tissue levels of FFAs in brain mPLA would account for the efflux of FFAs at the onset2
increase during the ischemic episode and then decline of reperfusion. It appears that FFAs generated intracellu-during reperfusion [1,3,33,41,45]. The discrepant patterns larly during ischemia are not readily able to penetrate the of change in FFA levels in the two groups of experiments plasma membrane, thus accounting for their absence in suggests a possibility that different pools of FFA are being cortical superfusates during the period of ischemia. measured. There is evidence that in vitro rat cortical Topically applied EIPA (25mM) inhibited the ischemia /
21
neurons possess four PLA2 activities, including Ca - reperfusion-evoked release of FFAs. This result is of
21
independent or Ca -dependent membrane bound PLA2 particular interest for comparisons with an earlier study on (mPLA )2 and cytoplasmic PLA2 (cPLA )2 activities the effects of EIPA (25mM) on amino acid release from
21
[32,37]. Only the activity of the Ca -dependent mPLA2 the ischemic / reperfused rat cerebral cortex [26]. In that
21 1 1
was inhibited at low pHs whilst Ca -independent mPLA2 study, the Na / H exchange inhibitor attenuated the activity and the cPLA activities were not [37]. The release2 ischemia-evoked release of several amino acids, including of FFAs observed in the present study at the onset of that of aspartate, glutamate, GABA, taurine and phos-reperfusion may therefore have been a consequence of the phoethanolamine. Amino acid release tends to be most
21
Fig. 4. Free fatty acid levels in cerebral cortical superfusates prior to, during and following a 30-min period of four-vessel occlusion cerebral ischemia (collection periods 5, 6, and 7).
1 1
duration of the experiment. The reductions in ischemia- recovery of pH by incubation with blockers of Na / Hi
evoked amino acid release were attributed to EIPA’s exchange (dimethylamiloride or harmaline) also had
1 1
inhibition of the Na / H exchanger which is presumed to protective effects. Measurements of pH confirmed that thei
have reduced ischemia-evoked cell swelling and a ‘regula- blockers slowed recovery from intracellular acidosis and tory volume decrease (RVD)’ amino acid release from that more rapid pH recovery was correlated with injury, asi
cortical cells [29,30]. In contrast, FFA efflux, with the measured by lactate dehydrogenase release. Ferimer et al. exception of a small ischemia-related increase in arach- [11] have established the ability of a systemically adminis-idonic acid, coincided with the initial phase of reperfusion tered amiloride derivative (methylisobutylamiloride) to and not with the ischemia itself. This observation was significantly reduce cardiac pH in rats subjected to arresti
reinforced by the additional delay in FFA efflux when the with 15 min of reperfusion. They also established that ischemia was extended to 30 min (Fig. 4). Evidence that methylisobutylamiloride did not change intracellular pH phospholipase activation is involved in both amino acid from its normal, non-ischemic, value.
and FFA efflux from the rat cerebral cortex has been An alternative explanation for the EIPA-induced attenua-forthcoming from experiments in which PLA , PLC or2 tion of FFA efflux described in this report could be a direct melittin (to activate PLA ) applied topically, in the2 inhibition of phospholipases by this amiloride derivative. absence of ischemia, elicited both amino acid and FFA We are not aware of such an action of this group of efflux [24,31]. The inhibitory effect of EIPA on FFA efflux compounds and furthermore, with the exception of
myris-1 1
can be attributed to its block of the Na / H exchanger tic acid, there was no evidence that EIPA administration and a prolongation of intracellular acidosis. affected basal, pre-ischemic levels of FFA efflux (Fig. 3). Intracellular acidosis during and after simulated is- EIPA has previously been reported to have a significant chemia / reperfusion protects cultured cardiac myocytes ability to block ionic currents through the NMDA receptor against injury [21]. The onset of injury is delayed for as activated channels but this effect was apparently not a long as the cells remain acidotic and injury is initiated as consequence of an action at the NMDA receptor, but was
1 1
reduces NMDA receptor activation, glutamate neurotoxicity, and into the mechanisms by which EIPA, an inhibitor of
1 1 oxygen–glucose deprivation neuronal injury in cortical cultures,
Na / H exchange may exert its neuroprotective action in
Brain Res. 506 (1990) 339–342.
1
the ischemic brain [25]. By inhibiting the exchange of Na [13] P.W. Hochachka, T.P. Mommsen, Protons and anaerobiosis, Science 1
for intracellular H , EIPA reduces ischemia-evoked cell 219 (1983) 1391–1397.
[14] T.L. Hoffman, J.C. La Manna, S. Pundik, W.R. Selman, T.S. swelling and delays normalization of brain intracellular pH
Whittingham, R.A. Ratcheson, W.D. Lust, Early reversal of acidosis during reperfusion. Acidosis, within limits, protects cells
and metabolic recovery following ischemia, J. Neurosurg. 81 (1994) from injury by inhibiting the activity of phospholipases 567–573.
and proteases which have pH optima in the neutral or [15] D. Kaku, R. Giffard, D. Choi, Neuroprotective effects of glutamate antagonists and extracellular acidity, Science 260 (1993) 1516. alkaline range. By preventing a rapid recovery of pH, EIPA
[16] H. Kalimo, S. Rehncrona, B. Soderfeldt, Y. Olsson, B. Siesjo, Brain effectively inhibits the rise in free fatty acid formation and
lactic acidosis and ischemic cell damage. 2. Histopathology, J. proteolytic processes which would otherwise occur during Cereb. Blood Flow Metab. 1 (1981) 313–327.
reperfusion, thus preserving plasma, mitochondrial and the [17] M. Karmazyn, X.T. Gan, R.A. Humphreys, H. Yoshida, K. 1 1
other intracellular structures necessary for recovery. In- Kusumoto, The myocardial Na –H exchange structure, regulation, and its role in heart disease, Circ. Res. 85 (1999) 777–786. tracellular calcium levels will eventually normalize
follow-¨
[18] O. Kempski, F. Staub, M. Jansen, F. Schodel, A. Baethmann, Glial ing ATP recovery, reducing damaging effects of the return
swelling during extracellular acidosis in vitro, Stroke 19 (1988) to a normal intracellular pH. These results further illustrate 385–392.
1 1
the therapeutic potential of Na / H exchange inhibitors [19] R.P. Kraig, C.K. Petito, F. Plum, W.A. Pulsinelli, Hydrogen ions kill at concentrations reached in ischemia, J. Cereb. Blood Flow Metab. for the treatment of cerebral ischemia following stroke or
7 (1987) 379–386. cardiac arrest.
[20] H.A. Krebs, H.F. Woods, K.G.M.M. Alberti, Hyperlactatemia and lactic acidosis, Essays Med. Biochem. 1 (1975) 81–103.
[21] J.J. Lemasters, J.M. Bond, E. Chacon, I.S. Harper, S.H. Kaplan, H. Obata, D.R. Trollinger, B. Herman, W.E. Cascio, The pH paradox in
Acknowledgements
ischemia-reperfusion injury to cardiac myocytes, EXS 76 (1996) 99–114.
1 21
Supported by USPHS award NS 26912. [22] T. Matsuda, K. Takuma, A. Baba, Na –Ca exchanger: physiology and pharmacology, Jpn. J. Pharmacol. 74 (1997) 1–20.
[23] N. Moskowitz, S. Puszkin, W. Schook, Characterization of brain synaptic vesicle phospholipase A2 activity and its modulation by
References calmodulin prostaglandin E , prostaglandin F , cyclic Amp, and
2 2a
ATP, J. Neurochem. 41 (1983) 1576–1586.
[1] K. Abe, K. Kogure, H. Yamamoto, M. Imazawa, K. Miyamoto, [24] M.H. O’Regan, M. Smith-Barbour, L.M. Perkins, J.W. Phillis, A Mechanism of arachidonic acid liberation during ischemia in gerbil possible role for phospholipases in the release of neurotransmitter cortex, J. Neurochem. 48 (1987) 503–509. amino acids from ischemic rat cerebral cortex, Neurosci. Lett. 185 [2] N. Andreeva, B. Khodorov, E. Stelmashook, S. Sokolova, E. (1995) 191–194.
Cragoe, L. Victorov, 5-(N-Ethyl-N-isopropyl) amiloride and mild [25] J.W. Phillis, A.Y. Estevez, L.L. Guyot, M.H. O’Regan, 5-(N-ethyl-N-1 1
acidosis protect cultured cerebellar granule cells against glutamate- isopropyl)-amiloride, an Na –H exchange inhibitor, protects ger-induced delayed neuronal death, Neuroscience 49 (1992) 175–181. bil hippocampal neurons from ischemic injury, Brain Res. 839 [3] N.G. Bazan, Effects of ischemia and electroconvulsive shock on (1999) 199–202.
fatty acid pool in brain, Biochim. Biophys. Acta 218 (1970) 1–10. [26] J.W. Phillis, M.H. O’Regan, D. Song, 5-(N-ethyl-N-isopropyl)-[4] J.M. Bond, E. Chacon, B. Herman, J.J. Lemasters, Intracellular pH amiloride inhibits amino acid release from the ischemic rat cerebral
21 1 1
and Ca homeostasis in the pH paradox of reperfusion injury to cortex: role of Na –H exchange, Brain Res. 812 (1998) 297–300. neonatal rat cardiac myocytes, Am. J. Physiol. 265 (1993) C129– [27] J.W. Phillis, M. Smith-Barbour, L.M. Perkins, M.H. O’Regan,
C137. Characterization of glutamate, aspartate and GABA release from
[5] S.F. Bronk, G.J. Gores, pH-dependent nonlysosomal proteolysis ischemic rat cerebral cortex, Brain Res. Bull. 34 (1994) 457–466. contributes to lethal anoxic injury of rat hepatocytes, Am. J. Physiol. [28] J.W. Phillis, D. Song, L.L. Guyot, M.H. O’Regan, Failure of 264 (1993) G744–G751. kynurenic acid to inhibit amino acid release from the ischemic rat [6] W.B. Busa, R. Nuccitelli, Metabolic regulation via intracellular pH, cerebral cortex, Neurosci. Lett. 273 (1999) 21–24.
Am. J. Physiol. 246 (1984) R409–R438. [29] J.W. Phillis, D. Song, M.H. O’Regan, Inhibition by anion channel [7] R.C.C. Chang, N. Plesnila, F. Ringel, C. Gronlinger, F. Staub, A. blockers of ischemia-evoked release of excitotoxic and other amino
Baethmann, Role of protein kinase C in acidosis induced glial acids from rat cerebral cortex, Brain Res. 758 (1997) 9–16. swelling — current understanding, Acta Neurochir. 70 (Suppl.) [30] J.W. Phillis, D. Song, M.H. O’Regan, Tamoxifen, a chloride channel (1997) 225–227. blocker, reduces glutamate and aspartate release from the ischemic [8] D.W. Choi, Excitotoxic cell death, J. Neurobiol. 23 (1992) 1261– cerebral cortex, Brain Res. 780 (1998) 352–355.
1276. [31] J.W. Phillis, D. Song, M.H. O’Regan, Melittin enhances amino acid [9] M. Erecinska, I.A. Silver, ATP and brain function, J. Cereb. Blood and free fatty acid release from the in vivo cerebral cortex, Brain
Flow Metab. 9 (1989) 2–19. Res. 847 (1999) 270–275.
[10] A.A. Farooqui, H.-C. Yang, T.A. Rosenberger, L.A. Horrocks, [32] D. Piomelli, P. Greengard, Bidirectional control of phospholipase A2 21
Phospholipase A2 and its role in brain tissue, J. Neurochem. 69 activity by Ca / calmodulin-dependent protein kinase II, cAMP-(1997) 889–901. dependent protein kinase, and casein kinase II, Proc. Natl. Acad. [11] H.N. Ferimer, K.L. Kutina, J.C. LaManna, Methyl isobutyl Sci. USA 88 (1991) 6770–6774.
[34] I. Saluja, M.H. O’Regan, D. Song, J.W. Phillis, Activation of cPLA2 [41] A. Umemura, H. Mabe, H. Nagai, F. Sugino, Action of phospholip-PKC and ERKs in the rat cerebral cortex during ischemia / reperfu- ases A and C on free fatty acid release during complete ischemia in2
sion, Neurochem. Res. 24 (1999) 669–677. rat neocortex. Effect of phospholipase C inhibitor and N-methyl-D -[35] R.M. Sapolsky, J. Trafton, G.C. Tombaugh, Excitotoxic neuron aspartate antagonist, J. Neurosurg. 76 (1992) 648–651.
death, acidotic endangerment, and the paradox of acidotic protec- [42] R.C. Vannucci, J. Towfighi, D.F. Heitjan, R.M. Brucklacher, Carbon tion, Adv. Neurol. 71 (1996) 237–244. dioxide protects the perinatal brain from hypoxic-ischemic damage: [36] R.P. Simon, M. Niiro, R. Gwinn, Brain acidosis induced by an experimental study in the immature rat, Pediatrics 95 (1995)
hypercarbic ventilation attenuates focal ischemic injury, J. Phar- 868–874.
macol. Exp. Ther. 267 (1993) 1428–1431. [43] P. Vigne, C. Frelin, E.J. Cragoe, M. Lazdunski, Ethylisopropyl-[37] N. Stella, L. Pellerin, P.J. Magistretti, Modulation of the glutamate- amiloride: a new and highly potent derivative of amiloride for the
1 1
evoked release of arachidonic acid from mouse cortical neurons: inhibition of the Na / H exchange system in various cell types, involvement of a pH-sensitive membrane phospholipase A , J.2 Biochem. Biophys. Res. Commun. 116 (1983) 86–89.
Neurosci. 15 (1995) 3307–3317. [44] J. Voronov, A. Thomas, D. Jo, Protective effects of extracellular [38] G.C. Tombaugh, R.M. Sapolsky, Mild acidosis protects hippocam- acidosis and blockade of sodium / hydrogen exchange during re-pal neurons from injury induced by oxygen and glucose deprivation, covery from metabolic inhibition in neuronal tissue culture, J. Brain Res. 506 (1990) 343–345. Neurochem. 67 (1996) 2379–2389.
[39] S.F. Traynelis, S.G. Cull-Candy, Proton inhibition of N-methyl-D- [45] S. Yoshida, K. Abe, R. Busto, B.D. Watson, K. Kogure, M.D. aspartate receptors in cerebellar neurons, Nature 345 (1990) 347– Ginsberg, Influence of transient ischemia on lipid-soluble anti-350. oxidants, free fatty acids and energy metabolites in rat brain, Brain [40] R. Tyson, J. Peeling, G. Sutherland, Metabolic changes associated Res. 245 (1982) 307–316.