Summary The maintenance of plane trees (Platanus acerfo-lia Wild) by regular curtain-like pruning during the vegetative period induced modifications in the distribution and seasonal patterns of carbohydrate reserves in the perennial parts. The unpruned trees were characterized by high and fairly constant concentrations of starch in roots > 5 cm in diameter and a decreasing gradient of starch from the base to the top of the trunk. Starch also accumulated at the trunk--branch junction and at the base of large branches. Curtain-like pruning caused the starch gradient in the trunk to disappear and induced well marked seasonal variations in the starch concentration of roots > 5 cm in diameter. Pruning also eliminated the accumulation of starch at the trunk--branch junction during summer, but it had no effect on the accumulation of starch at the base of large branches. Concentrations and seasonal fluctuations of carbo-hydrates in roots < 0.5 cm in diameter were similar in both pruned and unpruned trees. Repeated cuts or ‘‘short head prun-ing’’ induced the formation of excrescences at the tips of branches that accumulated starch.
Keywords: roots, soluble sugars, starch.
Introduction
Seasonal patterns of carbohydrates have been studied in many woody plant species. Total carbohydrate content of the peren-nial parts of trees of the temperate zone reaches a maximum in the autumn, begins to decrease in late winter and decreases rapidly in early spring. The summer minimum is followed by a phase of starch accumulation in autumn and early winter (Priestley 1964, Kramer and Kozlowski 1979).
We have obtained evidence that reserve compounds are compartmentalized within the tree (Clair-Maczulajtys and Bory 1988, Bory et al. 1991, Bory and Clair-Maczulajtys 1993). In Ailanthus glandulosa (Mill.) Swingle, several com-partments exist: the short orthotropic roots and the tuberized lateral roots are the main carbohydrate storage sites below ground, whereas the base of the trunk and the branches consti-tute the main zones of carbohydrate accumulation in the aerial part of the tree (Bory 1983, Clair-Maczulajtys 1984). In branches, the zones located between two successive growth
units accumulate both starch and proteins. In sweet cherry (Prunus avium L.) trees, the major carbohydrate storage sites are the trunk--branch junction, the trunk--epicormic shoot junc-tion and the visible leaf scars on the trunk (Bory and Clair-Maczulajtys 1991).
Storage sites are classified as permanent or temporary de-pending on whether the storage organs are perennial or decidu-ous. Temporary storage sites include flowers, fruits or seeds, and cataphylls or leaves. In Ailanthus, the cataphylls temporar-ily accumulate lipids and carbohydrates during heterotrophic development of the shoot (Clair-Maczulajtys and Bory 1986). Perry and Simons (1967) suggested that the scale buds of Carya tomentosa Nutt. and Liquidambar styraciflua L. serve as carbohydrate storage sites. In some species, root buds grouped in nodes are characterized by high contents of starch and proteins that are used during shoot development (Clair-Maczulajtys 1984). Permanent storage sites that maintain a high content of compounds for several years or throughout the life of a woody plant include the root system of pioneer trees and the ligneous rays of the trunk of forest trees (Kramer and Kozlowski 1979).
Because little information is available on the effects of pruning on the accumulation and distribution of reserve sub-stances, we have examined the physiological impact of cur-tain-like pruning of plane trees (Platanus acerfolia Wild) on the distribution and seasonal patterns of carbohydrate reserves.
Materials and methods
Twelve 60--70-year-old plane trees growing in urban areas (Champ de Mars, Paris, France) were used. Six of the trees were left unpruned and six trees were subject to curtain-like pruning in the previous July. Curtain-like pruning consisted of reducing the crown in height and spread by shortening branches at suitable points to shape the trees into a paral-lelepipedic form. Studies on the root system were superficial because of the difficulty of sampling tree roots in urban areas. We distinguished between roots having a diameter greater than 5 cm (large roots) and roots of diameter less than 0.5 cm (thin roots).
Effects of curtain-like pruning on distribution and seasonal patterns of
carbohydrate reserves in plane (
Platanus acerifolia
Wild) trees
Y. HADDAD,
1D. CLAIR-MACZULAJTYS
1,2and G. BORY
11 Laboratoire de Physiologie de l’Arbre, Université de Paris 7, 2 place Jussieu, 75005 Paris, France 2 Author to whom correspondence should be addressed
Received May 21, 1992
Once a month from May until the following March, samples were taken with an electric drill. The bit size was selected according to the volume of the organ under study. Trunk samples were taken from the base, the middle and the top of each tree. Three samples were taken from the large branches: the first sample was taken from the trunk--branch junction, the second sample was taken from 80 cm above this level, and the third sample was taken from 160 cm above the trunk--branch junction. These three large branch samples correspond to the trunk--branch junction, Large Branch 1 and Large Branch 2, respectively, in the tables. In the crown, we selected the base of branches of diameter 5--6 cm, the 3--4-cm diameter branches and the twigs. The twigs represented the current-year shoots and the 1-year-old shoots of diameter less than 1 cm. The whole branch samples were collected from both the lower and upper parts of the crown.
In trees subjected to curtain-like pruning, branches were repeatedly cut at the same level (short head pruning), which induced the formation of excrescences at the branch tips (Fig-ure 1). Samples were also taken from different parts of the short head including (1) from the branch supporting the head (5--9 cm diameter) at 30--240 cm below the head, (2) from the short head itself, and (3) from the base of the twigs developed on the short head (Figure 1).
For analysis, 2 g of fresh material was finely ground in 80% ethanol and then homogenized for 10 min with an Ultraturrax homogenizer. Soluble sugars were extracted three times with 50 ml of boiling 80% ethanol under reflux. The total soluble sugar concentration was estimated colorimetrically by the an-throne method (Ashwell 1957). After the extraction of soluble sugars followed by repeated washing with cold water, the residue was hydrolyzed with amyloglucosidase (A 7420, Sigma Chemical Co.) following the method of Thivend et al. (1965), and the glucose liberated by hydrolysis was then quan-tified with a biochemical analyzer (YSI 2700, Yellow Spring Instruments, Ohio).
Results
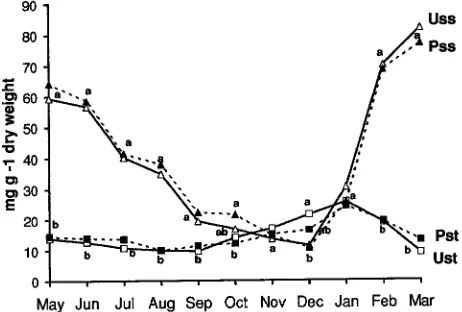
Roots
Concentrations and seasonal fluctuations of carbohydrates of thin roots (diameter 0.1--0.5 cm) were similar in pruned and unpruned trees (Figure 2). The concentration of soluble sugars in thin roots was higher than the concentration of starch except during autumn. Seasonal variations in soluble sugars in thin roots showed an increase from late autumn to early spring and a gradual decrease during the growing period until leaf fall (Figure 2). Starch concentrations of thin roots remained more or less constant throughout the year with a slight increase in late autumn (Figure 2). Large roots (diameter > 5 cm) were characterized by higher concentrations of starch than soluble sugars, especially in unpruned trees (Figure 3). Starch concen-trations of large roots were lower in pruned trees than in
Figure 1. Diagrammatic representation of a short head pruned branch showing different parts used for determining starch and soluble sugar concentrations (bh = branch supporting the head, sh = short head, and tb = twig base).
Figures 2. Seasonal variations in total soluble sugars and starch con-centrations of thin roots of unpruned and pruned plane trees. U = unpruned trees, P = pruned trees, ss = soluble sugars, and st = starch. Mean values (n = 6) followed by the same latter are not statistically different from others for the same month (Tukey’s test, P < 0.05).
unpruned trees except in late autumn. Seasonal variation of starch in large roots was more pronounced in pruned than in unpruned trees and was characterized by a decrease during the growing period and an increase during autumn. Throughout winter, the starch concentrations in large roots of pruned trees decreased, whereas the concentrations of soluble sugars in-creased. Soluble sugars in large roots of unpruned trees showed a similar pattern to that observed in pruned trees, but in winter the variations were less and the concentrations were lower (Figure 3).
Trunk and branches
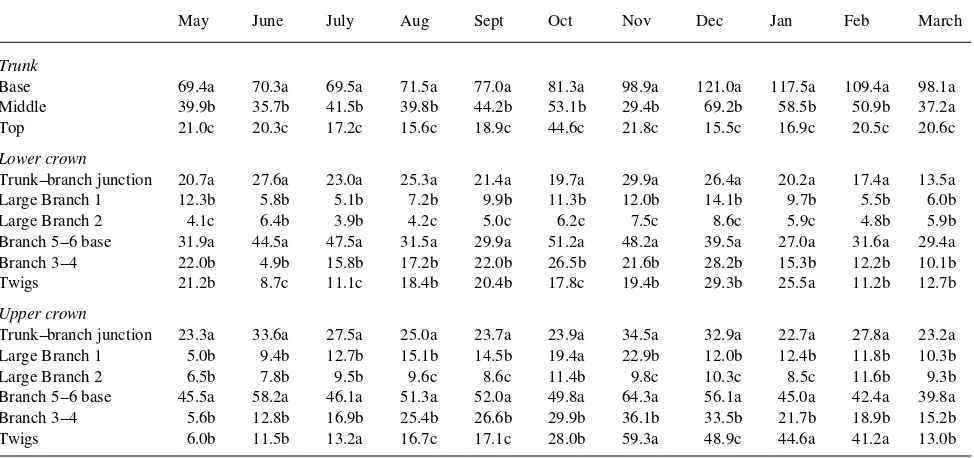
In the trunks of both pruned and unpruned trees, the concen-trations of soluble sugars were generally lower than that of starch (Tables 1 to 4). The trunks of unpruned trees were characterized by a gradient of starch that was stable throughout the year. Starch concentrations were always higher at the base than in the middle or at the top of the trunk. This gradient of starch was less apparent in trunks of pruned trees (Tables 1 and 3), although high concentrations of starch were observed in the upper parts of pruned trees, especially from January to March (Table 3).
In unpruned trees, the accumulation and disappearance of carbohydrate reserves in the upper and the lower parts of the crown were slightly out of phase. In the trunk--branch junction, branches and twigs, the lowest soluble sugar concentrations were observed in the upper crown during September and in the lower crown during October. Highest soluble sugar concentra-tions were observed in the upper crown during February and in the lower crown during March (Table 2). The trunk--branch junction and the bases of the 5--6-cm diameter branches had higher concentrations of starch than of total soluble sugars
except at the end of the winter (Tables 1 and 2). The carbohy-drate concentrations of the large branches were lower than those of the 3--4-cm diameter branches. More of the large branches in the upper part of the crown had high concentra-tions of starch than large branches in the lower part of the crown (Table 1). Seasonal variations in starch and soluble sugar concentrations were similar in the upper and lower crown, with starch concentrations increasing in summer and decreasing in winter and sugar concentrations decreasing dur-ing the summer and increasdur-ing throughout the winter (Table 2). Concentrations of soluble sugars in twigs were generally higher than in branches (Table 2). During the rest period, twigs located in the upper part of the crown had higher concentra-tions of starch than twigs located in the lower crown (Table 1). In pruned trees, there were few differences in carbohydrate concentrations of large branches, 3--4-cm diameter branches and twigs between the upper and the lower parts of the crown (Tables 3 and 4). Twigs had higher concentrations of soluble sugars than branches (Tables 2 and 4). Seasonal variations in carbohydrate reserves of large branches were similar to those at the top of the trunk (Tables 3 and 4). In contrast to unpruned trees, starch accumulation was observed in large branches and 3--4-cm diameter branches of pruned trees during winter (Ta-bles 1 and 3). Seasonal variations of carbohydrate reserves differed among compartments in pruned trees; for example, starch minima were observed during October in the 3--4-cm diameter branches and during May in the twigs (Table 3), and starch accumulation in the twigs occurred before accumulation in the branches (Table 3). In pruned trees, the trunk--branch junction was not a site of starch storage during summer but the bases of the 5--6-cm diameter branches were characterized by high concentrations of starch (Tables 1 and 3).
Table 1. Seasonal changes in concentrations of starch of different zones of unpruned plane trees. Results are expressed in mg gDW−1. For each type
of organ (trunk, trunk--branch junction and large branch, branch and twigs) mean values (n = 6) followed by the same letter are not statistically different for the same month (Tukey’s test, P≤ 0.05).
May June July Aug Sept Oct Nov Dec Jan Feb March
Trunk
Base 69.4a 70.3a 69.5a 71.5a 77.0a 81.3a 98.9a 121.0a 117.5a 109.4a 98.1a Middle 39.9b 35.7b 41.5b 39.8b 44.2b 53.1b 29.4b 69.2b 58.5b 50.9b 37.2a Top 21.0c 20.3c 17.2c 15.6c 18.9c 44.6c 21.8c 15.5c 16.9c 20.5c 20.6c
Lower crown
Trunk--branch junction 20.7a 27.6a 23.0a 25.3a 21.4a 19.7a 29.9a 26.4a 20.2a 17.4a 13.5a Large Branch 1 12.3b 5.8b 5.1b 7.2b 9.9b 11.3b 12.0b 14.1b 9.7b 5.5b 6.0b Large Branch 2 4.1c 6.4b 3.9b 4.2c 5.0c 6.2c 7.5c 8.6c 5.9c 4.8b 5.9b Branch 5--6 base 31.9a 44.5a 47.5a 31.5a 29.9a 51.2a 48.2a 39.5a 27.0a 31.6a 29.4a Branch 3--4 22.0b 4.9b 15.8b 17.2b 22.0b 26.5b 21.6b 28.2b 15.3b 12.2b 10.1b Twigs 21.2b 8.7c 11.1c 18.4b 20.4b 17.8c 19.4b 29.3b 25.5a 11.2b 12.7b
Upper crown
Short heads
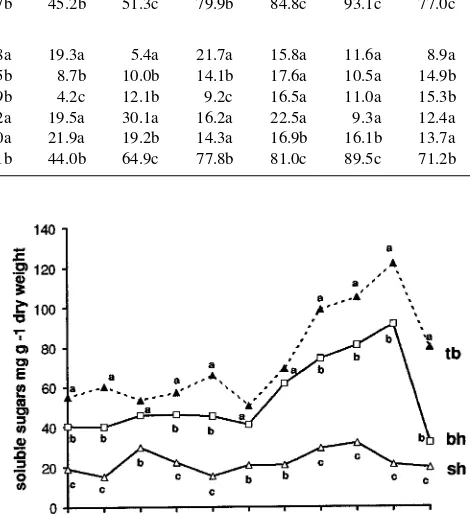
The short heads were distinguished by high concentrations of starch and low concentrations of soluble sugars throughout the year (Figures 4 and 5). The concentrations of starch in the short heads were often higher than in other crown compartments (Figure 4 and Table 3). Seasonal fluctuations of carbohydrates were less pronounced in the short head than in the branch supporting the head or in the twigs.
Discussion
Pruning resulted in qualitative and quantitative seasonal changes in carbohydrate reserves (cf. Clair-Maczulajtys and Bory 1988, Bory and Clair-Maczulajtys 1991, Bory et al. 1991). At the same crown level, large branches, 3--4-cm di-ameter branches and twigs of unpruned plane trees showed similar seasonal variations in carbohydrates. Pruning modified
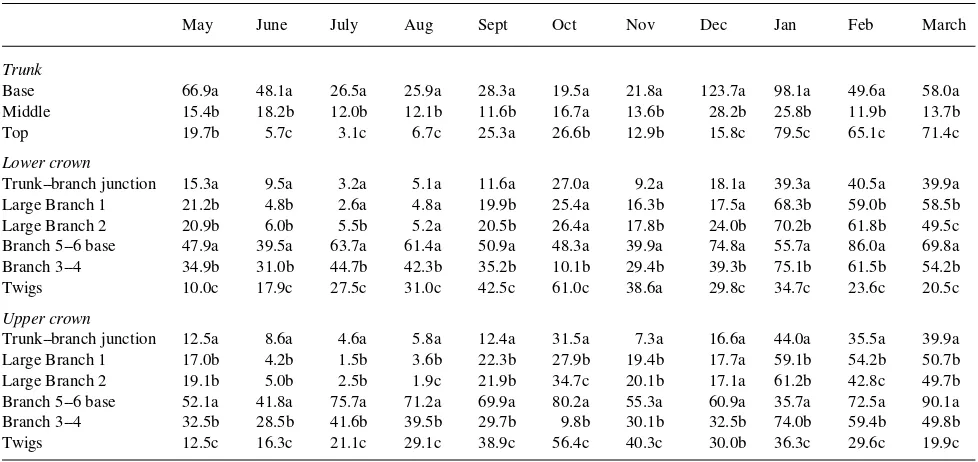
Table 3. Seasonal changes in concentrations of starch of different zones of pruned plane trees. Results are expressed in mg gDW−1. For each type
of organ (trunk, trunk--branch junction and large branch, branch and twigs) mean values (n = 6) followed by the same letter are not statistically different for the same month (Tukey’s test, P≤ 0.05).
May June July Aug Sept Oct Nov Dec Jan Feb March
Trunk
Base 66.9a 48.1a 26.5a 25.9a 28.3a 19.5a 21.8a 123.7a 98.1a 49.6a 58.0a Middle 15.4b 18.2b 12.0b 12.1b 11.6b 16.7a 13.6b 28.2b 25.8b 11.9b 13.7b Top 19.7b 5.7c 3.1c 6.7c 25.3a 26.6b 12.9b 15.8c 79.5c 65.1c 71.4c
Lower crown
Trunk--branch junction 15.3a 9.5a 3.2a 5.1a 11.6a 27.0a 9.2a 18.1a 39.3a 40.5a 39.9a Large Branch 1 21.2b 4.8b 2.6a 4.8a 19.9b 25.4a 16.3b 17.5a 68.3b 59.0b 58.5b Large Branch 2 20.9b 6.0b 5.5b 5.2a 20.5b 26.4a 17.8b 24.0b 70.2b 61.8b 49.5c Branch 5--6 base 47.9a 39.5a 63.7a 61.4a 50.9a 48.3a 39.9a 74.8a 55.7a 86.0a 69.8a Branch 3--4 34.9b 31.0b 44.7b 42.3b 35.2b 10.1b 29.4b 39.3b 75.1b 61.5b 54.2b Twigs 10.0c 17.9c 27.5c 31.0c 42.5c 61.0c 38.6a 29.8c 34.7c 23.6c 20.5c
Upper crown
Trunk--branch junction 12.5a 8.6a 4.6a 5.8a 12.4a 31.5a 7.3a 16.6a 44.0a 35.5a 39.9a Large Branch 1 17.0b 4.2b 1.5b 3.6b 22.3b 27.9b 19.4b 17.7a 59.1b 54.2b 50.7b Large Branch 2 19.1b 5.0b 2.5b 1.9c 21.9b 34.7c 20.1b 17.1a 61.2b 42.8c 49.7b Branch 5--6 base 52.1a 41.8a 75.7a 71.2a 69.9a 80.2a 55.3a 60.9a 35.7a 72.5a 90.1a Branch 3--4 32.5b 28.5b 41.6b 39.5b 29.7b 9.8b 30.1b 32.5b 74.0b 59.4b 49.8b Twigs 12.5c 16.3c 21.1c 29.1c 38.9c 56.4c 40.3c 30.0b 36.3c 29.6c 19.9c Table 2. Seasonal changes in concentrations of total soluble sugars of different zones of unpruned plane trees. Results are expressed in mg gDW−1.
For each type of organ (trunk, trunk--branch junction and large branch, branch and twigs) mean values (n = 6) followed by the same letter are not statistically different for the same month (Tukey’s test, P≤ 0.05).
May June July Aug Sept Oct Nov Dec Jan Feb March
Trunk
Base 11.2a 10.9a 12.5a 13.5a 15.0a 11.9a 9.5a 10.1a 8.4a 10.0a 9.7a Middle 10.6a 11.4a 12.8a 13.0a 10.9b 10.8a 9.4a 13.9b 11.6b 11.1a 10.4a Top 16.5b 18.2b 14.6b 14.1a 15.0a 16.7b 18.2b 13.7a 14.4c 16.8b 20.3b
Lower crown
Trunk--branch junction 12.2a 17.9a 13.6a 12.8a 10.9a 6.5a 12.7a 14.9a 21.4a 26.0a 29.9a Large Branch 1 13.4a 10.1b 9.5b 8.9b 7.2b 5.5a 10.9a 13.4a 17.9b 20.3b 22.7b Large Branch 2 8.9b 11.2b 10.7b 12.1a 8.3c 4.8a 11.0a 14.2a 16.7b 19.5b 23.8b Branch 5--6 base 11.5a 17.8a 19.9a 14.6a 15.2a 9.0a 15.5a 11.3a 14.7a 24.9a 38.6a Branch 3--4 13.3a 25.8b 21.4b 15.8a 16.2a 10.1a 27.0b 35.0b 39.8b 43.4b 51.0b Twigs 44.4b 41.9c 60.8c 55.7b 31.5b 19.5b 36.1c 44.2c 51.5c 69.3c 78.1c
Upper crown
this pattern by delaying starch accumulation in the whole branches and by inducing differences in seasonal fluctuations from one compartment to another. These modifications were probably related to pruning-induced changes in the phenology of the trees (Satoh and Ohyama 1977, Hayden and Emerson 1979, Rom and Ferree 1985, Marini 1986).
The unpruned trees were characterized by stable starch concentrations in the large roots, by a decreasing gradient of starch from the base to the top of the trunk and by differences in the amounts of reserves between the upper and lower crown. Pruning modified the distribution of reserve compounds by
eliminating the differences in reserve storage patterns between the upper and lower crown and by inducing a marked decrease in the carbohydrate reserves of the base of the trunk, which eliminated the starch gradient in the trunk, and of the large roots. These reductions could be associated with partial de-struction of foliage. Defoliation by summer pruning prevents the use of assimilates for plant growth, impedes assimilate accumulation and may hasten assimilate depletion (Bory and Clair-Maczulajtys 1993).
Starch reserves in the roots of Acer saccharum Marsh. (Parker and Houston 1971) and Pseudotsuga menziesii (Mirb.) Franco (Webb and Karchesy 1977) decrease when the leaves
Figure 5. Seasonal variations in total soluble sugar concentrations of different short head pruning parts. bh = branch supporting the head, sh = short head, and tb = twig base. Mean values (n = 6) followed by the same letter are not statistically different from others for the same month (Tukey’s test, P < 0.05).
Table 4. Seasonal changes in concentrations of total soluble sugars of different zones of pruned plane trees. Results are expressed in mg gDW−1.
For each type of organ (trunk, trunk--branch junction and large branch, branch and twigs) mean values (n = 6) followed by the same letter are not statistically different for the same month (Tukey’s test, P≤ 0.05).
May June July Aug Sept Oct Nov Dec Jan Feb March
Trunk
Base 17.2a 11.3a 8.2a 9.4a 19.6a 9.9a 7.5a 20.1a 15.6a 17.7a 22.8a Middle 13.0b 9.5a 8.0a 8.8a 10.8b 9.1a 10.9b 18.5a 17.2a 10.6b 12.9b Top 19.7a 12.8b 8.5a 9.1a 12.3b 9.4a 8.3ab 13.7b 16.0a 9.9b 15.3b
Lower crown
Trunk--branch junction 15.8a 25.4a 11.0a 13.8a 17.5a 11.7a 10.6a 24.8a 19.1a 10.5a 17.5a Large Branch 1 16.9a 9.5b 6.2b 7.2b 12.0b 10.5a 9.3a 13.9b 15.2b 9.9a 16.7a Large Branch 2 17.4a 10.3b 5.0b 6.7b 10.1b 11.2a 9.7a 12.5b 14.9b 11.3a 18.1a Branch 5--6 base 10.8a 3.4a 16.6a 17.6a 19.2a 25.5a 28.0a 14.1a 19.5a 10.2a 9.4a Branch 3--4 12.4a 2.5a 10.9b 9.4b 18.3a 24.0a 17.6b 15.1a 18.7b 15.3b 14.2b Twigs 78.9b 57.5b 50.6c 51.5c 49.7b 45.2b 51.3c 79.9b 84.8c 93.1c 77.0c
Upper crown
Trunk--branch junction 17.2a 14.0a 16.7a 15.8a 21.8a 19.3a 5.4a 21.7a 15.8a 11.6a 8.9a Large Branch 1 16.9a 12.7a 3.3b 4.0b 11.5b 8.7b 10.0b 14.1b 17.6a 10.5a 14.9b Large Branch 2 15.8a 14.5a 6.1a 4.4b 10.9b 4.2c 12.1b 9.2c 16.5a 11.0a 15.3b Branch 5--6 base 9.9a 8.8a 20.3a 19.5a 16.2a 19.5a 30.1a 16.2a 22.5a 9.3a 12.4a Branch 3--4 11.8a 4.9b 12.1b 13.1b 17.0a 21.9a 19.2b 14.3a 16.9b 16.1b 13.7a Twigs 84.2b 60.1c 51.3c 50.6c 47.1b 44.0b 64.9c 77.8b 81.0c 89.5c 71.2b
are removed. In aspen, defoliation causes a decrease in reserve accumulation in young organs and an increase in the mobiliza-tion of carbohydrates in the trunk xylem (Eliasson 1968). Removal of leaves at the beginning of October results in a reduction of root and stem carbohydrate reserves in young apple trees, but the reduction is less if defoliation occurs later in the year (Abusrewill et al. 1983). Removal of summer foliage of Quercus rubra L. induces a decrease in carbohy-drates of roots that is more serious if defoliation is rapidly followed by a new foliation (Parker 1979). Bamber and Hum-phreys (1965) observed the disappearance of starch in the stems of some Eucalyptus species that were defoliated several times.
Pruning often creates new sinks for metabolites (e.g., the development of cicatricial bulges or antifungal substances; Green et al. 1981, Clair-Maczulajtys 1984, Shigo 1985) or the development of epicormic shoots or suckers (Hinze and van Laar 1986, Bory and Clair-Maczulajtys 1991, Bory et al. 1991) that compete with the reconstitution of reserves. The reconsti-tution of the aerial parts that have been removed by pruning also results in depletion of reserves.
The base of the trunk was an important site of carbohydrate storage in the plane trees in the present study. Similar findings have been reported for species that are able to produce stump shoots (Kramer and Kozlowski 1979) and for several pioneer trees including Ailanthus altissima (Mill.) Swingle (Bory 1983), Cercis siliquastrum L. and Catalpa bignonioides Walt. (Bory and Clair-Maczulajtys 1993). The distribution of re-serves in the trunk may explain the ability of plane trees to withstand heavy and repeated pruning (Steinbeck and Nwoboshi 1980). In some species including Platanus, re-peated cuts made on large branches induce the development of large ‘‘short heads.’’ In P. acerifolia and Tilia platyphyllos Scop., the appearance of new zones of carbohydrate accumu-lation occur either in the head itself, in the large branch sup-porting the head, or in the base of the twigs developed on the head (Clair-Maczulajtys and Bory 1988). In pruned plane trees, new storage zones of carbohydrates in the crown in-cluded the small head and the branch supporting the head.
Acknowledgments
The authors thank the municipal Department of Parks and Gardens of Paris, France for technical collaboration.
References
Abusrewill, G.S., F.E. Larsen and R. Fritts. 1983. Prestorage and poststorage starch levels in chemically and hand defoliated ‘‘Deli-cious’’ apple nursery stock. J. Am. Soc. Hortic. Sci. 108:20--23. Ashwell, G. 1957. Methods in enzymology. In Methods in
Enzymol-ogy. Eds. S.P. Colowick and N.O. Kaplan. Academic Press, New York, 3:73.
Bamber, R.K. and F.R. Humphreys. 1965. Variation in sapwood starch levels in some Australian forest species. Aust. For. 29:15--23. Bory, G. 1983. Quelques aspects de la biologie de l’Ailanthus
altis-sima (Mill) Swingle. Mouvements de métabolites, croissance, développement, sécrétion et floraison chez divers types d’arbres. Thèse Doctorat d’Etat. Université Paris 7, 278 p.
Bory, G. and D. Clair-Maczulajtys. 1991. Gourmands et glucides de réserves dans le tronc du Prunus avium L. Rapport Ministère de la Recherche-Eurosylva, 52 p.
Bory, G. and D. Clair-Maczulajtys. 1993. Les métabolites de réserve: un outil de perception de la physiologie de l’arbre entier. In Physi-ologie des arbres et arbustes en zones arides et semi-arides. Eds. A. Riedacker, E. Dreyer, C. Pafdadnam and G. Bory. John Libbey Eurotext, France, pp 97--115.
Bory, G., M.D. Sidibe and D. Clair-Maczulajtys. 1991. Effets du recépage sur les glucides et les lipides de réserve du Faux-Vernis du Japon. Ann. Sci. For. 48:1--13.
Clair-Maczulajtys, D. 1984. Quelques aspects de la biologie de l’Ailanthus altissima. Etude de la double reproduction par graine et par drageonnement en relation avec les métabolites de réserve. Thèse Doctorat d’Etat. Université Paris 7, 441 p.
Clair-Maczulajtys, D. and G. Bory. 1986. Structure et fonction des cataphylles d’Ailanthus glandulosa au cours de l’ouverture du bourgeon. Phytomorphology 36:12--24.
Clair-Maczulajtys, D. and G. Bory. 1988. Modification de la réparti-tion des glucides de réserve sous l’effet de l’élagage, chez deux arbres d’ornement (Platanus acerifolia Wild et Tilia platyphyllos
Scop.). Bull. Soc. Bot. Fr. Actual. Bot. 135:81--90.
Eliasson, L. 1968. Dependence of root growth on photosynthesis in
Populus tremula. Physiol. Plant. 21:806--810.
Green, D.J., W.C. Shortle and A.L. Shigo. 1981. Compartmentaliza-tion of discolored and decayed wood in red maple branch stubs. For. Sci. 27:519--522.
Hayden, R.A. and F.H. Emerson. 1979. Pruning high density peach hedge plantings. Compact Fruit Tree 12:76--78.
Hinze, W.H.F. and A. van Laar 1986. Pruning studies in Pinus radiata. South. Afr. For. J. 137:1--8.
Kramer, P.J. and T.T. Kozlowski. 1979. Physiology of woody plants. Academic Press, New York, 811 p.
Marini, R.P. 1986. Growth and cropping of ‘‘Redhaven’’ peach trees following foliar applications of flurprimidol and paclobutrazol. J. Am. Soc. Hortic. Sci. 111:849--859.
Parker, J. 1979. Effects of defoliation and root height above a water table on some red oak root metabolites. J. Am. Soc. Hortic. Sci. 104:417--421.
Parker, J. and D.R. Houston. 1971. Effects of drought and defoliation on metabolites in roots of black oak seedlings. For. Sci. 17:91--95. Perry, T.O. and R.W. Simons. 1967. Growth of bud scales and leaves
during the winter. For. Sci. 13:400--401.
Priestley, C.A. 1964. The importance of autumn foliage to carbohy-drate status and root growth of apple trees. Rep. East Malling Res. Stn., 1963, pp 104--106.
Rom, C.R. and D.C. Ferree. 1985. Time and severity of summer pruning influences on young peach trees net photosynthesis transpi-ration and dry weight distribution. J. Am. Soc. Hortic. Sci. 110:455--461.
Satoh, M. and K. Ohyama. 1977. Studies on photosynthesis and translocation of photosynthates in mulberry tree. VI. Proc. Crop Sci. Jpn. 46:499--503.
Shigo, A.L. 1985. How tree branches are attached to trunks. Can. J. Bot. 63:1391--1401.
Steinbeck, K. and L.C. Nwoboshi. 1980. Rootstock mass of coppiced
Platanus occidentalis as affected by spacing and rotation length. For. Sci. 26:545--547.
Thivend, P., C. Mercier and A. Gilbot. 1965. Dosage de l’amidon dans les milieux complexes. Ann. Biol. Anim. Biochim. Biophys. 5:513--526.