I
n crown-gall disease, infection by an Agrobacterium tumefaciensstrain that harbors a large tumor-inducing (Ti) plasmid1,2causes a mass of mainly undifferen-tiated plant cells to form on a plant’s stem at the soil line (the crown). The transfer DNA (T-DNA) portion of the Ti plasmid and its delimiting ‘right’ and ‘left’ border sequences become integrated into the nuclear genome of a susceptible plant cell that is in contact with the bacterium. The T-DNA encodes, among other things, enzymes for synthesizing plant hormones that stimulate cell division and the proliferation of undif-ferentiated cells into the tumor. However, T-DNA from which the genes for hormone-synthesizing enzymes have been removed is an excellent vector for introducing foreign DNA into a nuclear chromosome of a plant cell.Nuclear transformation
There are several steps involved in A. tumefaciens -mediated insertion of DNA into a plant: (1) a foreign gene is inserted between the borders of the T-DNA cloned in a small plasmid; (2) this construct is intro-duced into an A. tumefaciens strain that lacks T-DNA and the engineered T-DNA inserts into the Ti plasmid; (3) the A. tumefaciens bacteria carrying the foreign DNA on the now-disarmed Ti plasmid are put into contact with plant cells; (4) the T-DNA is transferred into a plant cell; and (5) the new gene, as a component of the T-DNA, is incorporated into a chromosome in the nucleus of the plant cell.
The next step after producing a transformed cell is to obtain a fertile transgenic plant. Research into plant tissue culture started at the beginning of the 20th century. One aspect of this work culminated in the demonstration that single carrot cells, cultured on an appropriate medium, can divide, give rise to embryos and grow into entire plants3. The culture media used in the initial experiments included complex and poorly
characterized components but later, using information acquired in studies of plant-hormone action, entire plants could be regenerated by transferring cells in cul-ture through a series of culcul-ture media with different hormone contents. Even mature cells from, for exam-ple, tobacco leaves could be stimulated to multiply to form clumps of cells from which fertile plants formed. The results of these two lines of research – investi-gations of the Ti plasmid and the regeneration of whole plants from cells in culture – were used together in the early 1980s to produce fertile transgenic plants. The most significant discovery for the genetic engineering of plants was that if a gene was introduced via T-DNA into a cell that was then used to generate a fertile plant, that gene would propagate to tobacco progeny through the sexual cycle and seed. The commercial potential for crops was, of course, apparent immediately. At that time, the major limitation to producing a genetically transformed crop plant appeared to be the ability to regenerate that plant from cells or clumps of tissue in culture. Only a few species could be manipulated in this way then; now many can be.
Crop plants
A. tumefaciens-mediated nuclear transformation tech-nology has been used widely to create transgenic dicotyledonous plants, but monocotyledonous plants proved difficult to transform by this method. The results of early experiments led to the view that most mono-cotyledonous plants – including cereals – were beyond the host range of A. tumefaciens. However, experiments in the late 1980s showed that maize-streak-virus DNA can be delivered to maize via the A. tumefaciens T-DNA system and can be expressed in the plant. It was only in 1993 and 1994, after the processes involved in T-DNA transfer were better understood, that transgenic rice was produced via A. tumefaciens4. The efficient transformation of maize viaA. tumefacienswas described in 1996 (Ref. 5) and of wheat in 1997 (Ref. 6). Mean-while, however, and largely driven by the prospect of crop improvement, commercialization and profits, other techniques were developed for introducing foreign
Engineering chloroplasts: an alternative site
for foreign genes, proteins, reactions and
products
Lawrence Bogorad
Plant genetic engineering via the nucleus is a mature technology that has been used very productively for research and
com-mercial biotechnology. By contrast, the ability to introduce foreign genes at specific locations on a chloroplast’s chromosome
has been aquired relatively recently. Certain limitations of nuclear genome transformation methods might be overcome by
the site-specific introduction of genes into plastid chromosomes. In addition, plastids, mitochondria and other subcellular
organelles might provide more favorable environments than the nuclear–cytoplasmic compartment for certain biochemical
reactions and for accumulating large amounts of some gene and enzyme products.
DNA into cells. Among these techniques, microprojectile bombardment7has been broadly effective and has been particularly successful for producing transgenic maize8,9. The rapid increase, over the past few years, in the areas planted with Ti-plasmid-transformed cotton and microprojectile-transformed maize attests to the success of the methods for transforming plant nuclei. However, the current techniques have important limitations. One significant problem, which is at best an inconvenience and at worst a blocking impediment, is that the expres-sion level of an introduced gene can vary greatly from one transformed plant to another. Consequently, many transformants with the same gene usually need to be produced and tested to identify a strongly expressing plant strain suitable for further research or for com-mercialization. This effect is thought to be attributable, at least in part, to poorly understood ‘position effects’. The notion is that the location at which a gene is inserted on a chromosome – and on which chromo-some – affects how strongly it is expressed. Methods for directing the insertion of foreign genes to specific sites in the nuclear genomes of plants have not been devel-oped but a valuable method for creating small mutations in known genes in situhas been described recently10,11.
Multiple genes
The consequences of variable expression are com-pounded if more than one gene needs to be introduced. Although some useful traits result from the presence of a single gene (e.g. the production of Bacillus thuringiensis toxin to protect against insects, of a viral coat protein to protect against viruses or of an enzyme to inactivate a herbicide), the vast majority of agronomic traits are multigenic, that is, they result from the action of several genes. A biosynthetic pathway is perhaps the simplest example: a series of enzymes, each encoded by one or more genes, is needed to yield a product. Natural examples include the biosynthetic chains that produce cell-wall components, chlorophyll, amino acids, and so on. Indeed, most of the characters that concern plant breeders are quantitative traits; they are controlled polygenetically. As discussed above, intro-ducing a single gene usually yields some transformants that express the new gene strongly and others that express it poorly. The best expressing strain must be selected from among these. Suppose that this gene encodes one of several enzymes in a biosynthetic chain that is to be introduced into a plant. After selecting transformants that express the first gene well, the best of these would need to be transformed with a gene for an enzyme that catalyses the next step in the biosynthetic
chain, followed by another round of selections from among many transformants. Obviously, the more enzymes in the chain, the more times these steps must be repeated.
However, in an exciting and skilful seven-year-long effort12, Potrykus et al.recently introduced into rice a set of three genes for a short biosynthetic chain that resulted in ‘yellow rice’. (They were fortunate in being able to introduce two of these genes at one time.) This chain of genes produced the enzymes required to enable rice grains to form b-carotene, which humans convert into vitamin A13. The complications attendant on introducing a more extended biosynthetic chain and on attaining other major plant biotechnology goals are obvious. Two separate genes introduced into a plant in a single T-DNA fragment may be expressed to differ-ent relative extdiffer-ents in one transformant than in another. Thus, contrary to what one might have thought, cloning several genes into a single T-DNA does not generally avoid the compounded, variable expression problem.
An interesting single-gene approach for simulta-neously introducing several new proteins into a cell via nuclear gene transformation was taken by Dasgupta et al.14 They constructed and introduced a large single gene encoding a polyprotein containing three enzymes into a plant. The enzyme proteins were separated from one another by the amino acid sequence recognized by the tobacco-vein-mottling-virus N1a proteinase, which was one of the three enzymes in the polyprotein. The three enzymes were detected as individual active pep-tides in the transformant, showing that the N1a pro-teinase cut the individual enzymes out of the polyprotein and that all three enzymes were functional.
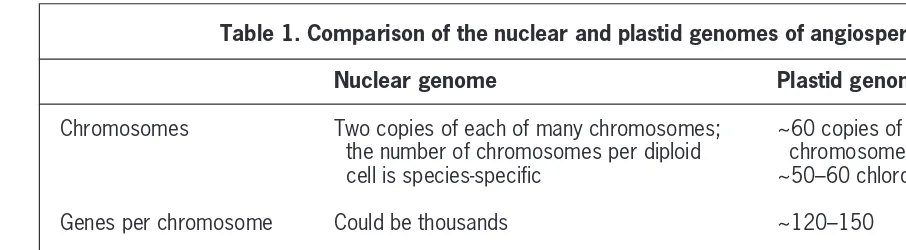
In nature, however, the problem is approached in various other ways. Operons (groups of genes with a single promoter), which often encode enzymes of the same biosynthetic chain, are common in bacteria. The single transcript of an entire multigenic operon carries information required for the production of several pro-teins. Nuclear genes are transcribed singly; they are not arranged in operons. However, chloroplast genes are often present in operons. Some pertinent features of nuclear and plastid genomes are compared in Table 1.
Chloroplasts for genetic engineering
Plant cells have three gene-containing compartments: the nuclear–cytoplasmic system, mitochondria and plastids. There are several types of plastid including: (1) chlorophyll-containing chloroplasts; (2) yellow, orange or red carotenoid-containing chromoplasts; (3) starch-storing amyloplasts; (4) oil-containing elaioplasts; Table 1. Comparison of the nuclear and plastid genomes of angiosperms
Nuclear genome Plastid genome
Chromosomes Two copies of each of many chromosomes; ~60 copies of a single circular the number of chromosomes per diploid chromosome per plastid cell is species-specific ~50–60 chloroplasts per cell
Genes per chromosome Could be thousands ~120–150
(5) proplastids (plastid precursors found in most plant cells); and (6) etioplasts (partially developed chloroplasts that form in dark-grown seedlings). The conversion of photosynthetic chloroplasts into yellow carotenoid-rich chromoplasts is seen in the ripening of bananas; the conversion of chloroplasts to lycopene-containing red chromoplasts is seen in the ripening of tomatoes. Each compartment of the eukaryotic cell is unique. A par-ticular biochemistry can be favored in one compartment (e.g. chloroplasts or chromoplasts) while the environ-ment in another compartenviron-ment (e.g. the cytoplasm) is unfavorable.
The photosynthetic apparatus of the chloroplast captures light energy and converts it into biologically useful energy that is, for example, available for: (1) manu-facturing sugars and amino acids; (2) reducing nitrate to ammonium; (3) making starch; (4) synthesizing com-plex organic compounds; and (5) assembling chloro-plast proteins that are required for the chlorochloro-plasts themselves to function. (Non-green plastids use energy produced elsewhere in the cell for metabolism.) It is easy to imagine modifying or adding to existing biosynthetic chains, which are already linked to the energy-converting machinery of the chloroplast, to produce and accumulate desirable new products. The chloroplast genomes of flowering plants encode per-haps 150 genes; the remainder of the hundreds or thou-sands of plastid proteins are the products of nuclear genes. The compositions of chloroplasts and other plas-tids can be modified by inserting genes into the nuclear genome; a chimeric gene can be constructed with an N-terminal sequence that targets its protein product to the chloroplast.
Why plastids?
However, why do we need to send a protein into the plastid? Some syntheses other than photosynthesis are only carried out in plastids, probably because one or another feature of the organelle’s environment does not exist elsewhere in the cell. Furthermore, the cytoplasm and chloroplast contain different proteases, and a pro-tein might survive better in one compartment than in the other. A specific type of plastid might be a good place to accumulate certain proteins or their biosyn-thetic products that would be harmful if they were present in large amounts in the cytoplasm or in a plastid of a different type. The various forms of plastid (amy-loplasts, chromoplasts, etc.) have desirable properties as places to conduct reactions and to accumulate proteins or products of enzymes, and they can be exploited using known nuclear gene transformation methods. Nevertheless, the limitations of plant nuclear transfor-mation technology discussed above remain. Fortunately, there is an alternative: new genes can be introduced directly into the plastid genome. This has several advan-tages, although some technical problems have still to be solved to simplify the procedure.
Methods for transforming plastids
In 1988, it was reported that a deletion in the chloro-plast gene atpBthat rendered the green alga Chlamydo-monas reinhardtiiincapable of carrying out photosyn-thesis could be corrected by introducing the wild-type gene15. The DNA was deposited on tungsten micro-projectiles ~1mm in size and propelled into cells spread
on an agar plate using a gunpowder charge7. Later, com-pressed gases were used for biolistic transformations16,17. The DNA became incorporated into the Chlamydomonas chloroplast chromosome by homologous recombination. Thus, unlike nuclear transformation, DNA can be directed to a specific site within the plastid chromo-some. Blowers et al.showed that foreign DNA bor-dered by chloroplast DNA sequences is incorporated and stably maintained in the Chlamydomonaschloroplast chromosome18.
Daniell et al.19and Svab et al.20took advantage of this knowledge and introduced DNA into tobacco chloro-plasts in cultured cells in situor in leaf tissue. Transient expression was observed19and, in addition, transformed cells were regenerated into plants with modified chloro-plasts20. The usefulness of plastid transformation for biotechnology was shown strikingly by McBride et al., who introduced the gene encoding the B. thuringiensis lepidopteran protoxin into the chloroplast chromo-somes of tobacco plants using the microprojectile method. In the resulting plants, this protoxin consti-tuted 2–3% of the soluble leaf protein21. This very high level of transgene expression was attributed to the pres-ence of ~50 chloroplasts per leaf cell and 60–100 chro-mosomes per plastid. Each leaf cell contains ~3000 copies of each gene, present once in each plastid chromosome. However, the large number of copies of the gene in the cell is unlikely to be the entire expla-nation: some chloroplast genes encode proteins that are amongst the most abundant in the cell, but others are much less highly expressed. Also, the mRNAs for some nuclear genes that are present in <10 copies per cell are as abundant as the most abundant plastid-encoded mRNAs.
Two additional methods for delivering DNA into plastids have recently been developed: a polyethylene glycol-mediated method and direct injection of DNA in situ. Nicotiana plumaginifolia22and Nicotiana tabacum23 with transformed chloroplasts have been produced by exposing protoplasts to polyethylene glycol and reporter DNA bordered by chloroplast sequences to enable homologous recombination to occur. The poly-ethylene-glycol method holds out the promise of the capacity to generate more cells with transformed plas-tids more readily than by the biolistic procedure. How-ever, the limiting step in this case is regenerating plants from protoplasts. For example, immature embryos, rather than protoplasts, are used as the starting material for successfully producing maize and rice varieties with nuclear transgenes. Although they have not yet regen-erated any plants, Knoblauch et al.have shown that DNA is expressed after it is injected directly into individual chloroplasts in photosynthetic leaf cells of tobacco24.
Operons
to having transgenes with the same kind of promoter at separate locations on the chromosome.
Because insertion is to a specified site on the chromo-some, if particular sites are found to affect the tran-scription of an inserted gene (e.g. through effects on DNA conformation that affect transcription25), the site of insertion of transgenes could be selected in order to take advantage of these effects. This is better than being at the mercy of undirected insertion and position effects. (Of course, uniform transcription does not assure uniform stability or equal translation of transcripts. But these are separate problems and are faced in whichever cellular compartment the gene is transcribed.)
The expression of some plastid genes, as is the case for some nuclear genes, is regulated by environmental factors (e.g. light) and other genes are expressed only in specific types of plastid and cell. Such specificities can be taken advantage of in genetic engineering. There is a lot of information available about plastid-gene DNA-dependent RNA polymerases, promoters and enhancers, and all of this knowledge can be exploited for genetically engineering the plant’s relatively simple plastid genome.
Inheritance
The chloroplasts of angiosperms are generally trans-mitted only by the maternal parent. This means that chloroplast genes are not present in pollen. Conse-quently, a trait introduced by genetically engineering the chloroplast genome would not be unintentionally transferred to sexually compatible relatives of the crops that might be growing nearby26. Furthermore, the plastid-chromosome-encoded protein would not be produced in the pollen and thus would not affect insects that feed on pollen or pollen-coated plant tissues.
Pros, cons and uncertainties
Plastids are important alternatives to the cytoplasm as sites in which to engineer enzymatic reactions or to make, process or store proteins or enzyme products. The internal environments of some differentiated forms of plastids (e.g. chloroplasts, chromoplasts and amylo-plasts) might be more favorable than those of the cyto-plasm for certain enzymatic reactions, for modifying or processing a particular protein and so on. A protein might be more stable in the plastid than in the cyto-plasm because of differences in proteases, ions and other constituents. It might also be advantageous to place energy-intensive biochemical reactions close to the site of photosynthesis. In addition, if the protein that accu-mulates or if the product of an enzymatic reaction is deleterious to the cell if present in the cytoplasm, it might not be harmful in some form of plastid. How-ever, the plastid could be the wrong place for a process if the product must leave the plastid and go to another place in the plant. Various small organic molecules are transported out of chloroplasts but very little is cur-rently known about the export of many molecules from this organelle.
Proteins made in the cytoplasm can be directed into the plastids so why consider putting genes directly into the plastid genome? First, the level of expression might be more predictable. Second, a group of genes can be introduced as an operon for transcription as a unit in the plastid or as a series of independently transcribed
DNA sequences, all in a single transformation. Third, foreign genes will not disperse to related species growing within pollination range of the crop because chloroplast genes are not present in most angiosperm pollens. In addition, locating genes in plastids might offer different possible expression patterns in the plant than nuclear localization (e.g. in all cells containing proplastids or chloroplasts or amyloplasts).
The present range for plastid transformation
Chloroplast transformation has been very successful for Chlamydomonas reinhardtii and tobacco. This has allowed important experiments to be done on the mol-ecular biology of chloroplasts and on photosynthetic processes. Among higher plants, fertile plants with genetically modified chloroplast chromosomes have been reported, to date, only in tobacco. Low-efficiency plastid transformation has been reported for Arabidopsis thaliana27but none of the transformed plants that regen-erated were fertile. Chloroplasts in potato-leaf cells have been transformed with the bacterial gene for spectino-mycin and streptospectino-mycin resistance (aadA) as well as with the green fluorescent protein (GFP) gene of the jelly-fish Aequorea victoria28. Plastids in suspension cultures of embryogenic rice cells have been transformed with an aadA–GFPfusion gene29. In these cases, DNA was deliv-ered on microprojectiles and transgenic plants were recovered, but in neither case were fertile plants recovered. However, these results are encouraging, especially when combined with the observation that GFP-encoding DNA attached to a plastid rRNA promoter (delivered on microprojectiles) is transiently expressed in non-green starch-storing amyloplasts in potato tuber tissue and in chromoplasts in marigold petals, carrot roots and pepper fruits30. First, these results show that foreign genes can be expressed in non-green plastids and, at least in the case of rice, that the DNA can be incorporated into the genome of non-green plastids. (This suggests that the range of target tissues for plastid transformation extends beyond chloroplast-containing cells.) Second, the results with potato and rice provide a good start for the broadening of the species range of the technology. These limited successes are heartening but a great deal remains to be done to extend the usefulness of chloroplast transformation technology to these and other economically important crops.
Heterogeneity, heteroplasmy and stability
Although the inability to regenerate fertile, trans-genic, economically important plants readily is a major impediment (just as it was in the early days of nuclear transformation), there is every reason to expect that this difficulty will be overcome with one species at a time in chloroplast genetic engineering, as it has been and continues to be in plant nuclear biotechnology.
Chlamydomonas, by contrast, is a single-celled flagel-late, each of whose cells contains a single chloroplast with ~60 chromosomes, and homoplasmic chloroplast transformants can generally be isolated after continued culture under selection. A wild-type plastid chromosome in a transplastomic crop plant could persist under the ‘protection’ of the product of the introduced gene present in other chromosomes in the same or other plastids and take over in the absence of selection for the transgene.
The current major approach to obtaining homoplas-mic plants is repeated selection from transformed tis-sues in culture, when possible, followed by regenerating plants. Unfortunately, repeated cell culturing puts a further restraint on the kinds of plant material that can be used for regeneration. A different strategy might be to make the transgene (or the transgene product fused to another protein coding sequence) essential for cell survival, thus providing constant selection and rendering the presence of wild-type chromosomes irrelevant. Another course might be temporarily (i.e. reversibly) reducing the number of chloroplasts per cell and the number of chromosomes per plastid. Antisense suppression of one of the genes controlling plastid divi-sion in A. thaliana results in the number of chloroplasts per leaf cell dropping from 100 to one31, and Chlamydo-monaschloroplast, but not nuclear, DNA is temporar-ily reduced to one-seventh its normal level (approxi-mately 10 rather than 60 chromosomes per plastid) after 6–7 doublings in the presence of the thymidylate-synthase inhibitor fluorodeoxyuridine32. However, thymine starvation-induced mutagenesis occurs in bac-teria, T4bacteriophage and, probably, Chlamydomonas chloroplast DNA33, which means that this might be a clue to how to reduce the chloroplast DNA rather than a literal solution.
It remains to be seen whether one or both of these sorts of approaches can be used to address the vexing problem of wild-type chromosomes lurking in the background but ready to ‘take over’. Concerted efforts are likely to overcome the present limitations but tech-nology that can easily solve or circumvent the problem has not yet been developed. It was recognized many
years ago that chloroplasts in the giant alga Acetabularia might not be entirely independent of one another and, indeed, might be a single fusing and dividing mass34. The recent observation of connections between chloro-plasts in cells of higher plants35is a striking reminder of how little we know about plastid biology. As we learn about the subject, we are likely to acquire new outlooks on how to approach the heteroplasmy problem directly and effectively. Some aspects of nuclear and plastid genome transformation are compared in Table 2.
New and renewed possibilities?
The development of multiple sites for genetically engineering plant cells raises new prospects for genetic engineering. It also prompts renewed consideration of two examples of major modifications of plant metab-olism involving plastids that could be very important for agriculture: (1) enabling plants to fix N2 and (2) improving photosynthetic CO2fixation. Although both possibilities might, in reality, still be a long way off, there are reasons to be hopeful. First, we continue to learn more about how to take advantage of multiple sites in the cell for introducing foreign genes and about how to control plant gene expression, and (particularly through proteomics) we should come to know more about the kinds of reactions favored in one cell com-partment over others. Second, at the same time, we are becoming increasingly better-informed about the bio-chemistry and physiology of N2 fixation and natural adaptations for increasing CO2fixation.
Nitrogen fixation
It was first proposed approximately 20 years ago that plastids might be suitable and appropriate sites for engi-neering into plants the capacity for nitrogen fixation36,37. Nitrogen fixation requires large amounts of energy in the form of ATP and chloroplasts make this as a prod-uct of photosynthetic energy transdprod-uction. The sensi-tivity of the enzyme nitrogenase to O2, a major prod-uct of photosynthesis, would appear to preclude N2 fixation by chloroplasts but cyanobacteria also conduct oxygenic photosynthesis despite fixing N2very effec-tively. Some cyanobacteria separate the two processes
Table 2. Introducing genes into nuclear and plastid genomes
Nuclear genome Plastid genome
Insertion of foreign DNA Undirected; multiple insertions are common. Directed to a specific site by homologous recombination. into genome
Transcription of Affected by the promoter, the type of cell Affected by the promoter, the type of plastid and the introduced genes and the site of insertion of the gene into type of cell. The location on the chromosome is not
the genome. Each introduced gene is known to affect transcription. A set of genes could be expressed individually. introduced as an operon or as individual transcription
units.
Current limitations The level of expression of an introduced Obtaining homoplasmic transformed strains can be gene is unpredictable. difficult: the development of easier new methods will be Each gene in a set required for a new important. Alternatively, methods are needed to retain
multigenic trait or biosynthetic pathway transgene(s) in the presence of untransformed plastid may have to be introduced separately chromosomes.
temporally and others produce heterocysts for N2 fixa-tion. Heterocysts have thickened walls that impede the passage of O2 but, of paramount importance, the heterocyst’s photosynthetic apparatus lacks the capacity to evolve oxygen but retains the ability to produce ATP.
CO2fixation in C3 plants
In C3 plants (including most crop species), CO2 is fixed to the five-carbon compound ribulose-1,5-bis-phosphate. Two molecules of 3-phosphoglycerate (a C3 compound) are produced from the product of this fixation. The phosphoglycerate is reduced to 3-phosphoglyceraldehyde using the ATP and reduced nicotinamide adenine dinucleotide phosphate (NADPH) that are produced photosynthetically. All chloroplasts in all the cells of a C3 leaf contain ribulose bisphos-phate carboxylase–oxygenase (Rubisco), the enzyme that catalyses this fixation of CO2. However, as well as fixing CO2 to ribulose-1,5-bisphosphate to produce two molecules of phosphoglycerate, Rubisco can cata-lyse the oxygenation of the same five-carbon compound using O2to produce one molecule of phosphoglycer-ate and one molecule of phosphoglycolphosphoglycer-ate; O2 and CO2 compete for the active site on Rubisco. As the rate of photosynthesis increases (e.g. as the light intensity increases), so does O2 production, resulting in more competition with CO2 for Rubisco. As a result, C3 photosynthesis saturates at about one third of the maximum light intensity under which many crop plants grow.
CO2fixation in C4 plants
C4 plants can take advantage of higher light inten-sities. In C4 plants, such as maize and sorghum, there are two types of photosynthetic leaf cell: bundle-sheath cells, which surround the vascular bundles, and meso-phyll cells, which occupy the space between the cylin-ders of bundle-sheath cells and the upper and lower epi-dermis of leaves. Bundle-sheath cells contain Rubisco; mesophyll cells do not. Carbon dioxide is fixed first in mesophyll cells by addition to phosphoenolpyruvate to form oxaloacetate (a C4 compound). Then, in maize,
for example, oxaloacetate produced in mesophyll cells is reduced to malate, which is transferred to the adjacent bundle-sheath cells. In the bundle-sheath cells, CO2is released and refixed by Rubisco in a low-O2environment, because bundle-sheath chloroplasts, like cyanobacterial heterocysts, have little or no oxygen-evolving capacity.
Improving CO2fixation
Could C3 plants be converted into C4 plants? C4 plants are better adapted to more arid conditions and to using higher light intensities than C3 plants. New information about the molecular basis for suppressing Rubisco production in the mesophyll cells of maize38 is one step towards making this possible. Of course, it would also be necessary to selectively eliminate O2 pro-duction in chloroplasts with Rubisco and to increase the activities of phosphoenolpyruvate carboxylase and other enzymes involved in the fixation of CO2 into oxaloacetate as in Rubisco-free mesophyll cells of C4 plants. An alternative and simpler way to improve the effectiveness of CO2fixation by Rubisco might be by genetically installing a CO2-concentrating mechanism39. Important knowledge has been gained about the genes and enzymes of nitrogen fixation since the first suggestions were made that bundle-sheath plastids and cyanobacterial heterocysts were excellent models and that bundle-sheath plastids could be good sites for engineering N2fixation36; this has been the subject of very thoughtful discussion by Dixon et al.40
Future prospects
Relatively few genes have been introduced into plants for commercial purposes. The importance of the availability of separate, metabolically unique compart-ments in cells will become increasingly evident as more diverse metabolic products and enzymatic reactions are introduced into plants. The possibility that a non-cyto-plasmic environment, such as the mitochondrion or a particular form of plastid, will favor a particular enzy-matic reaction and/or the accumulation of a protein or metabolic product will be considered with increasing frequency. (Tables 1 and 2, and Box 1, summarize the features of the nuclear and plastid genomes that are per-tinent to genetic transformation and recapitulate other considerations for judging whether a gene, a biosyn-thetic chain or an enzyme product is best targeted to plastids or to another cellular compartment.)
The major change in plant biotechnology, however, will come with the need to introduce sets of genes in order to express traits determined by multiple genes. In these cases, especially if large amounts of energy are needed to drive one or more of the reactions catalysed by enzymes encoded by newly introduced genes, the method of first choice is likely to be its introduction into the chloroplast genome. Indeed, even if the processes are not energy intensive, it might be easier to introduce multiple genes into the plastid genome than into the nuclear genome.
Current knowledge has broadened the possibilities for genetically engineering plants. The nuclear–cyto-plasmic system might be the most favorable place in a cell for some new biosynthetic processes but other lo-cations in the cell, among them plastids, might be better for others. As for accumulating metabolic products or proteins, the mantra is: the product might be safer in
Box 1. Additional considerations in targeting genes, biosynthetic paths and
enzyme products
• The stability and accumulation of a particular RNA or protein may be greater or lesser in the nuclear-cytoplasmic compartment than in a plastid compart-ment or vice versa.
• Conditions for a particular enzymatic reaction may be more or less favorable in the nuclear-cytoplasmic com-partment than in a plastid comcom-partment or vice versa. • More of the product(s) of an enzymatic reaction or of a chain of reactions may accumulate and not affect the cell in the nuclear-cytoplasmic compartment than in a plastid compartment or vice versa.
• If gene containment is important, the maternal trans-mission of plastids may be advantageous.
the plastid and the cell might be safer with the product in the plastid.
Acknowledgments
The work in the author’s laboratory and the prepar-ation of this paper were supported in part by research grants from the National Institute of General Medical Sciences of the National Institutes of Health of the USA.
References
1Van Larebeke, N. et al. (1974) Large plasmid in Agrobacterium tumefaciensessential for crown gall-inducing ability. Nature 252, 169–170
2Gordon, M.P. (1998) Discovery of the T-DNA of Agrobacterium tumefacieus. In Discoveries in Plant Biology (Vol. 1) (Kung, S.D. and Yang, S.F., eds), pp. 111–114, World Scientific Publishing
3Xu, Z-H. (1998) The discovery of plant tissue culture. In Discover-ies in Plant Biology(Vol. 2) (Kung, S.D. and Yang, S.F., eds), pp. 287–316, World Scientific Publishing
4Hiei, Y. et al. (1997) Transformation of rice mediated by Agrobacterium tumefaciens. Plant Mol. Biol.35, 205–218
5Ishida, Y. et al.(1996) High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat. Biotechnol.
14, 745–750
6Cheng, M. et al.(1997) Genetic transformation of wheat mediated by Agrobacterium tumefaciens. Plant Physiol.115, 971–980
7Klein, T.M. et al.(1987) High velocity microprojectiles for deliver-ing nucleic acids into livdeliver-ing cells. Nature327, 70–73
8Fromm, M.E. et al. (1990) Inheritance and expression of chimeric genes in the progeny of transgenic maize plants. Biotechnology8, 833–839
9Gordon-Kamm, W.J. et al.(1990) Transformation of maize cells and regeneration of fertile transgenic plants. Plant Cell2, 603–618
10Zhu, T. et al.(1999) Targeted manipulation of maize genes in vivo
using chimeric RNA–DNA oligonucleotides. Proc. Natl. Acad. Sci.
U. S. A.96, 8768–8773
11Beetham, P.R. et al. (1999) A tool for functional plant genomics: chimeric RNA–DNA oligonucleotides in vivo gene-specific mutations. Proc. Natl. Acad. Sci. U. S. A.96, 8774–8778
12Gura, T. (1999) New genes boost rice nutrients. Science 285, 994–995
13Ye, X. et al.(2000) Engineering the provitamin A (b-carotene) biosynthetic pathway into (carotenoid free) rice endosperm. Science
287, 303–305
14Dasgupta, S.et al. (1998) Co-ordinated expression of multiple enzymes in different subcellular compartments in plants. Plant J. 16, 107–116
15Boynton, J.E. et al. (1988) Chloroplast transformation in Chlamydo-monaswith high velocity microprojectiles. Science240, 1534–1538
16Sanford, J.C. et al.(1991) An improved helium-driven biolistic device. Technique 3, 3–16
17Finer, J.J. et al.(1992) Development of the particle inflow gun for DNA delivery to plant cells. Plant Cell Rep.11, 323–328
18Blowers, A.D. et al.(1989) Studies on Chlamydomonaschloroplast transformation: foreign DNA can be stably maintained in the chromosome.Plant Cell1, 123–132
19Daniell, H. et al.(1990) Transient foreign gene expression in chloro-plasts of cultured tobacco cells after biolistic delivery of chloroplast vectors. Proc. Natl. Acad. Sci. U. S. A.87, 88–92
20Svab, Z. et al.(1990) Stable transformation of plastids in higher plants.
Proc. Natl. Acad. Sci. U. S. A.87, 8526–8530
21McBride, K.E. et al.(1995) Amplification of a chimeric Bacillusgene in chloroplasts leads to an extraordinary level of an insecticidal protein in tobacco. Biotechnology13, 362–365
22O’Neill, C. et al.(1993) Chloroplast transformation in plants: poly-ethylene glycol (PEG) treatment of protoplasts is an alternative to biolistic delivery systems. Plant J.3, 729–738
23Koop, H.U. et al.(1996) Integration of foreign sequences into the tobacco plastome via polyethylene glycol-mediated protoplast trans-formation. Planta 199, 193–201
24Knoblauch, M. et al. (1999) A galinstan expansion femtosyringe for microinjection of eukaryotic organelles and prokaryotes. Nat. Biotechnol. 17, 906–909
25Stirdivant, S.M.et al. (1985) DNA supercoiling affects in vitro tran-scription of two maize chloroplast genes differently. Proc. Natl. Acad. Sci. U. S. A.82, 4886–4890
26Daniell, H. et al. (1998) Containment of herbicide resistance through genetic engineering of the chloroplast genome. Nat. Biotechnol. 16, 345–348
27Sikdar, S.R. et al.(1998) Plastid transformation of Arabidopsis thaliana.
Plant Cell Rep.18, 20–24
28Siderov, V. et al. (1999) Stable chloroplast transformation in potato: use of green fluorescent protein as a plastid marker. Plant J.19, 209–216
29Khan, M.S. et al. (1999) Fluorescent antibiotic resistance marker for tracking plastid transformation in higher plants. Nat. Biotechnol.17, 910–915
30Hibbard, J.M. et al.(1998) Transient expression of green fluorescent protein in various plastid types following microprojectile bombard-ment. Plant J. 16, 627–632
31Osteryoung, K.W.et al.(1998) Chloroplast divisions in higher plants requires members of two functionally divergent gene families with homology to bacterial ftZ. Plant Cell10, 1991–2004
32Wurtz, E.A. et al.(1977) Pertubation of chloroplast DNA amounts and chloroplast gene transmission in Chlamydomonas reinhardtiiby fluorodeoxyuridine. Proc. Natl. Acad. Sci. U. S. A. 74, 4552–4556
33Wurtz, E.A. et al.(1979) A specific increase in chloroplast gene mutations following growth of Chlamydomonas in 5-fluoro-deoxyuridine. Mol. Gen. Genet. 170, 235–242
34Woodcock, C.L.F. et al. (1970) Evidence for variation in the quan-tity of DNA among plastids of Acetabularia. J. Cell Biol.44, 361–375
35Kohler, R.H. et al.(1997) Exchange of protein molecules through connections between higher plant plastids. Science276, 2039–2042
36Bogorad, L. (1979) The chloroplast, its genome and possibilities for genetically manipulating plants. InGenetic Engineering(Vol. 1) (Setlow, J.K. and Hollaender, A., eds), pp. 181–203, Plenum Press
37Merrick, M. and Dixon, R. (1984) Why don’t plants fix nitrogen?
Trends Biotechnol. 2, 162–166
38Purcell, M. et al. (1995) Red/far-red and blue light-responsive regions of maize rbcS-m3are active in bundle sheath and mesophyll cells, respectively. Proc. Natl. Acad. Sci. U. S. A.92, 11504–11508
39Reiskind, J.B. et al.(1997) Evidence that inducible C4-type photo-synthesis is a chloroplastic CO2-concentrating mechanism in Hydrilla,
a submersed monocot. Plant Cell Environ.20, 211–220
40Dixon, R.et al.(1997) Nifgene transfer and expression in chloro-plasts: prospects and problems. Plant Soil194, 193–203
Reference lists in TIBTECH articles
The lists of references accompanying articles in TIBTECH are intended to provide readers with a general indication of where further information on the topic can be found. They are not complete lists of every
paper published on the topic.