Combination toxicology of metal-enriched soils:
physiological responses of a Zn- and Cd-resistant ecotype of
Silene
6
ulgaris
on polymetallic soils
Wilfried H.O. Ernst *, Hans J.M. Nelissen, Wilma M. Ten Bookum
Department of Ecology and Ecotoxicology of Plants,Faculty of Biology,Vrije Uni6ersiteit,De Boelelaan1087, 1081HV Amsterdam,The Netherlands
Received 2 July 1999; received in revised form 8 September 1999; accepted 10 September 1999
Abstract
Plants of an ecotype ofSilene6ulgaris(Caryophyllaceae) originating from a Cd-Pb-Zn mine at Plombie`re (Belgium)
were grown on 15 polymetallic soils for a full life-cycle to investigate physiological responses which can help explain previously reported disorders in plants. The degree of regulation of the metal concentration in the young seedlings was a very reliable indicator of the subsequent plant performance. Uptake of Zn could be regulated up to 200 nmol water-soluble Zn g−1dry soil without surpassing 7
mmol Zn g−1dry leaf tissue supporting the hypothesis of a high
regulating potential of Zn-resistant ecotypes. As soon as a certain ecotype-specific threshold was surpassed the Zn concentration in all plant tissues strongly increased. The Cu concentration in roots and shoots had no threshold and showed the tendency to increase near linearly with the external Cu soil concentration. Similar behaviours were found for Cd, Mn and Pb. The metal concentration of seeds was the lowest of all plant parts; nevertheless it increased linearly with increasing concentration of Fe and Zn in the soil. From visible symptoms the degree of chlorosis was positively related with the concentration of Zn, but not with that of Cu, and interrelated with Fe availability. High cyanidin concentrations in leaves were not indicating a surplus of heavy metals, but deficiency in phosphorus and to a lesser degree in nitrogen. Phytochelatins (PCs) were only present in measurable amounts in leaves of plants grown on soils rich in Cu; but PCs amounts in the early vegetative phase could not be related to vegetative and seed biomass at the reproductive stage. Therefore, it is concluded that PCs are a less reliable indicator of metal toxicity during a full life-cycle than the metal concentration of young seedlings. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Chlorosis; Copper; Cyanidin; Lead; Mine soil; Phytochelatin; Seedling
www.elsevier.com/locate/envexpbot
1. Introduction
Orogenic soils are the result of ore outcrops, mining, smelting, or refining activities with a co-occurrence of several heavy metals and a high variation in the concentration of each metal * Corresponding author. Tel.:+31-20-4447050; fax:
+31-20-4447123.
E-mail address:[email protected] (W.H.O. Ernst)
(Ernst, 1974). A restricted number of plant species which have evolved metal resistance mechanisms are able to colonize these polymetallic soils (Ernst et al., 1992). In the analysis of the impact of metal mixtures on plant performance, metal-resistant ecotypes can at least partly help to overcome the disadvantage of sensitive plants on metal-contam-inated soils. In addition to many short-term ex-periments with one metal (Sanita´ di Toppi and Gabbrielli, 1999), only a few experiments on soils artificially contaminated with one metal have been extended to a full life-cycle of plants (Sheppard et al., 1993). Metal-resistant plants offer an excellent opportunity to analyse the impact of complex metal mixtures on the performance of plants dur-ing a full life-cycle. In addition, such plants can be exposed to metal mixtures in a realistic environ-mental soil setting, i.e. with differences in pH, organic matter, Ca content and other soil parame-ters because these plants are also adapted to low levels of major nutrients in orogenic soils (Ernst, 1974). The few studies on combination toxicology of heavy metals analysed plants which were artifi-cially exposed to enhanced concentrations of these elements in hydroponics for a short period (Wal-lace and Abou-Zam Zam, 1989; Wal(Wal-lace and Berry, 1989; Sharma et al., 1999). For our study we have selected the perennial herbSilene6ulgaris (Caryophyllaceae) being characteristic for many metal-enriched soils in Europe (Ernst, 1974). In
contrast to many other metal-resistant plants, S.
6ulgaris has nearly no symbiosis with arbuscular
mycorrhizal fungi (Ernst et al., 1990; Pawlowska et al., 1996; Hildebrandt et al., 1999). Therefore the roots are directly exposed to the metal concen-tration of the soil and changes of metal speciation and benefits for the host by mycorrhizal fungi can be excluded (Dueck et al., 1986; Hildebrandt et al., 1999).
With regard to morphological and ecological parameters, we have shown that a Zn- and Cd-re-sistant ecotype of S.6ulgaris reacted quite differ-ent in the various phases of its life-cycle (Ernst and Nelissen, 1999). Here, we report on the phys-iological responses of this ecotype to an exposure to combinations of the heavy metals Cd, Cu, Fe, Mn, Pb and Zn throughout a full life-cycle of the Zn- and Cd-resistant plants (Ernst and Nelissen, 1999). We tested the following hypotheses:
1. As soon as the metal concentration of the soil solution exceeds the Zn- and Cd-resistance of the metal-resistant ecotype, disturbance of the nutrient uptake will occur, partly being visible by discoloration.
2. The metal concentration in young seedlings is an indicator for the survival chance up to seed maturity with a negative relationship between metal concentration and survival.
3. Due to low Cu concentration which diminishes the performance of this ecotype for Cu by
50%, i.e. EC50(Schat and Ten Bookum, 1992),
Cu-enriched soils will strongly impair the metabolism which will be expressed by an enhanced synthesis of phytochelatins (PCs).
2. Materials and methods
2.1. Soils and growth conditions
Seeds of a Zn- and Cd-resistant ecotype of S.
6ulgaris (Moench) Garcke were collected from a population of the mine tailings at Plombie`res/
Bel-gium with high EC50-values for Zn and Cd, and
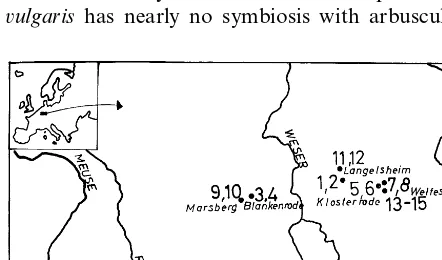
low values for Cu (Verkleij and Prast 1989; Schat and Ten Bookum 1992). Pots of 750 ml volume were filled with 1.3 kg air-dried metal-enriched soils collected on the following sites (Fig. 1):
Zn – Pb-mine tailings at Wildemann/D (1, 2), at
Blankenrode/D (3, 4) and Plombie`res/B (16), the Fig. 1. Sampling sites of soils (numbers 1 – 16) and seeds of the
abandoned Cu-mine at Marsberg/D (9, 10), the Bronze-Age smelting site at the banks of the river Innerste near Langelsheim (11, 12), the Zn –
Cu-mine tailings at Klosterrode/D (5, 6) and at
Welfesholz/D (7, 8, 13, 14, 15). After chemical
analysis it worked out that the sampling site of soil 4 was a mine pit which was filled up with clay material from other origin; therefore plants grow-ing on this soil were not incorporated in the analysis. The total and water-soluble metal con-centration of these soils was reported by Ernst and Nelissen (1999). Due to low organic matter of the orogenic soils, all water-soluble metals except Cu were present as free metal ions, as analysed by a batch-column-batch procedure using the cation exchange resins Amberlite CG 120 and Chelex 100 (cf. Ernst and Nelissen 1999). From water-soluble Cu 12 – 20% was bound to organic com-plexes; only in soils (7, 9) where birch leaves were blown in from adjacent woodlands, 35 – 45% of the water-soluble Cu was complexed.
For the analysis of seedling performance, 100 seeds were sown in each of three pots per sam-pling site; 20 plants were harvested 14 days after emergence. In another series, 30 seeds were sown per pot in triplicate per sampling site. Immedi-ately after emergence, they were thinned to ten seedlings per pot and kept under the below men-tioned conditions. After 5 weeks of growth (vege-tative phase of the life-cycle), five plants per pot were harvested for growth and mineral analysis and two plants were taken for the analysis of physiological parameters. The remaining three plants were grown up to seed maturity which was achieved 9 months after emergence.
The plants were kept in a greenhouse at a
temperature cycle of 20/15°C (day/night) from
November to April and then at 25/18°C for the
rest of the life-cycle. Additional radiation was supplied for 10 h daily from mercury iodide lamps
providing a radiation flux of 235 mmol m−2 s−1
at medium plant level.
2.2. Mineral elements
In the seedling stage (2 weeks), 20 seedlings from each pot were harvested, separated into root and shoot (hypocotyl and two cotyledons). The
shoots were washed twice for 30 s in demineral-ized water by slight brushing to remove poten-tially adhering soil particles, dried at 60°C for 48 h and pooled to two subsamples for analysis. The roots were discarded because it was not possible to clean them sufficiently from adhering soil parti-cles. At harvest (5 weeks and 9 months of growth), plants from each treatment at each age were separated into roots, stalks and leaves and at the reproductive phase additionally into calyx, capsule and seeds. Although roots are the first target of a metal surplus, chemical analysis of roots grown in soil is impeded by a high affinity to soil particles and adsorbed metals (Ernst, 1995). Therefore it was necessary to clean the roots first mechanically by a brush, with the risk of loosing fine roots which especially was the case in seedlings. As next step metals from the root surface were desorbed by a solution in 0.1 M
SrCl2-solution for 30 min at 4°C. In the present
study, SrCl2 was used because the more effective
Pb(NO3)2 (Harrison et al., 1979) would not allow
Pb analysis of the roots.
The various plant parts and roots were dried at 60°C for 48 h. Plant material (50 – 100 mg per sample, if present) was mineralised in Teflon
bombs at 140°C with aqua regia (HNO3/HCl, 3:1,
v/v) over night and the diluted solution analysed
by atomic absorption spectrometry (Perkin Elmer AAS 1100) or at low concentration of Cd, Cu and Pb by graphite furnace AAS (Perkin Elmer AAS
2100). La(NO3)2 was added to enhance
atomiza-tion of Ca and Mg. Phosphorus was determined by a spectrophotometric method after formation of a blue ascorbic acid-phosphorus complex (Chen et al., 1956). Carbon and N were analysed by column chromatography after burning the sample in pure oxygen (Kirsten, 1979) in a Perkin
Elmer CHN analyser. Mature leaves of Populus
nigraL. were used as internal laboratory reference material. Chemical analysis of soils are described by Ernst and Nelissen (1999).
2.3. Plant pigments
Table 1
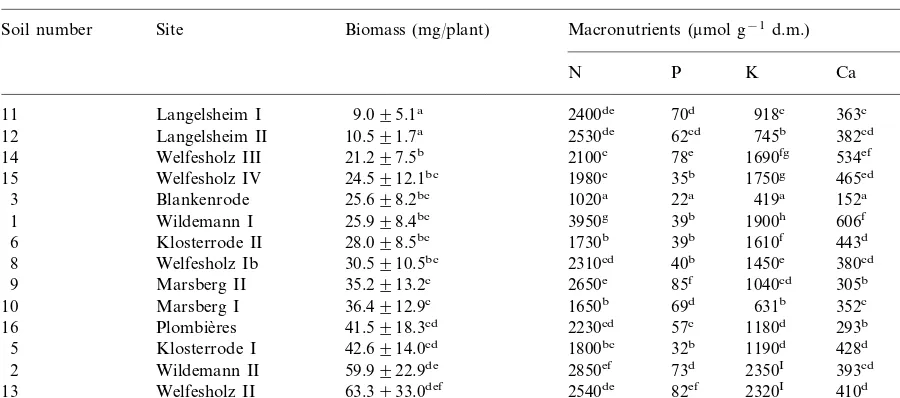
Mean plant biomass (91 SE) and mean concentration of macronutrients in leaves of the Zn–Cd resistant ecotype Plombie`res ofS. 6ulgarisgrown for 5 weeks on orogenic soils in a greenhousea
Biomass (mg/plant)
Welfesholz IV 1750g
15 465ed 378e
Klosterrode II 1610f
6 443d 529f
Marsberg I 631b
10 352c 137b
Wildemann II 2350I
2 393cd 173c
Welfesholz II
13 63.3933.0def 2540de 82ef 2320I 410d 356e
95.8928.4fg 2170c 87f 1790g 471e 215d
7 Welfesholz Ia
aThe plants are ranked according to biomass increase. Values with different superscripts indicate significant differences at least
atPB0.05. The biomass data are from Ernst and Nelissen (1999).
leaf pairs after extraction with 80% (v/v) aqueous acetone in the presence of small amounts of
quartz sand and Na2CO3 and centrifugation. The
absorption of the supernatant was measured at 470, 647 and 663 nm in a Pharmacia Ultraspec III. The concentration of chlorophyll a and b and carotinoids were calculated using the equations given by Lichtenthaler (1987).
Anthocyanins were extracted by grinding the oldest leaves of 5 week-old plants with mortar and pestle in the presence of quartz sand. The extraction medium was methanol/HCl (99/1, v/v). After centrifugation, absorbance spectra of the supernatant were recorded with a Pharmacia Ul-traspec III at 528 nm (Kakegawa et al., 1991), which was the wavelength with the maximum extinction. Cyanidinchloride was taken for cali-bration of the cyanidins.
2.4. Phytochelatin analysis
For the analysis of phytochelatins 3 – 5 pairs of mature leaves were excised from two 5-week-old plants per pot, three pots per soil, and immedi-ately frozen in liquid nitrogen. After
homogeniza-tion with quartz sand and centrifugahomogeniza-tion at
27 000×g for 20 min at 4°C the supernatant was
analysed by a HPLC assay using post-column derivatization or with 20 mM monobromobimane (Sneller, 1999) modified after Rijstenbil and Wijn-holds (1996). The samples were lyophilized and stored under vacuum until detection.
2.5. Statistics
Correlation between the various measured parameters were calculated and tested for
signifi-cance (PB0.05) by one way ANOVA. Multiple
comparison among means based on equal samples sizes were made by application of the T-method (Sokal and Rohlf, 1995).
3. Results
3.1. Mineral elements
3.1.1. Major nutrients
of the major nutrients in the leaves of plants grown on the various orogenic soils (Table 1) varied the least for P with a factor of 4.0 (from 22
to 87mmol P g−1 dry mass) and the most for K
and Mg with a factor of 5.6 (from 419 to 2350
mmol K g−1) and 6.5 (from 106 to 690mmol Mg
g−1), respectively; the concentration of N and Ca
varied by a factor of 4.0 (from 1020 to 3950mmol
Table 2
Change of the mean metal concentration in leaves during growth from seedling to mature plants of the Zn- and Cd-resistant ecotype Plombie`re ofS.6ulgarison four orogenic soilsa
Plant mass
Soil number Plant age (days) Metal concentration (mmol g−1dry mass)
Fe Mn Zn Cu Cd Pb
aOn the soil 3 and 12 the plants did not survive to maturity. Among each site and element data with different superscripts are
significantly different at least atPB0.05.
bn.d.; not determined.
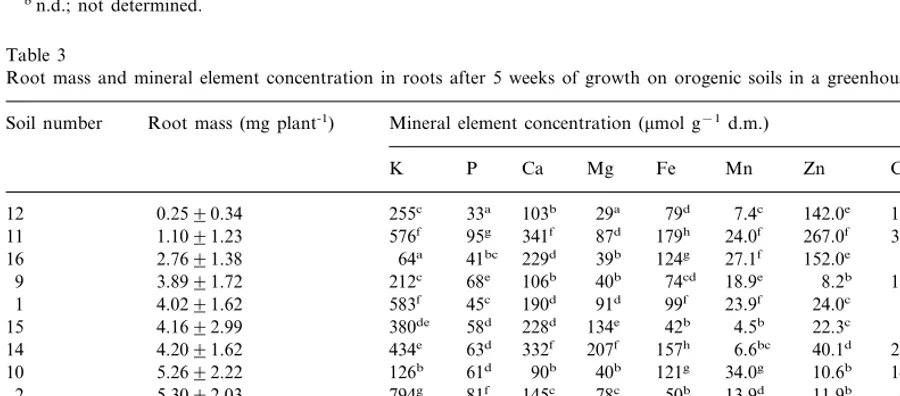
Table 3
Root mass and mineral element concentration in roots after 5 weeks of growth on orogenic soils in a greenhousea
Soil number Root mass (mg plant-1) Mineral element concentration (mmol g−1d.m.)
Zn
aThe mean root mass (91 SE) is based on six plants, two per pot of each soil. The analysis of mineral elements is the mean of
Table 4
Metal concentration in seeds of plants of the Cd–Zn-resistant ecotype ofS.6ulgarisgrown on orogenic soilsa
Element concentration (mmol g−1 d.m.) Soil number
aValues with different superscripts are significantly different
atPB0.05.
dons was quite different on the various soils already 14 days after emergence as shown by the selected data set comprising plants with the highest (soil 7) and lowest (soil 12) biomass and with a medium biomass, but dying prior (soil 3) or surviving up to (soil 6) seed ripeness (Table 2). Levels of Zn were the highest of all heavy metals; concentration below 5mmol g−1seedling dry mass allowed the finaliza-tion of the life-cycle. The regulafinaliza-tion of the Zn level was quite different on the various orogenic soils: it decreased significantly (PB0.01; soil 7) or doubled (PB0.01; soil 6) without surpassing the obviously critical level of 10mmol Zn g−1leaf dry mass (soil 3, 12) up to the end of the life-cycle. A decrease of the Zn level in seedlings on soil 3 by nearly 50% within 3 weeks was insufficient for survival. Levels
of approximately 1 mmol Cu g−1 seedling did
obviously not hamper the further development of the plants. Lead concentration above approxi-mately 0.3mmol g−1seedling may have contributed to seedling mortality. In all seedlings, the Cd
concentration strongly (PB0.001) decreased
be-tween 2 and 5 weeks after emergence.
Metal concentrations in roots are the result of uptake and translocation to the shoot. Root growth was severely diminished (Table 3) if the metal concentration in the roots was above approximately
20 mmol Zn, 5mmol Cu g−1 and/or 0.1 mmol Cd
g−1 dry mass (soil 11, 12, 16).
N g−1and from 152 to 606
mmol Ca g−1). None
of the nutrients in plant leaves, however, was significantly (PB0.05) related to the biomass pro-duction within 5 weeks after emergence (Table 1) or up to seed maturity (Table 5). The concentration of major nutrients in roots (Table 3) was also not related to growth performance.
3.1.2. Hea6y metals
The metal concentration of hypocotyl and
cotyle-Table 5
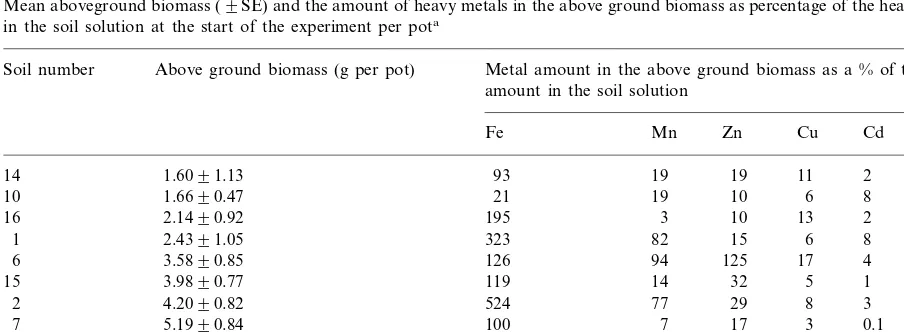
Mean aboveground biomass (9SE) and the amount of heavy metals in the above ground biomass as percentage of the heavy metals in the soil solution at the start of the experiment per pota
Soil number Above ground biomass (g per pot) Metal amount in the above ground biomass as a % of the amount in the soil solution
Fe Mn Zn Cu Cd Pb
Table 6
Concentration of chlorophylls (Chl a, Chl b) and cyanidin in the Zn- and Cd-resitant ecotype ofS.6ulgarisafter 5 weeks of growth on orogenic soils in a greenhousea
Chl a (mg g−1fresh wt.)
Soil number Chl b (mg−1fresh wt.) ratio Chl a/b cyanidin (
mmol g−1fr.wt.)
Chlorotic
2 0.36790.051 0.15290.020 2.41 1.7290.22
0.12090.008
7 0.38790.037 3.23 3.0890.41
0.04190.010 0.93
0.03890.009 n.d.b
11
0.04690.008
12 0.04890.009 0.96 n.d.
14 0.24490.024 0.16690.019 1.47 3.2790.37
Chlorotic and rich in cyanidin
1 0.26790.035 0.13190.014 2.04 6.0290.81
5 0.40690.044 0.18990.022 2.15 9.5091.02
0.23190.031 1.99
0.46090.040 5.4190.64
16
Rich in cyanidin
0.67890.043 1.20
0.81490.025 9.1490.87
3
0.68290.036
6 0.35890.028 1.91 6.7190.70
0.25990.026 2.81 8.6890.93 0.72990.042
15
Green plants
8 1.07090.098 0.28490.026 3.77 3.3790.45
0.29490.034 1.92
0.56590.041 2.3190.12
9
0.72490.055
13 0.21790.030 3.34 2.6690.23
10 0.66290.043 0.32590.021 2.04 2.9490.42
aData are the mean (91 SE) of three plants, one per pot, per soil. bn.d.; not determined.
Table 7
Mean concentration (91 SE) of phytochelatins (PC2, PC3) and heavy metals in mature leaves ofS.6ulgarisgrown for 5 weeks on orogenic soils in a greenhousea
Soil number PC2 (nmol g−1d.m.) PC3 (nmol g−1 d.m.) Element concentration (
mmol g−1d.m.)
Zn Cu Cd Pb
B2.0 5.5d
1 B2.0 0.16ab 0.049g 0.94e
2 B2.0 B2.0 4.3c 0.09a 0.034f 1.13ef
B2.0 16.5f 0.13a
B2.0 0.025e
3 0.81e
4.4942 5.3cd 1.02e
5 10.795.3 0.015d 0.09c
B2.0 7.5e 1.39e
5.791.7 0.016d
6 0.22d
7 B2.0 B2.0 2.6b 0.24b 0.008bc B0.01a
11.8915.2 1.6a 2.13g
18.194.5 0.001a
8 B0.01a
12.5915.2 2.7b 3.36h
9 103.0947.5 0.031ef 0.03b
B2.0 3.2bc 0.35c
B2.0 0.005b
13 0.05b
B2.0 7.0e 3.00h
14 6.690.2 0.010c 0.27d
B2.0 4.2c 0.60d
B2.0 0.016d
15 0.09c
B2.0 102.5g 0.38c
16 B2.0 0.820h 0.29d
Fig. 2. (Continued)
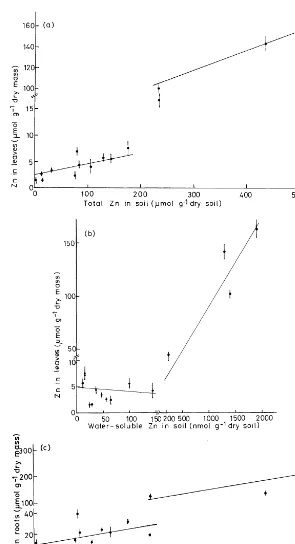
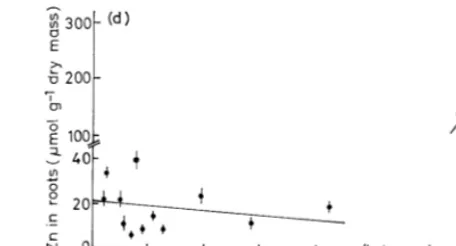
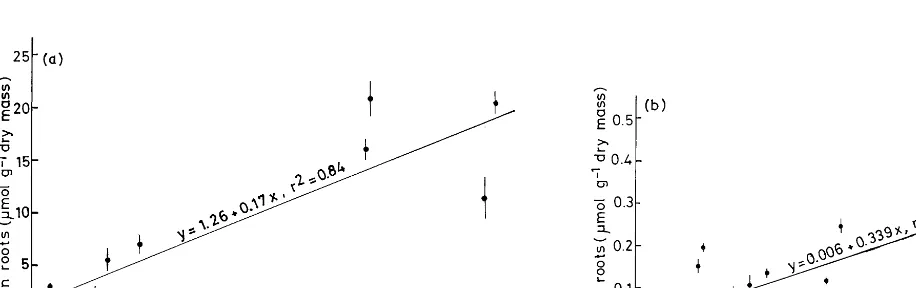
With regard to Zn concentration in plants, there were two response ranges. The Zn concen-tration in leaves and roots increased with
increas-ing total soil Zn up to 220mmol Zn g−1 dry soil
(Fig. 2a, c), but it was kept nearly constant if it was related to the water-soluble Zn up to 150
nmol Zn g−1dry soil in leaves (Fig. 2b) and 220
nmol water-soluble Zn g−1 in roots (Fig. 2d). In
this range the Zn concentration in roots remained
below 40 mmol, in leaves below 8 mmol g−1 dry
mass. On its soil of origin (16), the Zn uptake remained within this range, but the translocation into leaves resulted in very high Zn concentrations
(above 100 mmol Zn g−1 dry mass). The
regula-tion of Zn uptake by roots obviously failed on extremely Zn-enriched soils (3, 11, 12).
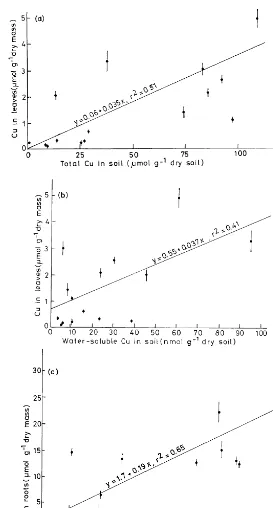
The Cu concentration of leaves increased more or less linearly with the water-soluble and total Cu concentration of the soil, however, with a low power of the regression function (Fig. 3a, b). Only Cu in roots and the total soil Cu concentration (Fig. 3c, d) had a significantly linear relationship
(PB0.02). The concentration of Cd and Pb in
roots (Fig. 4a, b) were linearly related with the total soil concentration of Cd (PB0.01,r2
=0.52)
and Pb (PB0.001, r2=0.84). In the case of the
above-ground plant parts, the concentration of Cd, Fe, Mn, and Pb was not correlated with the metal concentration in the soil (solution).
Enhanced metal exposure may affect the metal loading of seeds and thus burden the next genera-tion. Although the metal content in seeds was low compared to that in all other plant parts (roots, leaves, stalks, calyx and capsules) at the time of
seed maturity (data not shown), the metal concen-trations of the seeds (Table 4) varied by a factor of 2.4 for Fe, 4.3 for Cu and Zn, 6.4 for Mn between the various orogenic soils. The concen-tration of Cd and Pb in seeds remained below the
detection limit of 0.001 and 0.01 mmol,
respec-tively. For two elements there was a linear rela-tionship of the seed metal concentration with that of the soil: The Zn concentration of seeds
signifi-cantly (PB0.001) increased with total and
water-soluble Zn in the soil; the Fe concentration was significantly (PB0.001) related to the total iron level of the soil.
In our experiments with a defined soil mass and without leaching losses, availability of metals to and uptake of metals by plants can be estimated by the ratio of the amount of metals in plants and the amount of water-soluble metals in the experi-mental unit (Table 5). From the water-soluble amount of Cd and Pb less than 10 and 13%, respectively, was accumulated in the above-ground biomass. At maximum one third of water-soluble Cu was used by the plants, whereas the use of the water-soluble Zn varied between 10 and 240%; a value above 100% indicates that a replen-ishment of the water-soluble fraction was neces-sary to keep up with the accumulation in the plant. If plants took up more than 40% of the
amount of water-soluble Zn, they became
Fig. 3. (Continued)
parts. In all other soils, the Fe amount in the plant was nearly equal to the water-soluble amount in the pots (93 – 126%) except on soils from the Zn mines at Plombie`res (16) and Wilde-mann (1, 2) and the Cu mines from Klosterrode (5) where a replenishment up to a factor of 4 was necessary to cover the plant demand.
3.2. Cyanidins and chlorophylls
Plants growing on zinc-enriched soils at Wilde-mann (1), Plombie`res (16), and Blankenrode (3), and the Cu – Zn soils at Klosterrode (5, 6), and
Welfesholz (15) had dark-red leaves due to high concentrations of cyanidin varying from 5 to 10
mmol cyanidin g−1fresh weight (Table 6). Leaves
of plants grown on several of these soils (1, 5, 16) were chlorotic in the upper part of the shoot and rich in cyanidin in the lower part of the shoot. The concentration of cyanidin was negatively
re-lated with the P concentration (r2
=0.83, PB
0.001) and not significantly related with the leaf N concentration (r2
=0.25, P\0.05).
After 5 weeks of growth, plants on the poly-metallic soils from Wildemann (1, 2), Klosterrode (5), Langelsheim (11, 12) and Welfesholz (7, 14)
and on the Zn-soil from Plombie`res (16) developed symptoms of chlorosis (Table 6). The plants on the soils with the highest metal concentration (11, 12) contained very low chlorophyll a and b and were nearly yellow-whitish. They died prior to flowering. All other chlorotic plants except those on soil 5 were delayed in flowering and had a low biomass produc-tion. The degree of chlorosis, i.e. the chlorophyll concentration was negatively (r2=0.53,PB0.01) correlated with the Zn concentration of the leaves. In non-chlorotic plants, the mean chlorophyll
con-centration varied from 0.56 to 0.81 mg g−1
fresh weight. One exception to this rule were plants with very stunted growth on one of the Zn-enriched soils (3). The leaves had very high Zn concentrations combined with the highest chlorophyll content of all investigated non-chlorotic plants (1.49 mg chlorophyll g−1fr. wt. versus 0.85-1.35 mg chloro-phyll g−1fr. wt in normal green plants, Table 6).
3.3. Phytochelatins
Only leaves of plants grown on Cu mine soils (9) and on polymetallic soils with Cu concentration
above 38mmol Cu g−1dry soil had phytochelatin
(PC2) levels above 2 nmol SH equivalents per g dry mass (Table 7). Plants with leaf Cu concentration
above 1mmol Cu g−1dry mass contained also PC3
(5, 6, 9). All plants on soils high in Zn, but low in Cu had no detectable PC values. The Cd concentra-tion of the soils was obviously to low to induce PC synthesis.
4. Discussion
Heavy metal-resistant ecotypes are mostly resis-tant to those heavy metals which are in surplus in their natural environment (Ernst et al., 1992). It was expected that the Cd- and Zn-resistant ecotype of S.6ulgaris will thrive well on all soils high in Cd and Zn with the exception of the soils with Zn and Cd concentrations far above of that from the site of origin, i.e. Plombie`res (cf. the Bronze Age smelting sites 11 and 12). The death of seedlings 6 weeks after emergence on these latter soils (Ernst and Nelissen, 1999) confirmed this expectation in contrast to that on the Zn mine soil (3). On soil 3
the low pH of 3.9 enhanced the mobility of Cd, Pb and Zn and resulted in a very high uptake of these heavy metals within the first 2 weeks after seedling emergence (Table 2). Despite a very reasonable biomass of 5-week-old seedlings and a decrease of the metal concentration, the internal concentrations of Zn and Cd were obviously too high to ensure further growth.
Due to a lack of Cu-resistance of the Plombie`res ecotype (Schat and Ten Bookum, 1992) and due to the specificity of the various metal resistant mech-anisms (Ernst et al., 1992), it was expected that the Zn – Cd-resistant ecotype may have problems to perform well on Cu-enriched soils. But the biomass production on Cu-enriched soils was equal to or surpassed that of the Plombie`res ecotype on its soil of origin (cf. Tables 1 and 3). This unexpected result may indicate beneficial metal interactions on poly-metallic soils in contrast to the reported synergistic responses of a metal-sensitive ecotype ofS.6ulgaris to high Zn and Cu as well as high Zn and Cd (Sharma et al., 1999).
4.1. Metal impact on roots
The accumulation pattern of the various heavy metals in roots showed that the Cd- and Zn-resistant ecotype ofS.6ulgariswas quite differently affected by the water-soluble metal concentrations.
In contrast to Zn, the uptake of Cu can not regulated over a wide external concentration range. At mono-metal exposure to Cu the most rapid physiological disturbance of non-adapted ecotypes is that of the integrity of the root plasma membranes (De Vos et al., 1989, 1991). It resulted
in K+-leakage already a few minutes after
expo-sure as soon as the resistance limit to SH-reactive metals (Ag, As, Cu, Hg) was surpassed (Wain-wright and Woolhouse, 1977). As a result of this process, the K concentration should be dimin-ished in metal-affected roots. The consequences of this K+
-leakage for the K status of the plants has never been measured in short-term exposure ex-periments. In a medium-term hydroponic experi-ment with Cu-sensitive and Cu-resistant ecotypes of S. nutans, the K status of roots and shoots, however, was not affected by exposure to increas-ing Cu concentration (Ernst, 1975). In soil-grown plants, K+-efflux from roots to the soil solution,
however, cannot be directly measured due to the
generally high K+-concentration in soil solutions
of more than 1 mmol l−1 (Haby et al., 1990). In
the experiments of this study, there was no
signifi-cant (PB0.05) relationship between the K
con-centration and the Cu concon-centration of the roots (cf. Table 3) or the Cu concentration of the soil (cf. Fig. 3a, b). Obviously, plants exposed for a full life-cycle to enhanced Cu concentrations can
repair the K+-leakage of the root plasma
mem-brane. At multiple metal exposure, this impact of Cu may be mitigated by a moderate surplus of Zn because Zn is important for membrane integrity by protecting membrane lipids and proteins
against oxidative damage of a Cu excess
(Marschner, 1995). The relatively good growth of the Zn – Cd resistant ecotype on moderately Cu-enriched soils (Ernst and Nelissen, 1999) may be a prove of this statement.
4.2. Metal impact on shoots
A damaged root will impair the ion uptake and water economy, partially via the K metabolism of
the plant during the further development.
Subtoxic to toxic metal concentration in seedlings were already analysed prior or at the same time as the first chlorosis was visible. At a total and a
water-soluble Zn concentration of more than 200 mmol and 0.5 mmol g−1dry soil, respectively, the uptake and translocation of Zn to the shoots was too high to prevent the plant from severe toxicity and finally death. Although the translocation of metals from roots to shoots may be delayed in time (Lolkema et al., 1984), it was sufficiently
rapid in S. 6ulgaris so that within a few days or
weeks the metal concentration of stalks and leaves was a good indicator of the metal exposure. The Zn- and Cd-resistant ecotype had a relatively high
threshold for Zn, behaving up to 200 mmol Zn
g−1
dry soil as a shoot Zn excluder sensu Baker (1981) but perhaps better defined as Zn-regulator, whereas the linear relation between plant and soil Cu was similar to that of a metal indicator sensu Baker (1981).
Detoxification of metals in plant tissues and cells is necessary to ensure survival up to repro-duction and seed prorepro-duction as the ultimate scope for the maintenance of a population. With the exception of an enhanced Zn transport across the tonoplast in this Zn-resistant ecotype (Chardon-nens et al., 1999) most of the processes are related to metal transport via the xylem, i.e. preferential accumulation of Cd and Zn in the leaf epidermis of S. 6ulgaris (Chardonnens et al., 1998), precipi-tation of heavy metals in cell walls of various
ecotypes of S. 6ulgaris (Ernst and Weinert, 1972;
Bringezu et al., 1999) and the metallophytes Thlaspi caerulescens (Va´zquez et al., 1992) and Minuartia 6erna(Neumann et al., 1997), and in a few plant species decontamination by glandular tissue (Ernst, 1974; MacFarlane and Burchett, 1999).
4.3. Metal impact on seeds
con-tributed to the metal loading of the young seedlings. Therefore the relatively high concentra-tion of Cd, Cu, Pb and Zn in the seedling two weeks after emergence (cf. Table 2) may be not only the result of metal uptake and translocation to the cotyledons, but also the carry-over burden from the field-collected seeds. How this burden will affect germination and seedling establishment in the field is not known, but may explain the sometimes low germination percentage (Ernst and Nelissen, 1999).
4.4. Physiological biomarkers: plant pigments
The above-mentioned detoxification processes may impede a direct relation between metal
con-centration and metal toxicity. Physiological
biomarkers may be another reliable early warning system in plants (Ernst and Peterson, 1994), as long as they also predict plant performance up to reproduction. From the various metabolic pro-cesses which are known to give a good correlation between short-term metal exposure and toxicity (for an overview see Ernst, 1998) most are studied in roots of plants growing in nutrient solution. Visible changes of plant colours, i.e. increased anthocyanidin concentration and loss of chloro-phyll (chlorosis), may be a first indication of an insufficient detoxification of the metals resulting in deregulation of a plant’s physiology. These symptoms are expected to be non-specific, not necessarily related to a surplus (Ernst, 1974), but also to a shortage of metals (Khan et al., 1998).
4.4.1. Chlorosis
Chlorosis is a parameter of a disturbed metabolism by hampering chlorophyll synthesis. In severely chlorotic plants of the Zn- and
Cd-re-sistant ecotype Plombie`res of S. 6ulgaris the
chlorophyll content was decreased by more than 90% on soils with a surplus of many heavy metals, i.e. Cd, Cu, Pb and Zn (11, 12). A decrease in the chlorophyll content was reported from various plant species grown on soils naturally enriched with metals, especially Cu all over the world (Reilly and Reilly, 1973; Ernst, 1974; Vardaka et al., 1997). In the present study, low chlorophyll concentrations could not be related to Cu, but
partially to a surplus of Zn. This correlation may be indirect. A surplus of Zn can impede the Fe uptake as shown earlier for the
Zn-hyperaccumu-lator Cardaminopsis halleriand other Zn-resistant
plant species (Ernst, 1996). One of the conditions causing chlorosis may be the low availability of Fe in the soil solution. The water-soluble Fe concentration in soils 5 and 16 (cf. Ernst and Nelissen, 1999) and the Fe demand of the plants may indicate an imbalance resulting in Fe-chloro-sis due to a too slow replenishment of Fe in the soil solution. Iron shortage of the soil will stimu-late the roots of dicotyledonous plants to exudate protons and acidify the rhizosphere (Marschner, 1995), thus enhancing the uptake of other heavy metals (Ernst, 1996). Another aspect of chlorosis
may be a Fe/Zn interaction causing cytosolic Fe
shortage. The high Fe concentration in leaves of plants grown on soil 11 and 12 showed that the chlorosis was not due to Fe-deficiency in the soil (Marschner, 1995). It may be the result of an insufficient cellular availability of reduced Fe in the presence of a surplus of Zn or the competition of Zn for the Fe binding sites during heme synthe-sis (Van Assche et al. 1979). Plants with a medium degree of chlorosis on soils 1, 2, and 6 survived up to seed maturity, but with increasing plant age the
Fe/Zn ratio decreased from 1.5 to 0.2. Thus the
replenishment of iron in the soil solution and the competition of iron with zinc in the cellular
metabolism are two completely independent
parameters thus reducing the indicative value of chlorosis.
4.4.2. Cyanidin
Anthocyanins can be formed as a reaction to a lot of adverse environmental conditions. At nutri-ent shortage, such as N- and P-deficiency, a
sur-plus of carbohydrates can be stored as
con-centrations are not indicating a surplus of heavy metals.
4.5. Biochemical biomarkers: phytochelatins
Another sensitive indicator of (sub)lethal
metal exposure may be the level of tochelatins (PCs). The relationship between phy-tochelatin synthesis and metal exposure was mainly tested in roots grown in solution culture under controlled conditions (Inouhe et al., 1994; De Knecht et al., 1995; Klapheck et al., 1995; Meuwly et al., 1995; Keltjens and Van Beu-sichem, 1998; Sneller 1999). PCs were the result of metal-imposed strain and were not related to the metal-resistance (Schat and Kalff, 1992). Therefore PC levels increased also in Cd- or Cu-resistant ecotypes as soon as the exposure level exceeded the resistance level to that specific metal (De Knecht et al., 1992). Only a few stud-ies have analysed the PC level of metal-exposed plants in the field. Grill et al. (1988) found very
low PC levels in roots of Acer pseudoplatanus
and S. 6ulgaris on a Zn mine, and the PC levels
in the current-year needles of Picea rubens
af-fected by air pollution did not surpass 8 nM (Gawel et al., 1996). The present study with the
Zn – Cd-resistant ecotype of S. 6ulgaris
confi-rmed the general picture of an initiation of PC synthesis by SH-reactive heavy metals (Grill et al., 1987): only plants exposed to high
environ-mental concentrations of copper and/or
cad-mium synthesized measurable concentrations of PCs being in good agreement with a high stimu-lation of the PC synthase by Cd and Cu, and a low one by Zn (Grill et al., 1987; Klapheck et al., 1995).
Can the degree of metal exposure in the field be related to PC levels of leaves when the plants have not evolved tolerance to environmentally
high Cu and/or Cd concentrations (De Knecht
et al., 1995)? The high concentration of PC2 and PC3 in mature leaves of plants grown on the Cu soil from Marsberg (9, 10) did fit well with the suggested low Cu tolerance of the Plombie`res ecotype (Schat and Ten Bookum,
1992) and confirmed field studies on Betula pen
-dula and Brassica rapa grown on some of the
soils of this study (Ernst, 1999). But the high PC-levels were not indicative for the perfor-mance of the plants during a full life-cycle be-cause plants with high PC-levels were able to survive and produce a good seedlot. Therefore PCs may be indicative for a transient distur-bance of the metabolism with some protection of metal-sensitive enzymes (Kneer and Zenk, 1992), but they do not allow a prediction on the performance of the plant up to reproduction.
5. Conclusions
The moderate biomass production, the occur-rence of chlorosis and P-deficiency are indica-tions that the ecotype Plombie`res is obviously not fully adapted to its habitat, neither to the metal concentration of the soil nor to the low levels of major nutrients. Nevertheless, the data do support the hypothesis that plants from a metal-resistant ecotype can regulate the uptake of that metal over a wide metal range thus re-sulting in very low increase of that metal for which they are resistant. Enhanced metal con-centration in the seedlings can be a very sensi-tive and early indicator of metal-exposed plants. The inverse relation between the metal concen-trations in young seedlings and the life-expec-tancy is a reasonable indicator for survival up to seed maturity. Due to the complex ecological situation in soils, the degree of metal resistance of ecotypes, as analysed in mono-metal solutions (Schat and Ten Bookum, 1992), is not in full agreement with plant performance in soils and
should be modified considering the low
availability of major nutrients in orogenic soils.
Acknowledgements
References
Baker, A.J.M., 1981. Accumulators and excluders-strategies in the response of plants to heavy metals. J. Plant Nutr. 3, 643 – 654.
Bergmann, W., 1983. Erna¨hrungssto¨rungen bei Kultur-pflanzen. G. Fischer Verlag, Stuttgart.
Bringezu, K., Lichtenberger, O., Leopold, I., Neumann, D., 1999. Heavy metal tolerance of Silene 6ulgaris. J. Plant Physiol. 154, 536 – 546.
Chardonnens, A.N., Ten Bookum, W.M., Kuijper, L.D.J., Verkleij, J.A.C., Ernst, W.H.O., 1998. Distribution of cad-mium in leaves of cadcad-mium tolerant and sensitive ecotypes ofSilene6ulgaris. Plant Physiol. 104, 75 – 80.
Chardonnens, A.N., Koevoets, P.L.M., van Zanten, A., Schat, H., Verkleij, J.A.C., 1999. Properties of enhanced tonoplast zinc transport in naturally selected zinc-tolerant
Silene6ulgaris. Physiol. Plant 120, 779 – 785.
Chen, T.S., Toribara, T.Y., Warner, U., 1956. Microdetermi-nation of phosphorus. Anal. Chem. 28, 1756 – 1758. Davies, M.S., Francis, D., Thomas, J.D., 1991. Rapidity of
cellular changes induced by zinc in a zinc tolerant and non-tolerant cultivar ofFestuca o6inaL. New Phytol. 117, 103 – 108.
De Knecht, J.A., Koevoets, P.L.M., Verkleij, J.A.C., Ernst, W.H.O., 1992. Evidence against a role for phytochelatins in naturally selected increqased cadmium tolerance inSi
-lene6ulgaris(Moench) Garcke. New Phytol. 122, 681 – 688. De Knecht, J.A., Van Baren, N., Ten Bookum, W.M., Fong Sang, H.W.W., Koevoets, P.L.M., Schat, H., Verkleij, J.A.C., 1995. Synthesis and degradation of phytochelatins in cadmium-sensitive and cadmium-tolerantSilene6ulgaris. Plant Sci. 106, 9 – 18.
De Vos, C.H.R., Vooijs, R., Schat, H., Ernst, W.H.O., 1989. Copper-induced damage to the permeability barrier in roots ofSilene cucubalus. J. Plant Physiol. 135, 165 – 169. De Vos, C.H.R., De Waal, M.A.M., Vooijs, R., Schat, H.,
Ernst, W.H.O., 1991. Increased resistance to copper-in-duced damage of the root cell plasmalemma in copper tolerantSilene cucubalus. Plant Physiol. 82, 523 – 528. Dueck, T.A., Visser, P., Ernst, W.H.O., Schat, H., 1986.
Vesicular-arbuscular mycorrhizae decrease zinc-toxicity to grasses growing in zinc-polluted soil. Soil Biol. Biochem. 18, 331 – 333.
Ernst, W., 1968. Der Einfluss der Phosphatversorgung sowie die Wirkung von ionogenem und chelatisierten Zink auf die Zink- und Phosphataufnahme einiger Schwermetall-pflanzen. Plant Physiol. 21, 323 – 333.
Ernst, W., Weinert, H., 1972. Localisation of zinc in the leaves ofSilene cucubalus. Z. Pflanzenphysiol. 66, 258 – 264. Ernst, W.H.O., 1974. Schwermetallvegetation der Erde. G.
Fischer Verlag, Stuttgart.
Ernst, W.H.O., 1975. Physiology of heavy metal resistance in plants. In: Hutchinson, T.A., (Ed.), International Confer-ence on Heavy Metals in the Environment, Toronto, pp. 121 – 136.
Ernst, W.H.O., 1995. Sampling of plant material for chemical analysis. Sci. Tot. Environ. 176, 15 – 24.
Ernst, W.H.O., 1996. Schwermetalle. In: Brunold, C., Ru¨eggsegger, A., Bra¨ndle, R. (Eds.), Stress bei Pflanzen. Haupt Verlag, Bern, pp. 191 – 219.
Ernst, W.H.O., 1998. Effects of heavy metals in plants at the cellular and organismic level. In: Schu¨u¨rmann, G., Mark-ert, B. (Eds.), Ecotoxicology. Ecological fundamentals, Chemical exposure and Biological effects. Wiley, Heidel-berg, pp. 587 – 620.
Ernst, W.H.O., 1999. Biomarkers in plants. In: Peakall, D.B., Walker, C.H., Migula, P. (Eds.), Biomarkers: A pragmatic basis for remediation of severe pollution in Eastern Eu-rope. Kluwer Academic Publishers, Dordrecht, pp. 135 – 151.
Ernst, W.H.O., Schat, H., Verkleij, J.A.C., 1990. Evolutionary biology of metal resistance inSilene6ulgaris. Evol. Trends Plants 4, 45 – 51.
Ernst, W.H.O., Verkleij, J.A.C., Schat, H., 1992. Metal toler-ance in plants. Acta Bot. Neerl. 41, 229 – 248.
Ernst, W.H.O., Peterson, P.J., 1994. The role of biomarkers in environmental assessment. (4) Terrestrial plants. Ecotoxi-cology 3, 180 – 192.
Ernst, W.H.O., Nelissen, H.J.M., 1999. Life-cycle phases of a zinc- and cadmium-resistant ecotype ofSilene 6ulgarisin risk assessment of polymetallic soils. Environ. Pollut. in press.
Gawel, J.E., Ahner, B.A., Friedland, A.J., Morel, F.M.M., 1996. Role of heavy metals in forest decline indicated by phytochelatin measurements. Nature 381, 64 – 65. Gregory, R.P.G., Bradshaw, A.D., 1965. Heavy metal
toler-ance in populations of Agrostis tenuis Sibth. and other grasses. New Phytol. 64, 131 – 143.
Grill, E., Winnacker, E.L., Zenk, M.H., 1987. Phytochelatins, a new class of heavy-metal-binding peptides from plants are functionally analogous to metallothioneins. Proc. Natl. Acad. Sci. USA 84, 439 – 443.
Grill, E., Winnacker, E.L., Zenk, M.H., 1988. Occurrence of heavy metal binding phytochelatins in plants growing in a mining refuse area. Experientia 44, 539 – 540.
Haby, V.A., Russelle, M.P., Skogley, E.A., 1990. Testing soils for portassium, calcium, and magnesium. In: Westerman, R.L. (Ed.), Soil testing and plant analysis. Soil Science Society of America, Madison, pp. 181 – 227.
Harrison, S.I., Lepp, N.W., Phipps, N.A., 1979. Uptake of copper by excised roots. II. Copper desorption from the free space. Z. Pflanzenphysiol. 94, 27 – 34.
Hildebrandt, U., Kaldorf, M., Bothe, H., 1999. The zinc violet and its colonization by arbuscular mycorrhizal fungi. J. Plant Physiol. 154, 709 – 717.
Inouhe, M., Ninomiya, S., Tohoyama, H., Joho, M., Mu-rayama, T., 1994. Different characteristics of roots in the cadmium-tolerance and Cd-binding complex formation be-tween mono- and dicotyledonous plants. J. Plant Res. 107, 201 – 207.
activities in cell cultures ofCentaurea cyanusby UV-light irradiation. Phytochemistry 30, 2271 – 2273.
Khan, H.R., Mcdonald, G.K., Rengel, Z., 1998. Chickpea genotypes differ in their sensitivity to Zn deficiency. Plant Soil 198, 11 – 18.
Keltjens, W.G., Van Beusichem, M.L., 1998. Phytochelatins as biomarkers for heavy metal toxicity in maize: single metal effects of copper and cadmium. J. Plant Nutr. 21, 635 – 648. Kirsten, W.J., 1979. Automatic methods for the simultaneous determination of carbon, hydrogen, nitrogen and sulphur, and sulphur alone in organic and inorganic materials. Anal. Chem. 51, 1173 – 1175.
Klapheck, S., Schlunz, S., Bergmann, L., 1995. Synthesis of phytochelatins and homo-phytochelatins inPisum sati6um L. Plant Physiol. 107, 515 – 521.
Kneer, R., Zenk, M.H., 1992. Phytochelatins protect plant enzymes from heavy metal poisoning. Phytochemistry 31, 2663 – 2667.
Lichtenthaler, H.K., 1987. Chlorophylls and carotenoids: Pig-ments of photosynthetic biomambranes. Methods Enzy-mol. 148, 350 – 382.
Lolkema, P.C., Donker, M.H., Schouten, A.J., Ernst, W.H.O., 1984. The possible role of metallothioneins in copper toler-ance ofSilene cucubalus. Plant Physiol. 162, 174 – 179. MacFarlane, G.R., Burchett, M.D., 1999. Zinc distribution
and excretion in the leaves of the grey mangrove,A6icennia
marina(Forsk.) Vierh. Environ. Exp. Bot. 41, 167 – 175. Macnair, M.R., Cumbes, Q., 1987. Evidence that arsenic
tolerance in Holcus lanatus L. is caused by an altered phosphate uptake system. New Phytol. 107, 387 – 394. Marschner, H., 1995. Mineral nutrition of higher plants,
sec-ond ed. Academic Press, Lsec-ondon.
Meuwly, P., Thibault, P., Schwan, A.L., Rauser, W.E., 1995. Three families of thiol peptides are induced by cadmium in maize. Plant J. 7, 391 – 400.
Neumann, D., Zur Nieden, U., Schwieger, W., Leopold, I., Lichtenberger, O., 1997. Heavy metal tolerance ofMinuar
-tia6erna. J. Plant Physiol. 151, 101 – 108.
Otte, M.L., Ernst, W.H.O., 1994. Arsenic in vegetation of wetlands. In: Nriagu, J.O. (Ed.), Arsenic in the environ-ment. Part I. Cycling and characterization. Wiley, New York, pp. 365 – 379.
Paulsen, I.T., Saier Jr, M.H., 1997. A novel family of ubiqui-tous heavy metal ion transport proteins. J. Membrane Biol. 156, 99 – 103.
Pawlowska, T.E., Blaszkowski, J., Ru¨hling, A., 1996. The mycorrhizal status of plants colonizing a calamine spoil mound in southern Poland. Mycorrhiza 6, 499 – 505. Reilly, A., Reilly, C., 1973. Copper-induced chlorosis inBe
-cium homblei(De Wild.) Duvign. and Plancke. Plant Soil 38, 671 – 674.
Rijstenbil, J.W., Wijnholds, J.A., 1996. HPLC analysis of nonprotein thiols in planktonic diatoms: pool size, redox state and response to copper and cadmium exposure. Mar. Biol. 127, 45 – 54.
Sanita` di Toppi, L., Gabbrielli, R., 1999. Response to cad-mium in higher plants. Environ. Exp. Bot. 41, 105 – 130. Schat, H., Kalff, M.M.A., 1992. Are phytochelatins involved
in differential metal tolerance or do they merely reflect metal-imposed strain? Plant Physiol. 99, 1475 – 1480. Schat, H., Ten Bookum, W.M., 1992. Metal-specificity of
metal tolerance syndromes in higher plants. In: Baker, A.J.M., Proctor, J., Reeves, R.D. (Eds.), The vegetation of ultramafic (serpentine) soils. Intercept, Andover, Hamp-shire, pp. 337 – 352.
Sharma, S.S., Schat, H., Vooijs, R., Van Heerwaarden, L.M., 1999. Combination toxicology of copper, zinc, and cad-mium in binary mixtures: Concentration-dependent antag-onistic, nonadditive, and synergistic effects on root growth inSilene6ulgaris. Environ. Toxicol. Chem. 18, 348 – 355. Sheppard, S.C., Evenden, W.G., Abboud, S.A., Stephenson,
M., 1993. A plant life-cycle biassay for contaminated soil, with comparison to other bioassays: mercury and zinc. Arch. Environ. Contam. Toxicol. 25, 27 – 35.
Sneller, F.E.C., 1999. Phytochelatins as biomarker of metals in terrestrial plants. Doctorate Thesis, Vrije Universiteit, Am-sterdam.
Sokal, R.R., Rohlf, F.J., 1995. Biometry. third ed. W.H. Freeman and Company, San Francisco.
Van Assche, F., Clijsters, H., Marcelle, R., 1979. Photosynthe-sis inPhaseolus6ulgarisL., as influenced by supra-optimal zinc nutrition. In: Marcelle, R., Clijsters, H., Van Pouckel, M. (Eds.), Photosynthesis and plant development. W. Junk Publishers, The Hague, pp. 175 – 184.
Van der Zaal, B.J., Neuteboom, L.W., Pinas, J.E., Chardon-nens, A.N., Schat, H., Verkleij, J.A.C., Hooykaas, P.J.J., 1999. Overexpression of a novel Arabidopsis gene related to putatitive zinc-transporter genes from animals can lead to enhanced zinc resistance and accumulation. Plant Phys-iol. 119, 1047 – 1055.
Vardaka, E., Cook, C.M., Lanaras, T., 1997. Interelemental relationships in the soil and plant tissue and photosynthesis of field-cultivated wheat growing in naturally enriched copper soils. J. Plant Nutr. 20, 441 – 453.
Va´zquez, M.D., Barcelo´, J., Poschenrieder, C.H., Ma´dico, J., Hatton, P., Baker, A.J.M., Cope, G.H., 1992. Localization of zinc and cadmium inThlaspi caerulescens(Brassicaceae), a metallophyte that can hyperaccumulate both metals. J. Plant Physiol. 140, 350 – 355.
Verkleij, J.A.C., Prast, J.E., 1989. Cadmium tolerance and co-tolerance in Silene 6ulgaris (Moench) Garcke (S. cu
-cubalus(L.) Wib.). New Phytol. 111, 637 – 645.
Wainwright, S.J., Woolhouse, H.W., 1977. Some physiological aspects of copper and zinc tolerance in Agrostis tenuis
Sibth.: cell elongation and membrane damage. J. Exp. Bot. 28, 1029 – 1038.
Wallace, A., Abou-Zam Zam, A.M., 1989. Cobalt – zinc inter-actions in bush beans grown in solution culture. Soil Sci. 147, 436 – 438.
Wallace, A., Berry, W.L., 1989. Dose-response curves for zinc, cadmium and nickel in combination of one, two or three. Soil Sci. 147, 401 – 410.