L
Journal of Experimental Marine Biology and Ecology 247 (2000) 85–97
www.elsevier.nl / locate / jembe
Ecology and energetics of two Antarctic sponges
* Jens Kowalke
Alfred Wegener Institute of Polar and Marine Research, Columbusstrasse, 27515 Bremerhaven, Germany Received 8 October 1999; received in revised form 2 December 1999; accepted 20 December 1999
Abstract
Retention efficiencies, pumping and respiration rates of the two Antarctic sponge species
Mycale acerata and Isodictya kerguelensis from Potter Cove, King George Island, were measured.
None of the species reached a 100% retention efficiency at any given particle size. This is probably due to the sediment-laden environment in which the animals were dwelling. A less efficient retention decreases the risk of the filtering structures being clogged. Both species filter
21
down into the bacterial size range. Pumping rates of the species were 180 ml h (M. acerata) and 21
220 ml h (I. kerguelensis) per g ash free dry mass (T518C), being lower than measured in 21
temperate water species. Oxygen consumption was 0.088 ml O h2 (M. acerata; T51.88C) and 21
0.035 ml O h2 (I. kerguelensis; T518C) per g ash free dry mass. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Antarctica; Sponges; Retention efficiency; Pumping rate; Respiration rate
1. Introduction
Sponges account for a major proportion of Antarctic soft and hard bottom dwellers (Voss, 1988; Barthel and Gutt, 1992; Starmans, 1997) and more than 300 species have been described for the Southern Ocean of which 50% are endemic (Koltun, 1970). Six species of hexactinellids dominate the Weddell Sea shelves (Barthel and Tendal, 1994). These animals structure the shelf habitats forming ‘multi-storied assemblages’ (Gutt and Schickan, 1998) which form important secondary substrata for holothurians, crinoids,
¨
crustaceans and fishes (Dearborn, 1977; Wagele, 1988; Barthel et al., 1991; Kunzmann, 1996; Barthel, 1997). Sponges have described as important benthos constituents from
*Tel.: 149-471-4831-313; fax:149-471-4831-149.
E-mail address: [email protected] (J. Kowalke)
shallow waters around the Antarctic Peninsula (King George Island: Sahade et al., 1998; Livingston Island: Saiz-Salinas et al., 1997; Signy Island: Barnes, 1995) and the McMurdo Sound (Dayton, 1978), but are, with the exception of McMurdo Sound, less numerous.
Sponges can be considered as a singular filter system, as the whole body functions as a filter. The water flows via numerous ostia (formed by porocytes) into branching channels, which end in choanocyte chambers generating the water flow. From these chambers the water is discharged into outflowing channels into one or several osculae. Particle uptake takes place at three different levels (Johnston and Hildemann, 1982). The maximum size of filterable particles, around 50mm, is determined by the diameter of the ostia. Food particles as small as 1mm are taken up through more or less specialised cells (archaeocytes) in the transmitting channels. Particles around 1 mm are filtered by the bases of the choanocytes and particles down to 0.1mm from microvilli, which form the choanocyte collar. Sponges are obviously unable to filter selectively and take up particles regardless of their nutritive value (Kilian, 1952; Reiswig, 1971; Wolfrath and Barthel, 1989).
Schmidt (1970) and Reiswig (1990) have shown that assimilation of dissolved organic material by choanocytes may also take place.
The food spectrum of Antarctic sponges is little known through a paucity of studies. Gaino et al. (1994) found diatoms, both of pelagic and benthic origin, in the cortex of two Antarctic species, which shall act as an additional skeleton and, during the nutrient
¨
poor Antarctic winter, as a food reserve. Kloser (personal communication) found up to 70% of benthic diatoms in different sponges of nearby Maxwell Bay, King George Island.
Potter Cove is not a favourable habitat for sponges, as inorganic particles are abundant in the water column throughout the year (Kowalke, 1998, 1999). The same could be stated for the entire Antarctic Peninsula region, as glacial melting during spring and summer bring sediments into the water column. Sponges, which are not able to close their inflowing channels, suffocate more easily than ascidians or bivalves, which can better manage these concentrations of inorganic suspended material. Sponges thus play a major role in habitats without heavy sedimentation — the shelf areas and high Antarctic sites like McMurdo Sound (Dayton and Oliver, 1977; Barry, 1988).
Nevertheless six species of sponges were found to dwell on the soft bottoms of Potter Cove (Kowalke, 1998). Mycale acerata, an important and fast growing species in Antarctic shallow waters (Dayton et al., 1974), occurs in densities of 0.02 individuals
22 22
m and has a biomass of 5.83 g m ash free dry mass (unpublished data). It grows sporadically, but in colonies up to 1.5 m in height (Kowalke, 1998). Although M. acerata contains secondary metabolites, which are at least highly toxic for fishes, it is predated by asteroids (McClintock, 1987). Isodictya kerguelensis occurs in densities of
22 22
0.10 individuals m and its ash free dry mass is 0.69 g m in 30 m depth (unpublished data).
2. Material and methods
The investigation was conducted at the Dallmann Laboratory, Potter Cove, King George Island, Antarctica (Fig. 1). Potter Cove is an inlet of the larger Maxwell Bay
2
system and stretches over 2 km . Maximum water depth is 50 m and the bottom is mainly composed of fine sediments imported by glacial meltwater. Hard substrata, such as rocky shores, cobbles and pebbles border the mouth of the cove. The soft bottom communities are characterised by a wide variety of suspension and deposit feeding animals (Kowalke and Abele, 1998; Sahade et al., 1998). The hydrography of the cove
is governed by strong, mostly westerly winds, which block the water circulation inside ¨
the cove and hamper the outflow of sediment-laden water (Kloser et al., 1994). Between December 1995 and February 1996 divers collected the experimental animals which were immediately placed in aerated flow-through aquaria of 25 l volume. The sponges were individually kept for several days in order to acclimate before starting the experiments. The water was drawn directly from the cove from 10 m depth.
2.1. Filtration
At the beginning of the experiment, the water supply was cut off for 40 h, however, aeration provided sufficient turbulence for stirring and thus keeping particles in suspension. At 8-h intervals, in the vicinity of each animal, 20 ml of water were siphoned off and immediately analysed on a Elzone Particle Counter 280 equipped with a 60-mm aperture tube. The time-span of 40 h was used to allow for maximum retention. To determine pumping rates the indirect method was employed because low pumping rates rendered flow through methods unsuccessful. For further discussion, see Kowalke (1999).
The aquaria were artificially cooled to maintain a temperature of 18C. Size spectra analysis took place between 1 and 15mm. The abundance of particles above 9mm was to small to allow for any statistical calculation and, therefore, excluded. Two aquaria without animals were used as blanks for the calculation of cell sedimentation and fission rates. After the correction for blanks, the retention efficiency and the standard deviation was calculated as the percentage of particles remaining in suspension.
The often used simple exponential model for the calculation of pumping rates requires a complete filtration of all particles after a given time for a correct evaluation of rates. Thus, the Williams (1982) formula, which allows for correction for unfiltered particles in each size group, was used:
(2k?t )
Ct5y0 1z0
PR5k?V
where k5filtration constant, Ct5particle concentration of a given size at time t, y05particle concentration of a given filterable size at time 0, z05particle concentration of a given unfilterable size (constant) at time 0, PR5pumping rate, V5volume of water in aquaria.
2.2. Respiration
Four hours prior to the experiment the tube shaped M. acerata were cut in rings, halved and maintained at 1.88C in aerated 0.2-mm filtered flowing seawater to clean off debris and sediment, which fill the cavities of the animals. These sediments are out-washed prior to the measurements and cannot influence the results.
and maintained at 1.08C in aerated 0.2-mm filtered seawater. The use of halved rings and branches made it possible to operate with small experimental chambers, which were accommodated in a cooling system. The use of sponge parts is common in methods in order to make very large individuals manageable (Cotter, 1978; Burlando et al., 1992). This handling did not seem to have any negative influence on the status of the animals. A one-electrode open flow system was used. A permanently aerated reservoir contained the water, to which a membrane electrode was calibrated to 100% oxygen saturation. This water passed through a 175-ml experimental chamber. The O -depletion2
in the outflowing water was continuously recorded by an oxygen electrode (Eschweiler, Kiel) and plotted on a Linseis printer in % against the 100% saturation of the inflowing
21
water. The consumption in ml h was calculated by using an oxygen solubility table (Grasshoff, 1983) for a salinity of 3.7% and a temperature of 1.8 / 1.08C, respectively. The difference in the O -concentration is dependent on the metabolic rate of the2
animal and the flow speed of the water but not on the experimental chamber size. Animals reduce the oxygen content in the chamber until a steady state concentration is reached. As bacterial-free seawater was used, a correction for background respiration was not necessary.
Here the curves showed an acclimatisation of the animals to the experimental conditions after 3 h and analysis readings were done after this time.
For the experiments the animals were inserted into the cooled experimental chambers and recleaned with a continuous stream of 0.2-mm filtered seawater. The measurements
21
were done in darkness at a flow speed of 108 ml h at 1.88C (M.acerata) and 1.08C (I. kerguelensis) for 8–10 h.
2.3. Computations
After the experiments, dry mass and ash free dry mass of each animal was determined by drying at 608C for 48 h and burning at 5008C for 5 h, respectively.
The metabolic rates (MR; pumping and respiration rates) are expressed as a function of body mass using the allometric equation, which was fitted using a least-squares regression with ln-transformed values:
ln MR5a1b?M
where M is the body mass and b the linear regression coefficient.
3. Results
Fig. 2. Retention efficiencies [%] of the two species (A) Mycale acerata, (B) Isodictya kerguelensis; points refer to means, bars refer to standard deviation of each measurement.
efficiency for smaller particles drops to 30%. A sharp decline can be observed around 1 mm and a depression between 2 and 4 mm in both species.
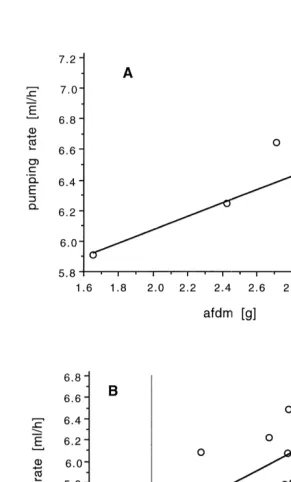
21
Pumping rates are shown in a double-log plot (Fig. 3). M. acerata pumps 180 ml h
21
and I. kerguelensis 220 ml h (standardised to an animal of 1 g ash free dry mass). The regression coefficients are below the theoretical value of 0.75.
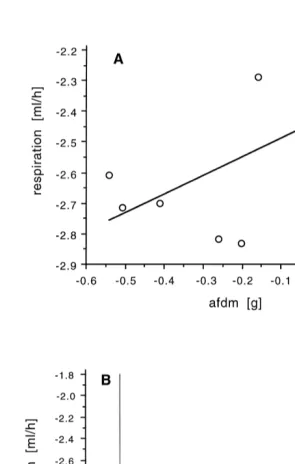
Fig. 4 shows the respiration rates in a double-log plot. M. acerata consumes 0.088 ml
21 21
O h2 at 1.88C and I. kerguelensis 0.035 ml O h2 at 18C (standardised to an animal of 1 g ash free dry mass).
4. Discussion
21
Fig. 3. Pumping rates (ml h ) in relation to ash free dry mass, ln-transformed. (A) Mycale acerata,
21
Fig. 4. Respiration rates (ml h ) in relation to ash free dry mass, ln-transformed. (A) Mycale acerata,
Reiswig (1971) and Pile et al. (1996) showed for different demosponges that this capture system supplied up to 80% of the total carbon diet. Antarctic sponges as well are able to take a part of their food from minute particles such as bacteria. So far, nothing is known, whether other Antarctic suspension feeding taxa are able to exploit this size range, but this might explain why sponges dominate vast areas of the Antarctic shelves (e.g. Dayton, 1978; Gutt and Starmans, 1998).
The decline of efficiency around 1mm, which was measured in all experiments, is a result of either a negative selection of this particle size range or a production of faeces which lowers the apparent efficiency. Stuart and Klumpp (1984), who observed the same phenomena between 2 and 4 mm in the species Haliclona anonyma, showed a particle production in this size range, but this can not be validated for these Antarctic species as data are still lacking.
Retention efficiencies are important when considering the food availability, especially during times of food limitation. Antarctic winter is believed to be such a food-limited period due to ice coverage and unfavourable light conditions (e.g. Gruzov, 1977). Yet at least in the Antarctic Peninsula region food in the nano and picoplankton size ranges may be abundant for a considerable period of the year (Barnes and Clarke, 1994; Leakey et al., 1996). Barnes and Clarke (1995) observed various taxa ceasing feeding only for a short time in winter. In McMurdo Sound, Berkman et al. (1986) observed viable benthic micro-algae during winter and argued that resuspension of these particles make them available for suspension feeding animals in near-shore environments of the Southern Ocean. Another food source during winter might be decaying algae debris, which fuels the growth of bacteria populations (Delille, 1993). No data on bacteria dynamics are available for Potter Cove, but debris of the brown algae Desmarestia sp. is abundant at least in autumn (personal observations). As M. acerata exploits the bacterial size range more efficiently as I. kerguelensis does, there might be more food available for the former species during winter.
The pumping rates of the sponges M. acerata and I. kerguelensis are below those of ˚
animals of boreal waters (for a compilation of rates, see Thomassen and Riisgard, 1995). This was shown for ascidians and a bivalve as well and is not only correlated with the low temperatures but also with inorganic sediment concentrations, which prevail in Potter Cove during spring and summer (Kowalke, 1998, 1999). Gerrodette and Flechsig (1979) showed a negative influence of rising sediment concentration on the pumping
21
rate of the tropical sponge Verongia lacunosa. Even the small concentration of 11 mg l
21
The regression coefficients are below the postulated value of 0.75 (Hemmingsen, 1960). This could be due to the suppression of water transport in bigger animals, which
˚
might be more sensitive to experimental conditions (Jørgensen, 1996). Riisgard et al. ˚
(1993) and Thomassen and Riisgard (1995) doubted the applicability of the allometric equation for sponges due to their singular morphology: they grow simply by increasing their number of water-pumping choanocytes. But the uptake of particles is not limited to the chambers. The numerous channels, which as well phagocytise particles, are subject to allometric growth. Another rejection of this view might be the increase of dead choanocyte chambers in older (and thus larger) animals, which possess in relation to smaller individuals fewer ‘engines’ of water processing.
The standard metabolism, which is defined as metabolisms without food intake, growth and reproduction, could be measured by the use of bacteria-free filtered seawater. The only published data of polar sponges concern stem from three deep-sea species from the Greenland Sea (Witte and Graf, 1996). Consumption was between 0.407 and 0.478 ml O per h and g ash free dry mass, at a temperature of2 20.58C. These values are tenfold higher than the respiration data of I. kerguelensis and fivefold higher than those of M. acerata. An explanation of this difference might be a depression-related artifact concerning the deep-sea species, as those values seem to be too high. Highly mobile Antarctic amphipods consumed less oxygen than the Greenland sponges (Rakusa-Suszczewski and Klekowski, 1973; Rakusa-(Rakusa-Suszczewski, 1980). Respiration data of
21
sessile Antarctic benthos showed values between 0.07 ml and 0.144 ml O2 h (ascidians, unpublished data; bivalve Laternula elliptica: Ahn and Shim, 1998; gorgo-nian Ainigmaptilon antarcticus: Gili et al., 1999), which are in the same range as are the Antarctic sponges.
The filtration effectiveness (amount of water pumped per ml O consumed) of both2
species lies within the range of sponges from warmer waters and is even higher than the effectiveness of the bivalve L. elliptica (Table 1). This confirms the findings of
˚
Thomassen and Riisgard (1995) who stressed the importance of the habitat, which dominates biological factors. As 0.46 mg C correspond to 1 ml O , a minimum of 0.222
Table 1
Filtration efficiency of different sponge species and Antarctic invertebrates
21
Halichondria panicea 2.7 Thomassen and Riisgard (1995)
Mycale acerata 2.1 this study
Isodictya kerguelensis 6.3 this study
Antarctic invertebrates
Cnemidocarpa verrucosa (Ascidiacea) 15.1 Kowalke, unpublished data
Molgula pedunculata (Ascidiacea) 6.0 Kowalke, unpublished data
21 21
mg C l must be available for M. acerata and 0.07 mg C l for I. kerguelensis to fulfill the maintenance requirements. These values are easily reached in Potter Cove during this time of the year (Kowalke, 1998).
5. Conclusions
The success of M. acerata (the biomass is tenfold higher) compared to I. kerguelensis has a number of possible explanations. The ability of M. acerata to exploit the bacterial size range more efficiently may be vital in periods of low availability of nano and microplankton. If the members of the microbial food chain are important nutritive sources during winter, as Barnes and Clarke (1995) have suggested, M. acerata simply gets more food. The second reason is the production of mucus by M. acerata. A sediment-covered surface can be cleaned with great efficiency and does not hamper filtration and respiration for long times. Slattery and Bockus (1997) suggested the success of the soft coral Alcyonium paessleri in McMurdo Sound to be due to the production of mucus. This seems advantageous even taking the metabolic costs into account. Furthermore, a lower pumping rate reduces the intake of particulate material and the risk of subsequent suffocation of the animal.
However, species like I. kerguelensis are able to dwell in environments like Potter Cove due to their low metabolic costs, even if the sediment-laden habitat does not favour species without proper cleaning mechanisms. Their biomass is consequently lower.
Acknowledgements
I would like to thank the Alfred Wegener Institute for Polar and Marine Research and the staff of the Dallmann Laboratory for logistical support. Dr V. Koltun (St. Petersburg) helped with identification of species. Professor W. Arntz, Dr T. Brey and two anonymous referees gave valuable comments on the manuscript. This work was partially financed from the Deutsche Forschungsgemeinschaft. This is AWI publication no. 1721. [RW]
References
Ahn, I.Y., Shim, J.H., 1998. Summer metabolism of the Antarctic clam, Laternula elliptica (King and Broderip) in Maxwell Bay, King George Island and its implications. J. Exp. Mar. Biol. Ecol. 224 (2), 253–264.
Barnes, D.K.A., 1995. Sublittoral epifaunal communities at Signy Island, Antarctica. 2. Below the ice-foot zone. Mar. Biol. 121 (3), 565–572.
Barnes, D.K.A., Clarke, A., 1994. Seasonal variation in the feeding activity of four species of Antarctic bryozoan in relation to environmental factors. J. Exp. Mar. Biol. Ecol. 181, 117–131.
Barry, J.P., 1988. Hydrographic patterns in McMurdo Sound, Antarctica and their relationship to local benthic communities. Pol. Biol. 8 (5), 377–391.
Barthel, D., 1997. Fish eggs and pentacrinoids in Weddell Sea hexactinellids: further examples for the structuring role of sponges in Antarctic benthic ecosystems. Pol. Biol. 17, 91–94.
Barthel, D., Gutt, J., 1992. Sponge associations in the Weddell Sea. Antarct. Sci. 4, 137–150. ¨
Barthel, D., Tendal, O., 1994. Antarctic hexactinellida. In: Wagele, J.W., Sieg, J. (Eds.), Synopsis of the Antarctic Benthos, Champaign Scientific Books, Loenigstein 6.
Barthel, D., Gutt, J., Tendal, O., 1991. New information on the biology of Antarctic deep water sponges derived from under water photography. Mar. Ecol. Prog. Ser. 69, 303–307.
Berkman, P.A., Marks, D.S., Shreve, G.P., 1986. Winter sediment resuspension in McMurdo Sound, Antarctica, and its ecological implications. Pol. Biol. 6, 1–3.
Burlando, B., Bavestrello, G., Arillo, A., 1992. Seasonal changes in the metabolism of the calcareous sponge
Clathria clathrus (Schmidt). Comp. Biochem. Physiol. 2, 341–344.
Cotter, A.J.R., 1978. Re-investigation of size, axial gradients and light as factors affecting the respiration of certain marine sponges. Comp. Biochem. Physiol. 60A, 117–122.
Dayton, P.K., 1978. Observations of growth, dispersal and population dynamics of some sponges in McMurdo ´
Sound, Antarctica. In: Levi, C., Boury-Esnault, N. (Eds.), Biologie Des Spongiaires, Coll. Int. du C.N.R.S, Vol. 291, pp. 271–282.
Dayton, P.K., Oliver, J.S., 1977. Antarctic soft bottom benthos in oligotrophic and eutrophic environments. Science 197, 55–58.
Dayton, P.K., Robillard, G.A., Paine, R.T., Dayton, L.B., 1974. Biological accommodation in the benthic community at McMurdo Sound, Antarctica. Ecol. Monogr. 44, 105–128.
Dearborn, J.H., 1977. Foods and feeding characteristics of Antarctic asteroids and ophiurids. In: Llano, G.A. (Ed.), Proc. 3rd SCAR Symp. on Antarctic Biology, Adaptations within Antarctic Ecosystems, Smithsonian Institute, Washington, pp. 293–326.
Delille, D., 1993. Seasonal changes in the abundance and composition of marine heterotrophic bacterial communities in an Antarctic coastal area. Pol. Biol. 13, 463–470.
`
Gaino, E., Bavestrello, G., Cattaneo-Vietti, R., Sara, M., 1994. Scanning electron microscope evidence for diatom uptake by two Antarctic sponges. Pol. Biol. 14, 55–58.
Gerrodette, T., Flechsig, A.O., 1979. Sediment-induced reduction in the pumping rate of the tropical sponge
Verongia lacunosa. Mar. Biol. 55, 103–110.
´ ´
Gili, J.M., Arntz, W.E., Filipe, P., Lopez, P., Orejas, C., Ros, J., Teixido, N., 1999. The role of Benthic suspension feeders in Antarctic communities. Rep. Pol. Res. 301, 30–93.
Grasshoff, E., 1983. Methods of Seawater Analysis, Verlag Chemie, Weizenheim.
Gruzov, E.N., 1977. Seasonal alterations in coastal communities in the Davis Sea. In: Llano, G.A. (Ed.), Proc. 3rd SCAR Symp. on Antarctic Biology, Adaptations within Antarctic Ecosystems, Smithsonian Institute, Washington, pp. 263–278.
Gutt, J., Schickan, T., 1998. Epibiotic relationships in Antarctic benthos. Antarct. Sci. 10, 398–405. Gutt, J., Starmans, A., 1998. Structure and biodiversity of megabenthos in the Weddell and Lazarev Seas
(Antarctica): ecological role of physical parameters and biological interactions. Pol. Biol. 20, 229–247. Hemmingsen, A.M., 1960. Energy metabolism as related to body size and respiratory surfaces, and its
evolution. Rep. Steno Memorial Hosp. Nord. Insulinlab. 9, 1–110.
Johnston, I.S., Hildemann, W.H., 1982. Cellular organization in the marine demosponge Callyspongia diffusa. Mar. Biol. 67, 1–7.
Jørgensen, C.B., 1996. Bivalve filter feeding revisited. Mar. Ecol. Prog. Ser. 142, 287–302.
¨ ¨
Kilian, E., 1952. Wasserstromung und Nahrungsaufnahme beim Susswasserschwamm Ephydatia fluvatilis. Z. Vgl. Physiol. 34, 407–447.
¨
Kloser, H., Ferreyra, G., Schloss, I., Mercuri, G., Laturnus, F., Curtosi, A., 1994. Hydrography of Potter Cove, a small, fjord-like inlet on King George Island (South Shetlands). Estuar. Coast. Shelf Sci. 38, 523–537. Koltun, V.M., 1970. Sponges of the Arctic and Antarctic: a faunistic review. Symp. Zool. Soc. Lond. 25,
285–297.
¨
Kowalke, J., 1999. Filtration in Antarctic ascidians — striking a balance. J. Exp. Mar. Biol. Ecol. 242 (2), 233–244.
Kowalke, J., Abele, D., 1998. A first record of the soft bottom infauna community of Potter Cove. Rep. Pol. Res. 299, 106–112.
¨ ¨
Kunzmann, K., 1996. Die mit ausgewahlten Schwammen (Hexactinellida und Demospongiaea) aus dem Weddellmeer, Antarktis, vergesellschaftete Fauna. Ber. Pol. Forsch. 210, 1.
Leakey, R.J.G., Archer, S.D., Grey, J., 1996. Microbial dynamics in coastal waters of East Antarctica: bacterial production and nanoflagellate bacterivory. Mar. Ecol. Prog. Ser. 142, 3–17.
McClintock, J.B., 1987. Investigation of the relationship between invertebrate predation and biochemical composition, energy content, spicule armament and toxicity of benthic sponges at McMurdo Sound, Antarctica. Mar. Biol. 94, 479–487.
Pile, A.J., Patterson, M.R., Witman, J.D., 1996. In situ grazing on plankton ,10mm by the boreal sponge
Mycale lingua. Mar. Ecol. Prog. Ser. 141, 95–102.
Rakusa-Suszczewski, S., 1980. Hypostenothermic organisms. Pol. Pol. Res. 1 (4), 231–241.
Rakusa-Suszczewski, S., Klekowski, R.Z., 1973. Biology and respiration of the Antarctic amphipoda (Paramoera walkeri Stebbing) in the summer. Pol. Arch. Hydrobiol. 20, 475–488.
Reiswig, H.M., 1971. Particle feeding in natural populations of three marine demosponges. Biol. Bull. 141, 568–591.
Reiswig, H.M., 1974. Water transport, respiration and energetics of three tropical marine sponges. J. Exp. Mar. Biol. Ecol. 14, 231–249.
Reiswig, H.M., 1975. Bacteria as food for temperate-water marine sponges. Can. J. Zool. 53, 582–589. ¨
Reiswig, H.M., 1990. In situ feeding in two shallow water hexactinellid sponges. In: Rutzler, K. (Ed.), New Perspectives in Sponge Biology, Smithsonian Institute, Washington, DC, pp. 504–510.
˚
Riisgard, H.U., Thomassen, S., Jakobsen, H., Weeks, J.K., Larsen, P.S., 1993. Suspension feeding in marine sponges Halichondria panicea and Haliclona urceolus: effects of temperature on filtration rate and energy cost of pumping. Mar. Ecol. Prog. Ser. 96, 177–188.
´ ¨
Sahade, R., Tatian, M., Kowalke, J., Kuhne, S., Esnal, G., 1998. Benthic faunal associations on soft substrates at Potter Cove, King George Island, Antarctica. Pol. Biol. 19, 85–91.
´ ´
Saiz-Salinas, J.I., Ramos, A., Garcıa, F.J., Troncoso, J.S., San Martin, G., Sanz, C., Palacin, C., 1997. Quantitative analysis of macrobenthic soft bottom assemblages in South Shetland waters (Antarctica). Pol. Biol. 17 (4), 393–400.
Schmidt, I., 1970. Phagocytose et pinocytose chez les Spongillidae. Z. Vergl. Physiol. 66, 398–420. Slattery, M., Bockus, D., 1997. Sedimentation in McMurdo Sound, Antarctica: a disturbance mechanism for
benthic invertebrates. Pol. Biol. 18, 172–179.
¨ ¨
Starmans, A., 1997. Vergleichende Untersuchungen zur Okologie und Biodiversitat des Megaepibenthos der Arktis und Antarktis. Rep. Pol. Res. 250, 1.
Stuart, V., Klumpp, D.W., 1984. Evidence for food-resource partitioning by kelp-bed filter feeders. Mar. Ecol. Prog. Ser. 16, 27–37.
˚
Thomassen, S., Riisgard, H.U., 1995. Growth and energetics of the sponge Halichondria panicea. Mar. Ecol. Prog. Ser. 128, 239–246.
Voss, J., 1988. Zoogeographie und Gemeinschaftsanalyse des Makrozoobenthos des Weddellmeeres (Antar-ktis). Ber. Polarforsch. 45, 1.
¨ ¨
Wagele, J.W., 1988. Aspects of the life cycle of the Antarctic fish parasite Gnathia calva Vanhoffen (Crustacea, Isopoda). Pol. Biol. 8, 287–291.
Williams, L.G., 1982. Mathematical analysis of the effects of particle retention efficiency on determination of filtration rate. Mar. Biol. 66, 171–177.
Witte, U., Graf, G., 1996. Metabolism of deep-sea sponges in the Greenland-Norwegian Sea. J. Exp. Mar. Biol. Ecol. 198, 223–235.
![Fig. 2. Retention efficiencies [%] of the two species (A) Mycale acerata, (B) Isodictya kerguelensis; pointsrefer to means, bars refer to standard deviation of each measurement.](https://thumb-ap.123doks.com/thumbv2/123dok/3099657.1375825/6.612.84.387.74.402/retention-efciencies-isodictya-kerguelensis-pointsrefer-standard-deviation-measurement.webp)