Variation of phosphorus loss from a small
Catchment in south Devon, UK
Richard McDowell
∗,1, Stephen Trudgill
Department of Geography, University of Cambridge, Downing Place, Cambridge CB2 3EN, UK
Received 16 June 1999; received in revised form 23 November 1999; accepted 23 November 1999
Abstract
The application of fertilizers and manure in excess of plant requirements has resulted in an accumulation in soil phosphorus (P), and increased potential for P loss. To develop P-based catchment management plans, we need to be able to estimate the impact of soil P on water draining a catchment. A 12-month investigation (August 1997–July 1998) determined the temporal change of soil P forms and soluble reactive P (SRP) in stream discharge in a small catchment of mixed landuse (cereal crops (Triticum aestivum and Hordeum sativum), root crops (Solanum tuberosum and Brassica sp.), grassland (Phleum
pratense) and woodland (largely Castanea sativa)), in south Devon, UK. This included monthly sampling of soils for sodium
bicarbonate extractable P (Olsen P), calcium chloride extractable P (CaCl2-P) on wet and air-dry soil, organic carbon and
pH. Also available were weekly data for stream discharge and SRP concentration during 1987–1989 and 1994–1998, which enabled an 8-year mean to be calculated for each month. All forms of soil P exhibited seasonal variation, with a late summer maximum and late winter minimum. Olsen P and CaCl2-P were related by a quantity–intensity relationship. Above a certain
value of Olsen P, termed the change point, CaCl2-P increased more per unit Olsen P than below this point. The change point
remained virtually constant throughout the year, never deviating more than 5 mg kg−1from a mean value of 31 mg kg−1Olsen
P. Changes in stream SRP concentrations for the monthly means for 8 years data were correlated only with CaCl2-P from dry
soil. Plots of cumulative SRP export against cumulative discharge over the 8-year data set suggested that SRP loss was limited by the supply of SRP from the soil matrix. Olsen P for root and cropping soils was twice that needed for maximum yields. Thus, to reduce SRP loss, P fertilizer applications should be stopped to allow Olsen P to decrease below the change point. The use of CaCl2-P and the change point has the potential to form the basis of simple environmental management planning
at the catchment scale. © 2000 Elsevier Science B.V. All rights reserved.
Keywords: Phosphorus loss; Catchment; Change point; Soil P; SRP
∗Corresponding author. Tel.:+44-1223-333399; fax:+44-1223-333392.
E-mail address: [email protected] (R. McDowell) 1Present address: USDA-ARS, Posture Systems and Watershed Management Laboratory, Building 3702, Curtin Road, University Park, PA 16802-3702, USA.
1. Introduction
Phosphorus (P) is a primary factor in the nutrition of plants and the eutrophication of surface waters. Soils with a high soil P concentration will generally give rise to high P concentrations in runoff (surface and sub-surface). Pote et al. (1996) showed that P concen-tration in surface runoff was closely correlated (r≈1) to water extractable P in the topsoil. Smith et al. (1995)
related the increase in soluble P in drainflow from a grassland catchment to a rise in soil Olsen P concentra-tions. Heckrath et al. (1995) showed that the concen-tration of dissolved (<0.45mm) reactive P, dissolved organic P and total P were linearly related to Olsen P above a certain point (at about 60 mg kg−1Olsen P). This point, termed the ‘change point’, is also evident when comparing molybdate reactive P (MRP), essen-tially inorganic P, in 0.01M CaCl2extracts with Olsen P in laboratory extractions (Brookes et al., 1997). This comparison has been suggested, in the absence of data for P concentrations in drainage water, as an indicator to predict soil P concentrations above which P losses to drainage water become environmentally significant. Seasonal changes in inorganic P (Pi) concentrations extractable from field soils under various vegetation types is well known. A 3-fold increase in extractable Pi during summer was found in an unfertilized plot in Scotland by Smith (1959). Concentrations of Pi extracted using anion exchange resin were greatest during the summer in arable and grassland ley plots at Rothamsted and Woburn, UK (Garbouchev, 1966). Similar variation occurred in arable and grassland plots sequentially extracted with resin, NaHCO3and NaOH from sandy soils in Denmark (Magid and Nielsen, 1992). Saunders and Metson (1971) and Goodwin et al. (1998) noted that seasonal variation of Pi extracted by anion exchange resin and Olsen’s reagent (NaHCO3) from improved grassland soils exhibited a similar trend to Pi in 0.01M CaCl2 soil extracts and soil solution. A seasonal variation in wa-ter extractable Piconcentrations occurred in an arable soil (Kuo and Jellum, 1987). Shand et al. (1994) showed the maximum concentration of Pi in soil so-lution of three P-deficient Cambisols in NE Scotland occurred in summer (August). Such seasonal varia-tion could affect the timing of soil sampling to assess fertilizer requirements, the amount of P available for plant uptake and the loss of P in runoff (surface or sub-surface).
The Slapton Wood catchment in south Devon, UK is a small (0.94 km2) second order tributary of the River Gara which drains into Slapton Ley, the largest body of freshwater in south-west England. Since late 1969, continual monitoring of stream discharge and weekly water sampling at this site, along with three other catchments has occurred, aimed at quantifying inputs of water, sediments and solutes into the lake. Of
the total annual runoff, only 1.15% is surface-runoff (Troake and Walling, 1973). During winter, a lag time of several days occurs between rainfall and the maxi-mum baseflow in stream discharge. This is indicative of a catchment where sub-surface runoff is favorable (Burt et al., 1983; Burt, 1988). It is therefore likely that P concentrations in runoff may reflect soil P con-centrations if little water bypasses the soil in macro-pore flow. However, it is recognised that a significant amount of total P loss and stream P concentration may be as particulate P (PP) or desorbed from PP in sur-face runoff during intense rainfall.
Seasonal dynamics in catchments cause changes in stream P concentrations. Many researchers have es-tablished a linear relationship between the log of flow rates and the log of stream dissolved or soluble reac-tive P (SRP) concentrations (e.g., Lennox et al., 1997). Large SRP concentrations occur during periods of low flow in summer (Leinweber, 1998). However, most SRP load is lost during periods of high flow in winter. Xue et al. (1998) used the theory of first-order kinet-ics in a plot of cumulative P export versus cumulative flow to describe P loss in tile drainage. They suggested that the exhaustion of P from the readily leachable soil P pool caused a decrease in cumulative P export compared to cumulative flow. Hodgkinson and With-ers (1998) used the same type of plot to show that most P was lost during winter. During a single runoff event, P concentrations will be affected by soil wa-ter residence times and dilution, but the potential for phosphorus loss in runoff and for plant nutrition will always be affected by the amount of soil P, and that which can be rapidly released into soil solution.
2. Materials and methods
2.1. Site
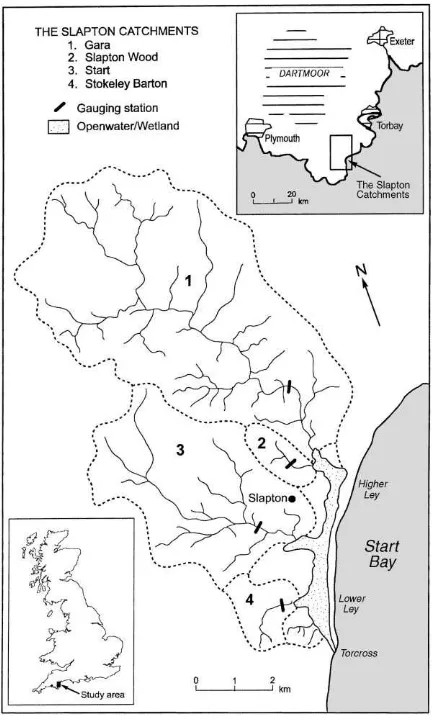
The Slapton Wood catchment in south Devon, England, is the lowest tributary of the River Gara (Fig. 1). Impermeable Dartmouth slates underlie the small drainage basin (0.94 km2). The slates have weathered to give a regolith of 1–2 m and freely draining acid brown earth soils (Eutric Cambisol), of pH 4–5, with 300–400 g kg−1 clay, 300–400 g kg−1 silt and the remainder being sand. The catchment is dominated above a 90 m contour by a plateau with slopes below 5◦, largely under arable farming. Below
Fig. 1. The Slapton Catchments.
90 m the valley side slopes are much steeper up to 25◦under grass or wood.
Climate data (rainfall and temperature) representa-tive of the catchment is measured at a nearby Field Centre 800 m away. The catchment has a mild, moist, maritime climate with mean temperatures of 5.7◦C in February and 15.8◦C in July and an annual mean tem-perature of 10.5◦C. On average, temperatures fall be-low freezing only 22 times a year. The long-term mean rainfall is quoted by Ratsey (1975) as 1051 mm per an-num. Mean annual rainfall for the period 1961–1985 was 1035 mm. On average, 1 mm of rainfall falls 135 days of the year and 10 mm of rainfall falls 35 days of the year (Ratsey, 1975). Although the catchment receives enough moisture for agriculture, mean an-nual evaporation exceeds 400 mm resulting in drought conditions during some warm months (Troake and Walling, 1973).
2.2. Stream analysis
Stream discharge was measured using a 120◦ thin plate V-notch weir and an Ott R16 stage recorder. Spot samples of stream water, taken at weekly intervals are filtered through a glass fibre filter paper (GF/C) and stored at 4◦C until analyzed for P. This is defined as SRP, essentially orthophosphate.
A continuous discharge and SRP data set for the period 1987–1989 and 1994–1998, without missing values of longer than 2 weeks is used in this study. Outliers in SRP and discharge (defined as >3 standard deviations from the geometric mean) were removed from the data set. This accounted for less than 0.5% of all observations. Monthly SRP load averaged over the 8 years was calculated using an interpolation pro-cedure (Walling and Webb, 1982):
Pn
i=1(CiQi) Pn
i=1Qi
QrK (1)
where Ci is the instantaneous individual SRP concen-tration (mg l−1), Q
i is the instantaneous discharge at time of sampling (l s−1), Qris the mean discharge for the time of record (l s−1) and K is the conversion fac-tor to take account of period of record.
Walling and Webb (1982) of calculating loads. How-ever, given the poor nature of rating curves between SRP and flow which could be used to estimate load by extrapolation (r2generally<0.10, data not given) and the frequency of sampling, this method was seen as most appropriate. However, it must be recognized that without more intensive sampling, some amount of error is to be expected.
2.3. Soil sampling and analyses
Six soil samples of the 0–7.5 cm depth of 10 fields (three grassland (Phleum pratense), cereal cropping (Triticum aestivum and Hordeum sativum), root crop-ping fields (Solanum tuberosum and Brassica sp.) and one woodland field (largely Castanea sativa)) were taken at monthly intervals from August 1997 to July 1998, excluding January 1998. Each sample was sieved to 2 mm. SRP was determined in filtered 0.01M CaCl2 extracts of wet and air-dried soils (CaCl2-P) and 0.5M NaHCO3 (adjusted to pH 8.5 with NaOH [Olsen P]) extracts of air-dried soils. Soils had been shaken for 30 min using soil:solution ratio of 1:20 for Olsen P (Olsen et al., 1954) and 1:5 for CaCl2-P (Schofield, 1955). Soils were extracted field moist with 0.01M CaCl2 in order to minimize disturbance of soil solution chemistry. Results for 0.01M CaCl2 extracts of wet soils are presented on an air-dry basis following a gravimetric moisture determination. Mi-crobial biomass P was determined on moist soils of the cereal, root and grassland landuses from the June sampling (not enough woodland soil was available), using the method of Brookes et al. (1982). Organic carbon (C) was determined on oven dry soil (Grewal et al., 1991) and soil pH in water using a 1:2.5 soil: solution ratio. The kinetics of SRP release in 0.01M CaCl2extracts of air-dry soils at either low and high Olsen P for each landuse from the June sampling was determined on filtered extracts after 2, 10, 30, 120, 300 and 1440 min and fitted to an expanded Elovich equation (Polyzopoulos et al., 1986):
Q=[ln(ab)+ln(t+c)]
b (2)
where Q is the amount (mg kg−1) of released P at time t, t is the desorption time (min) and a, b and c are constants.
The equation was fitted using nonlinear regression (SPSS v6.0) and the fit assessed through a linear plot of observed versus predicted values giving an r2value. The r2value is given, since nonlinear regression does not yield true r2values.
A preliminary analysis of variance using Minitab (v8.0) showed that four groups of data could separate according to their landuse. The management of each field has remained relatively unchanged during the 8 years of stream data. Data from each landuse were pooled (three fields each for soils under cereal crops, root crops and grassland), resulting in an acceptable standard error of the mean commonly less than 5% from 18 samples covering an area of 1.2 ha. Samples from the woodland landuse commonly had a standard error less than 9%.
3. Results
3.1. Soil characteristics
Table 1 gives the mean, standard error of the mean and range of pH and organic C (g kg−1) for each lan-duse during the study period and microbial biomass P (mg kg−1 Olsen P) for the cereal, root and grassland soils from the June sampling. No systematic or sig-nificant variation in pH of organic C between months was noted. Microbial biomass P was greatest in the grassland soils and greater in the root soils than the cereal soils. During the study period, pH ranged from 3.47 in the woodland soil to 7.10 in soil under cere-als. Organic C ranged from 0.26 g kg−1in a grassland soil to 1.87 g kg−1 in a woodland soil. On average, soils under root and cereal cropping had a significantly higher pH (p<0.01) and lower organic C than soils under grassland or woodland. One field that had been used for cropping 3 years before being sown to grass caused low values of organic C in grassland.
Table 1
Mean (±standard error of the mean), with range given below, for topsoil (0–7.5 cm) pH in water (1:2.5 ratio), organic C and microbial biomass P for each landuse
Landuse pH Organic C (g kg−1) Microbial biomass P
(mg kg−1 Olsen P)
Cereals 6.23 (0.08) 0.41 (0.20) 26.9 (2.9)
5.22–7.10 0.28–0.52 18.7–39.5
Root 6.01 (0.06) 0.38 (0.17) 32.7 (2.4)
5.12–6.97 0.29–0.51 28.5–44.5
Grassland 5.68 (0.04) 0.52 (0.20) 42.5 (5.4)
4.83–6.03 0.26–0.85 38.3–55.1
Woodland 4.63 (0.12) 1.08 (0.58) n.d.a
3.47–5.17 0.74–1.87
an.d.=not determined.
0.951. The quantity of desorbed P and the rate of P desorbed was assessed through the quantities and rates of P released after 30 and 1440 min (Table 2). These data show that more P is released and at a faster rate with increasing Olsen P.
Fig. 2. Concentration of CaCl2-P with time for low and high Olsen P grassland, cereal and root soils (symbols) fitted to the expanded Elovich equation (line).
3.2. Soil moisture and temperature
Table 2
The initial and final quantity and rate of desorbed P for soils of each landuse at one or two Olsen P concentrations Landuse r2value Olsen P Desorbed soil P Soil P desorption rates
(mg kg−1) (mg kg−1h−1) (mg kg−1h−1)
Initiala Finalb Initial Final
Grassland 0.972∗∗∗ 20 0.376 0.940 0.752 0.039
0.969∗∗∗ 39 0.865 1.729 1.316 0.054
Cereal 0.989∗∗∗ 23 0.418 0.964 0.836 0.040
0.951∗∗∗ 47 1.234 1.512 2.468 0.063
Root 0.990∗∗∗ 26 0.451 0.977 0.902 0.040
0.982∗∗∗ 55 1.429 1.767 2.857 0.074
Woodland 0.997∗∗∗ 8 0.376 0.602 0.752 0.025
aInitial=after 30 min. bFinal=after 1440 min.
∗∗∗Indicates significant at the p<0.01 level.
compared to a long-term mean of 1051 mm (Ratsey, 1975). Despite the greater than average annual rain-fall, only 9.4 mm fell during February compared to a long-term average of 86 mm. This caused low soil
decreasing evaporative soil moisture loss. In contrast to February, greater than average rainfall (120 mm for November and 122 mm for January) fell during November and January affecting soil moisture espe-cially in the grassland landuse. The seasonal pattern in soil temperature at 25 cm was normal, except for a lower than average temperature for April. Soil tem-perature was greatest in August and lowest in January.
3.3. Soil P forms
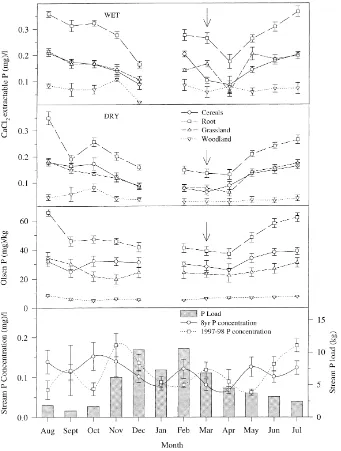
The monthly concentrations and variation in Olsen P and wet and dry CaCl2-P for each landuse, is shown in Fig. 4. Mean values of Olsen P within each landuse generally decreased in order from root>cereal>grassland>woodland for each month. Within each landuse there is a clear seasonal pattern in Olsen P concentration, which has been noted pre-viously, and related to the control and release of P by the microbial biomass (Seeling and Zasoski, 1993). The maximum concentration of Olsen P occurred in either July or August, while the lowest concentration occurred in winter.
Phosphorus extracted from wet and dry soil by 0.01M CaCl2 was an order of magnitude lower than Olsen P. Mean values of wet and dry CaCl2-P within each landuse generally decreased in order from root>cereal>grassland>woodland for each month. Both wet and dry CaCl2-P concentrations exhibited a seasonal variation, with a summer maximum and a winter minimum. The exception was the wood-land soil that had no obvious pattern. The variation and difficulty in detecting the low concentrations ex-tracted may have caused this. There was a rise in wet CaCl2-P in the root, cereal and grassland soils, which corresponded to the application of superphosphate fertilizer in March. However, the seasonal variation and summer maxima in dry CaCl2-P and Olsen P concentrations for root, cereal and grassland soils is probably not caused by a spring fertilizer application since neither rose during March or April.
3.4. Change points
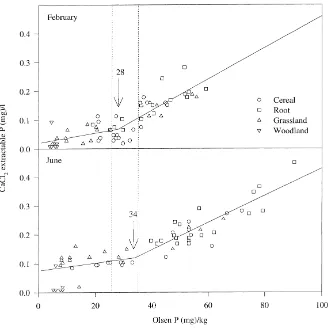
From Fig. 4 it is evident that an approximate 6-fold increase in Olsen P between woodland and root soils for August is paralleled by a 6-fold increase in dry
CaCl2-P. However, an approximate 5-fold increase in Olsen P between woodland and grassland soils in February only gives an approximate 3-fold increase in dry CaCl2-P. This is caused by the non-linear quantity (Olsen P)–intensity (CaCl2-P) (Q/I) relationship that exists between the two measurements (McDowell and Condron, 1999). This relationship can be described by sorption isotherms (e.g. Freundlich), but a split-line model has also been used (Brookes et al., 1997) which gives a change point above which environmen-tally significant levels of P may be lost. This change point is attractive as a management tool for farmers and policy makers alike. Fitting a split-line model to monthly dry CaCl2-P data shows that the change point has a mean value of 31 mg kg−1 Olsen P and a range of 26–36 mg kg−1 Olsen P (Table 3). There is no apparent seasonal variation in the value of the change point. However, the number of soil samples that lie above the change point varies according to season. For example, Fig. 5 shows the split line model fitted to data from February and June. The summer maximum associated with Olsen P and dry CaCl2-P results in more soil samples above the change point, and consequently at risk of accelerated P loss (but requiring moving water to be lost), than soils above the change point in winter. However, high flow rates in winter means that more P is lost than in summer months (Figs. 4, 6 and 7). Although following the same pattern as dry CaCl2-P, much more variation was associated with fitting the split-line model to cal-culate change points for wet CaCl2-P against Olsen P (data not shown).
3.5. Seasonal variation in stream P concentration and load
Fig. 4. Monthly concentration (±standard error of the mean (SEM) I) and variation in Olsen P and wet and dry CaCl2-P for each landuse, along with the monthly SRP concentration and load for the mean of 8 years stream discharge and monthly SRP concentrations for the 1997–1998 water year.
January and February generated 40% of the annual load. In contrast, low discharge rates during the same months in 1998 generated 19% of the annual P load (Fig. 6). The mean annual loss of P over 8 years is equivalent to about 7.5% of the P fertilizer applied to the catchment.
Fig. 5. Split line model fitted to CaCl2-P vs Olsen P for the February and June soil sampling. Numbers indicate the change point for each month and the dotted lines the total range of change points over the 12-month sampling period.
Table 3
Monthly calculated change points for the Q/I relationship of Olsen P vs dry CaCl2-P
Month Change point value
(mg kg−1 Olsen P)
August 1997 33
September 1997 31
October 1997 26
November 1997 26
December 1997 36
January 1998 n.d.a
February 1998 28
March 1998 33
April 1998 32
May 1998 30
June 1998 34
July 1998 33
an.d.=not determined.
extracts is given in Table 4. These data show that al-though the cause and effects relationships are not clear, and that P in soil extracts do not necessary represent the catchment as a whole, they suggest that changes in mean stream SRP concentration over an 8 year pe-riod were related to changes in CaCl2-P concentra-tion. Correlations of P in soil extracts with stream SRP concentrations during the August 1997–July 1998 pe-riod were low and may have been caused by a combi-nation of unusually low rainfall during February and high variation in stream SRP concentration associated with the low number of samples (n=47) compared to the 8 year period (n=300).
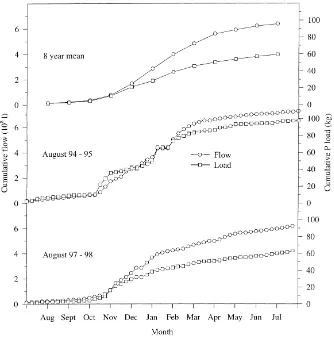
Fig. 6. Cumulative SRP export and flow for the mean of 8 years, the 1994–95 and 1997–98 water years.
against cumulative discharge. Variation from a linear relationship occurs twice around late autumn and late winter. Cumulative SRP export plateaus where P sup-ply is becoming exhausted. This effect is not as dis-tinct during the 1997–1998 water year compared to the 1994–1995 water year possibly due to unusually low rainfall and discharge in February 1998.
4. Discussion
4.1. Seasonal variation of P forms
Fluctuations in the concentration of soil P are caused by several factors. Increases arise from the addition of inorganic fertilizers or organic manures
and the decomposition of plant material and organic matter by microbial activity. Decreases are caused by the uptake of P by crops, by leaching and the immobi-lization of P in microbes and soil constituents. These in-turn are affected by climatic conditions such as temperature and rainfall and soil characteristics such as pH and variation in soil mineral constitution.
Fig. 7. Cumulative SRP export against cumulative discharge for the mean of 8 years data (A), the 1994–95 and 1997–98 water years (B).
minimize any extreme changes in soil solution chem-istry (Qian and Wolt, 1990). The least disturbance to soil solution chemistry would be expected in winter and spring months when soil moisture content is great-est (Fig. 3).
Air-drying soil is known to affect the release of P. The death of microorganisms has been reported to cause an increase in NaHCO3-extractable P (Brookes
Table 4
Correlation coefficients for either mean monthly stream P discharge of 8 years of the 1997–1998 year and Olsen P and wet and dry CaCl2-P for each landuse
Landuse Olsen P (mg kg−1) Dry CaCl
2-P (mg l−1) Wet CaCl2-P (mg l−1) 8 year mean monthly stream P discharge
Cereal 0.36 0.66∗ 0.64∗
Root 0.49 0.68∗ 0.63∗
Grassland 0.15 0.60∗ 0.59
Woodland 0.11 0.64∗ 0.32
1997–1998 mean monthly stream P discharge
Cereal 0.30 0.09 0.30
Root 0.09 0.12 0.27
Grassland 0.04 0.05 0.31
Woodland 0.05 0.20 0.12
∗Indicates significant at the p<0.05 level.
(Birch, 1964). Grassland soils contained more organic C and extractable microbial biomass P than either the cereal or root soils (Table 1). However, in general CaCl2-P from dry soils was less than CaCl2-P from wet soils. Dehydration of soil has been shown to in-crease P adsorption (Haynes and Swift, 1985; Bram-ley et al., 1992; Baskaran et al., 1994). Olsen and Court (1983) suggested that re-wetting exposes new surfaces containing native P and unreacted adsorption sites. Marked increases in CaCl2-P are therefore not expected, unless unreacted sites near saturation.
In the field, soil moisture content changes slowly, especially in those landuses with a crop canopy or root mat. This would allow the microbial biomass time to adapt. Indeed, Tate et al. (1991) has shown that no seasonal variation occurred in the microbial biomass of two pasture soils of different P fertility. Magid and Nielsen (1992) proposed that physiochemical changes associated with soil moisture might mask any bio-logical cycling. Several authors have noted a winter minimum and summer maximum in P concentrations occur in coarse textured soils where a large part of the soil volume dries out during the summer (Smith, 1959; Weaver et al., 1988; Magid and Nielsen, 1992). A winter maximum and summer minimum in soil P concentrations have been noted in fine textured soils, where P concentrations may be controlled by the re-duction and release of P from ferric hydroxides during wet months (Jensen et al., 1998). The soils at Slapton Wood are weekly structured, loose and friable, but also contain well-marked biopores and are free draining. Both preferential flow and soil moisture deficits are known to occur at Slapton Wood (Coles and Trudg-ill, 1985). These physical effects coupled with slow plant growth or even death during periods of low soil moisture in warmer months will cause P to become concentrated in the soil solution.
4.2. Seasonal variation in P runoff
Soluble reactive P in stream runoff is a direct function of the concentration of available soil P for leaching and water flow (surface and sub-surface). If neither is present then SRP loss will not occur. Fig. 7 shows that loss of SRP into the stream is directly affected by discharge and Table 4 shows that the con-centration of SRP in the stream from an 8 year period
is correlated to the concentration of CaCl2-P extracted from dry soil and to a lesser extent CaCl2-P extracted from cereal and root wet soils. Neither, Olsen P or CaCl2-P extracted from wet grassland or woodland soils were significantly correlated to the concentra-tion of SRP in stream discharge during 1997–1998 or the 8 year mean, nor was CaCl2-P extracted from wet or dry soil correlated to the concentration of SRP in stream discharge from 1997–1998 (Table 3). When a soil is enriched with P fertilizer or leached of P, there are changes in both the concentration of P in solution (intensity) and the supporting labile P pool (quantity). However, the amount of change in either differs by the slope of the sorption isotherm or Q/I relationship between the two, which is an expression of the soils P buffer capacity (Bache and Williams, 1971). Olsen P represents a quantity measurement of P associated with Al and Fe hydroxides and Ca phases (Schoenau and Karamanos, 1993), whereas CaCl2-P represents the concentration of P immediately available for plant uptake or lost by leaching.
Since soil extracted with CaCl2-P measures the con-centration of P in the soil solution, we may then expect this to be sensitive to short term changes in soil condi-tions and therefore better correlated to the 1997–1998 year than the mean of the 8 years SRP stream data. This was not the case. Rainfall during the 1997–1998 period was unusual, characterized by very low rain-fall during February and higher than average rainrain-fall in November and January. This was reflected in dis-charge (Fig. 6). However, concentrations of CaCl2-P were similar to SRP in stream discharge for the mean of 8 years. This implies that readily leachable soil P has maintained a memory of soil physical conditions analogous to the mean of 8 years. Discharge from the Slapton Wood catchment is characterized by a large amount of base flow, supplied with P by the soil ma-trix. Physical conditions in the soil matrix control-ling P concentration such as soil moisture will change slowly especially in those soils with a root mat or crop canopy (Fig. 3).
to SRP concentrations in stream discharge. However, while this suggests that soils under root cropping are most closely linked to SRP in stream discharge, with-out knowing the proportion of water flowing through root soils and the influence of grassland soils nearer the stream (Fig. 1), we cannot make any prediction for the amount of SRP supplied by them.
Plots of cumulative SRP export against cumu-lative flow show two contrasting situations for the 1994–1995 water year and the 1997–1998 water year compared to the mean of 8 years (Fig. 7). The shape of the plot for the 1994–1995 water year is best de-scribed by linking two or three first order kinetic curves, where the rapid loss of SRP is stemmed by SRP supply and the curve begins to plateau. The pool of readily leachable P then begins to be replenished by desorption, mineralization and fertilization ready for the next period of rapid loss. The major period of loss during the 1994–1995 year and the mean of 8 years occur during late autumn, when soil P concentrations and rainfall are high. Compared to 1994–1995 and the mean of 8 years data, the plot for the 1997–1998 water year is nearly linear, especially during winter when most SRP is lost. This suggests that SRP export from the catchment in 1997–1998 is not related to the amount of SRP that was in the catchment.
4.3. Management implications
It is possible to present correlations between changes in landuse, climate and management on the amount and concentration of SRP in streams (Smith et al., 1995). However, if we are to create predictive models or policy tools then the causal links, which depend upon the hydro-chemical processes within the soil, must be understood. For practical reasons there is also a need to maximize land area described by the least number of soil samples, but within an acceptable standard of error. This is critical if the results are to be used in a farm scale environmental management tool such as a ‘P index’ (Gburek et al., 1996), or used at larger scales within a GIS system. The results presented here show that for cereal and root landuses there is a common change point, above which P intensity increases greatly compared to plant available Olsen P, than if below. There is some ev-idence to show that a change point also exists for
grassland soils, which is affected by pH (McDowell and Condron, 1999). Any predictive tool that is based on plant-available (Olsen) P is presented with a prob-lem, namely ‘what happens once the change point is exceeded?’ Data presented here shows that while the increase or decrease in P intensity (CaCl2-P) and quantity (Olsen P) varies according to season, there is a constant change point.
Further work is required to assess the variability of P intensity with topography and the sensitivity of P losses. Clearly we need to understand where within a catchment a soil sample of high P intensity is of concern. The data from this site suggests that on av-erage (over 8 years), P transport within the catchment is limited by SRP supply rather than by water flow (Fig. 6.). Soils, which are ‘high risk’ in terms of sup-plying P, are those above the change point with rapid P release kinetics. In general, the root and some of the cereal soils were well above the Olsen P recom-mended for maximum yield for potatoes (Solanum tuberosum) (25 mg kg−1 Olsen P), sugar beet (Beta vulgaris) (20 mg kg−1 Olsen P) and winter wheat (Triticum aestivum) (20 mg kg−1 Olsen P) (Johnston et al., 1986). Consequently, the first step to mitigat-ing P losses within this catchment would be to allow Olsen P to decrease to half their present values by stopping P fertilizer application.
5. Conclusions
33% greater than the change point. Thus, to reduce SRP loss, P fertilizer applications should be stopped to allow Olsen P to decrease below the change point. The use of CaCl2-P and the change point has the po-tential to form the basis of simple environmental man-agement planning at the catchment scale.
Acknowledgements
We thank AGMARDT (New Zealand) and St. Johns College, Cambridge (UK) for funding and J.J. Cocks and sons for land access.
References
Bache, B.W., Williams, E.G., 1971. A phosphate sorption index for soils. J. Soil Sci. 22, 289–301.
Baskaran, S., Bolan, N.S., Rahman, A., Tillman, R.W., Macgregor, A.N., 1994. Effect of drying of soil on the adsorption and leaching of phosphate and 2,4-Dichlorophenoxyacetic acid. Aust. J. Soil Res. 32, 491–502.
Birch, H.F., 1964. The effect of 2:4-Dinitro-phenol on phosphorus transformations during humus decomposition. Plant Soil 21, 391–394.
Blakemore, M., 1966. Seasonal changes in the amounts of phosphorus and potassium dissolved from soils by dilute calcium chloride solutions. J. Agric. Sci. 66, 139–146. Bramley, R.G.V., Barrow, N.J., Shaw, T.C., 1992. The reaction
between phosphate and dry soil I. The effect of time, temperature and dryness. J. Soil Sci. 43, 749–758.
Brookes, P.C., Heckrath, G., De Smet, J., Hofman, G., Vanderdeelen, J., 1997. Losses of phosphorus in drainage water. In: Tunney, H., Carton, O.T., Brookes, P.C., Johnston, A.E. (Eds.), Phosphorus Loss from Soil to Water. CAB International Press, Cambridge, England, pp. 253–272.
Brookes, P.C., Powlson, D.S., Jenkinson, D.S., 1982. Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 14, 319–329.
Burt, T.P., Butcher, D.P., Coles, N., Thomas, A.D., 1983. The natural history of Slapton Ley nature reserve XV. Hydrological processes in the Slapton Wood catchment. Field Stud. 5, 731– 752.
Burt, T.P., Arkell, B.P., Trudgill, S.T., Walling, D.E., 1988. Stream nitrate levels in a small catchment in south west England over a period of 15 years (1970–1985). Hydrol. Process 2, 267–284. Burt, T.P., 1988. Seasonality of subsurface flow and nitrate
leaching. Catena Sup. 12, 59–65.
Coles, N., Trudgill, S.T., 1985. The movement of nitrate fertiliser from the soil surface to drainage waters by preferential flow in weakly structured soils, Slapton, South Devon. Agric. Ecosyst. Environ. 13, 241–259.
Garbouchev, I.P., 1966. Changes occurring during a year in soluble phosphorus and potassium in soil under crops in rotation experiments at Rothamsted, Woburn and Saxmundham. J. Agric. Sci. 66, 399–412.
Gburek, W.J., Sharpley, A.N., Pionke, H.B., 1996. Identification of critical source areas for phosphorus export from agricultural catchments. In: Anderson, M.G., Brooks, S.M. (Eds.), Advances in Hillslope Processes Vol. 1. Wiley, Chichester, UK, pp. 263–282.
Goodwin, M.J., Parkinson, R.J., Williams, E.N.D., Tallowin, J.R.B., 1998. Soil phosphorus extractability and uptake in Cirsio-Molinietum fen-meadow and an adjacent Holcus lanatus pasture on the culm measures, north Devon, UK. Agric. Ecosyst. Environ. 70, 169–179.
Grewal, K.S., Buchan, G.D., Sherlock, R.R., 1991. A comparison of three methods of organic carbon determination in some New Zealand soils. J. Soil Sci. 42, 251–257.
Haynes, R.J., Swift, R.S., 1985. Effects of air-drying on the adsorption and desorption of phosphate and levels of extractable phosphate in a group of New Zealand acid soils. Geoderma. 35, 145–157.
Heckrath, G., Brookes, P.C., Poulton, P.R., Goulding, K.W.T., 1995. Phosphorus leaching from soils containing different phosphorus concentrations in the Broadbalk experiment. J. Environ. Qual. 24, 904–910.
Hodgkinson, R.A., Withers, P.J., 1998. Tile drains as a pathway for phosphorus loss in small catchments. Practical and Innovative measure for the control of agricultural phosphorus losses to water. OECD Workshop, Greenmount College of Agriculture and Horticulture, Northern Ireland, pp. 150–151.
Hunt, H.W., Elliot, E.T., Walter, D.E., 1989. Inferring trophic transfers from pulse-dynamics in detrital food webs. Plant Soil 115, 247–259.
Jensen, M.B., Hansen, H.C.B., Nielsen, N.E., Magid, J., 1998. Phosphate mobilization and immobilization in two soils incubated under simulated reducing conditions. Acta Agric. Scand., Sect. B, Soil Plant Sci. 48, 11–17.
Johnston, A.E., Lane, P.W., Mattingly, G.E.G., Poulton, P.R., 1986. Effects of soil and fertilizer P on yields of potatoes, sugar beet, barley and winter wheat on a sandy clay loam soil at Saxmundham Suffolk. J. Agric. Sci. Camb. 106, 155–167. Kuo, S., Jellum, E.J., 1987. Influence of soil characteristics
and environmental conditions on seasonal variations of water-soluble phosphate in soils. Soil Sci. 143, 257–263. Larsen, S., Widdowson, A.E., 1968. Chemical composition of soil
solution. J. Sci. Food Agric. 19, 693–695.
Leinweber, P., 1998. Phosphorus in soil and surface water from the Harle catchment: results of a two-year monitoring programme. Practical and Innovative measure for the control of agricultural phosphorus losses to water. OECD Workshop, Greenmount College of Agriculture and Horticulture, Northern Ireland, pp. 80–81.
Magid, J., Nielsen, N.E., 1992. Seaonal variation in organic and inorganic phosphorus fractions of temperature-climate sandy soils. Plant Soil 144, 155–165.
McDowell, R.W., Condron, L.M., 1999. Developing and predictor for phosphorus loss from soil. In: Currie, L.D. (Ed.), Best Soil Management Practices for Production (Fertilizer and Lime Research Centre 12th Annual Workshop). Massey University, Palmerston North, New Zealand, pp. 153–164.
Olsen, R., Court, M.N., 1983. Effect of wetting and drying of soils on phosphate adsorption and resin extraction of soil phosphate. J. Soil Sci. 33, 709–717.
Olsen, S.R., Cole, C.V., Watanabe, F.S., Dean, L.A., 1954. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circ. 939, US Gov. Print Office, Washington, USA.
Polyzopoulos, N.A., Keramidas, V.Z., Pavlatou, A., 1986. On the limitations of the simplified Elovich equation in describing the kinetics of phosphate sorption and release from soils. J. Soil Sci. 37, 81–87.
Pote, D.H., Daniel, T.C., Sharpley, A.N., Moore Jr, P.A., Edwards, D.R., Nichols, D.J., 1996. Relating extactable soil phosphorus to phosphorus losses in runoff. J. Environ. Qual. 60, 855–859. Qian, P., Wolt, J.D., 1990. Effects of drying and time of incubation on the composition of displaced soil solution. Soil Sci. 149, 367–374.
Ratsey, S., 1975. The climate at Slapton Ley. Field Stud. 4, 191– 206.
Saunders, W.M.H., Metson, A.J., 1971. Seasonal variation of phosphorus in soil and pasture. NZ J. Agric. Res. 14, 307–328. Schoenau, J.J., Karamanos, R.E., 1993. Sodium bicarbonate-extractable P, K, and N. In: Carter, M.R. (Ed.), Soil Sampling and Methods of Analysis, Lewis Publishers, Boca Raton, pp. 51–58.
Schofield, R.K., 1955. Can a precise meaning be given to ‘avialable’ soil phosphorus. Soils Fertil. 18, 373–375.
Seeling, B., Zasoski, R.J., 1993. Microbial effects in maintaining organic and inorganic solution phosphorus concentrations in a grassland topsoil. Plant Soil 148, 277–284.
Shand, C.A., Macklon, A.E.S., Edwards, A.C., Smith, S., 1994. Inorganic and organic P in soil solutions from three upland soils. Plant Soil 159, 255–264.
Smith, A.M., 1959. Soil analysis and fertilizer recommendation. Proc. Fertil. Soc. 57, 1–40.
Smith, R.V., Lennox, S.D., Jordan, C.J., Foy, R.H., McHale, E., 1995. Increase in soluble phosphorus transported in drainflow from a grassland catchment in response to soil phosphorus accumulation. Soil Use Manage. 11, 204–209.
Sparling, G.P., Milne, J.D.G., Vincent, K.W., 1987. Effect of soil moisture regime on the microbial contribution to Olsen phosphorus values. NZ J. Agric. Res. 30, 79– 84.
Tate, K.R., Speir, T.W., Ross, D.J., Parfit, R.L., Whale, K.N., Cowling, J.C., 1991. Temporal variations in some plant and soil P pools in two pasture soils of widely different P fertility status. Plant Soil 132, 219–232.
Troake, R.P., Walling, D.E., 1973. The natural history of Slapton Ley nature reserve VII. The hydrology of the Slapton Wood stream. Field Stud. 3, 719–740.
Walling, D.E., Webb, B.W., 1982. The design of sampling programmes for studying catchment nutrient dynamics. Proc. Hydrology of Research Basins, Bern, Germany. Sonderh. Landeshydrologie, 747–758.
Weaver, D.M., Ritchie, G.S.P., Anderson, G.C., Deeley, D.M., 1988. Phosphorus leaching in sandy soils I. Short-term effects of fertilizer applications and environmental conditions. Aust. J. Soil Res. 26, 177–190.