www.elsevier.com / locate / bres
Short communication
GABAergic modulation of neurons in the nucleus of the solitary tract
with ascending projections to the subfornical organ in the rat
a ,
*
a a aJunichi Tanaka
, Hiroko Miyakubo , Sachiko Nomura , Kazuhiro Sakamaki ,
a b
Takefumi Okumura , Yasushi Hayashi
a
Department of Human Development, Naruto University of Education, Naruto, Tokushima 772-8502, Japan
b
Department of Education for Handicapped Children, Naruto University of Education, Naruto, Tokushima 772-8502, Japan
Accepted 19 September 2000
Abstract
Twenty-five neurons in the region of the nucleus of the solitary tract (NTS) were antidromically activated by electrical stimulation of the subfornical organ (SFO) in male rats under urethane anesthesia. Microiontophoretically applied bicuculline, ag-aminobutyric acid (GABA)A antagonist, but not phaclofen, a GABAB antagonist, attenuated the post-antidromic inhibitory response evoked by SFO stimulation of approximately two-third (n517) of identified neurons, indicating the existence of recurrent inhibitory systems through GABA receptors. Iontophoretically applied GABA decreased the spontaneous activity of all identified neurons, and the GABA-inducedA inhibition was prevented by simultaneously applied bicuculline, but not by phaclofen. Activation of peripheral baroreceptors, achieved by rising arterial blood pressure with an intravenous infusions of phenylepherine, suppressed the activity of the majority (n520) of identified neurons. The inhibitory response of identified neurons (n57) to baroreceptor activation was partially antagonized by iontophoretically applied bicuculline, but not by phaclofen. These results imply that GABAergic mechanisms may modulate the baroreceptor reflex acting on GABA receptors of NTS neurons with ascending projections to the SFO in the region of the NTS.A 2001 Elsevier Science B.V. All rights reserved.
Theme: Endocrine and autonomic regulation
Topic: Cardiovascular regulation
Keywords: Nucleus of the solitary tract; Subfornical organ;g-Aminobutyric acid; Bicuculline; Phaclofen; Baroreceptor activation
The subfornical organ (SFO) is an important neural the SFO from the region of the NTS are important for structure for the control of cardiovascular function and carrying peripheral baroreceptor information to the SFO body fluid homeostasis. Electrical or chemical stimulation [18,21–23].
of the SFO elicits pressor responses [5,14,15], drinking The NTS region of the rat contains a large amount of [4,14,15], and enhanced release of vasopressin from the g-aminobutyric acid (GABA) and GABA receptors [1,8]. posterior pituitary [10,12]. Activation of SFO efferents Microinjections of muscimol, a GABAA agonist, and causes changes in neuronal activities of neurons in the baclofen, a GABA agonist, into the NTS produce pressorB several brain sites where are involved in the regulation of and tachycardic effects and inhibit the baroreflex bradycar-arterial pressure and plasma osmolality [6,24–26]. Ana- dia, and the effects of muscimol and baclofen were tomical tracing observations have revealed that neurons in prevented by the GABAA antagonist bicuculline and the the nucleus of the solitary tract (NTS), the primary site of GABAB antagonist phaclofen, respectively [2,7,9,17,20]. termination of cardiovascular afferent fibers [2,11], project In contrast, bicuculline decreases the arterial blood pres-directly to the SFO [3,18,21,27]. Our findings have sure and heart rate and enhances the baroreflex bradycardia demonstrated that noradrenergic ascending projections to [13]. Thus, it is postulated that the GABAergic system modulates the neuronal activity of the baroreflex circuits in the NTS, and the two types of GABA receptors are *Corresponding author. Tel. / fax:181-88-687-6243.
E-mail address: [email protected] (J. Tanaka). involved in the modulation.
The objectives of current study were to elucidate the participation of GABAergic mechanisms in the modulation of the excitability of neurons projecting directly to the SFO in the region of the NTS, and to clarify the role of GABA receptor subtypes in the regulatory mechanism of the neurons.
Successful experiments were conducted on 18 male Wistar rats weighing 300–410 g. The animals were anesthetized with an intraperitoneal injection of urethane (1.4 g / kg). The femoral artery and vein were catheterized to record blood pressure and administer phenylephrine, respectively. The animals were then placed in a stereotaxic frame.
Electrical stimulation of the SFO, single-unit recordings in the region of the NTS, and microiontophoretic applica-tion of the drugs were performed by means of procedures described elsewhere [22,24–26]. Briefly, a coaxial bipolar electrode (tip-ring separation less than 0.3 mm, resistance 50–100 kV) was positioned in the region of the SFO (Fig. 1B). The electrode was connected to an isolated stimulat-ing unit programmed to deliver cathodic monophasic pulses with 0.2 ms duration (intensity less than 900 mA) antidromically. The criteria for antidromic response in-cluded a constant latency, ability to follow stimuli de-livered above 60 Hz, and collision between spontaneous action potential and a stimulus-evoked potentials (Fig. 1A). Glass microelectrodes filled with 0.5 M sodium acetate solution containing 2% Pontamine Sky Blue (DC resist-ance 10–15 MV) were used to make extracellular single-unit recording from the NTS area. Five-barrel micropipet-tes with tip diameters of 2–4 mm were constructed from pyrex glass tubes of 2.0 mm outer diameter and glued to
the recording electrode so that the tip of the recording Fig. 1. (A) Antidromic identification of neurons in the region of the nucleus of the solitary tract (NTS) projecting to the subfornical organ electrode protruded by 20–30 mm. Four barrels of the
(SFO). Five superimposed oscilloscope records from a neuron in the micropipette contained solutions of the following agents
region of the NTS illustrate the features of antidromic activation: all or for microiontophoresis (DC resistance 10–30 MV): 1 M
none constant latency at the threshold (top trace); constant latency GABA (Sigma), pH 4.0; 0.5 M sodium glutamate (Wako), responses following two SFO stimuli presented with an interstimulus pH 8.0; 20 mM l-bicuculline methiodide (Sigma), pH 4.0; interval less than 15 ms (second trace); collision cancellation of antid-romic action potentials by spontaneous action potentials (*) (lower trace). 13.3 mM phaclofen (Tocris Neuramin), pH 4.0. The
(B) Symbols on schematic transverse sections (8.0 and 7.6 mm anterior to remaining barrel of the micropipette was filled with 4 M
the interaural line) depict the location of the electrode tips in the SFO and sodium acetate solution for autonomic current balancing to
its surrounding region where stimulation evoked antidromic spikes (s) or prevent tip polarization artifacts. Microiontophoretic ejec- was without effect (d). (C) The location of neurons antidromically tion (current 10–50 nA) of the drugs was achieved with a identified as projecting to the SFO plotted on representative transverse sections of the medulla (obex to 2.0 mm rostral to obex). Circles and constant current unit (Dia Medical, DPI-25). Between
squares indicate the locations of inhibitory and unresponsive neurons to successive drug applications retaining currents of 25 nA
baroreceptor activation, respectively. AP, area postrema; Cu, cuneate were passed. Activation of peripheral baroreceptors was
nucleus; Gr, gracile nucleus; S, solitary tract; SFO, subfornical organ; SL, evoked by increasing arterial blood pressure following an lateral solitary nucleus; SM, medial solitary nucleus; TS, triangular septal intravenous infusion of phenylepherine (10mg / kg, Sigma). nucleus; 3V, third ventricle; 4V, fourth ventricle; 10, dorsal motornucleus
of vagus; 12, hypoglossal nucleus. Peristimulus time histograms (PSTHs) were constructed
from 200 stimuli applied at 1 / 3 Hz (a resolution, 5 ms) with a signal processor (Nihon Koden, ATAC-450) and
utilized to evaluate orthodromic effects on neuronal activi- durations refer to the time these stimulus-evoked changes ty. A stimulus-evoked 30% increase or decrease in the were noted to be in effect.
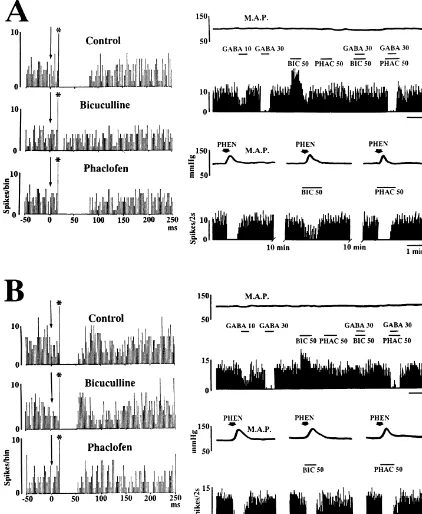
(inhibition), or no change in the frequency of action The activity of all identified neurons (n525) was tested potentials. A minimum 20% change in the frequency of the for a response to microiontophoretic application of GABA burst as compared with that of activity during 30 s before and to baroreceptor activation produced by rising arterial application of a test stimulus in each neuron was chosen as blood pressure with an intravenous injection of phenylep-the minimum arbitrary level of change. To evaluate phenylep-the hrine. The spontaneous activity of all neurons tested was effects of microiontophoretic application of the antagonists inhibited by iontophoretically applied GABA, and the on the evoked responses, the mean frequency of response inhibition was prevented by simultaneously applied induced by the application of GABA or baroreceptor bicuculline, but not by phaclofen (Fig. 2). These results activation during the antagonist application was compared indicate that the GABAergic system acts to suppress the with that of the evoked responses without the antagonist excitability of neurons with ascending projections to the application. A minimum 30% change in the frequency of SFO in the region of the NTS through a GABA receptorA the burst as compared with that of the evoked response mechanism. Bicuculline applied iontophoretically, but not without the antagonist application was classified as an phaclofen, increased the spontaneous activity of almost all efficacy of the antagonist. (n522) of the identified neurons (Fig. 2), suggesting the At the end of each experiment, the stimulation and possibility that neurons projecting to the SFO in the region recording sites were marked by depositing a small amount of the NTS are tonically inhibited by endogenous GABA of iron and dye, respectively. The animals were then acting on GABAA receptors. Of the identified neurons sacrificed with an overdose of urethane and perfused with tested, 20 displayed a reduction in neuronal firing that 10% formalin containing 3% potassium ferro-ferricyanide accompanied a 30 to 55 mmHg elevation in mean arterial mixture. The brain was removed, immersed in physiologi- pressure (M.A.P.) (Fig. 2), while five were unresponsive cal saline containing 30% sucrose for a few days. The (data not shown). The baroreceptor activation-induced marking sites were confirmed histologically in 50-mm inhibitory response of 7 out of 20 identified neurons was sections stained with Neutral Red. The stereotaxic coordi- partially antagonized by ionphoretically applied bicucul-nates for marking sites were determined according to the line, but not by phaclofen (Fig. 2A), while the response of atlas of Paxinos and Watson [16]. the remaining neurons was not affected by either bicucul-Data in the text are presented as mean6S.E.M. line or phaclofen (Fig. 2B). These data imply that Action potentials (Fig. 1A) evoked antidromically by GABAergic mechanisms suppressively modulate the baro-electrical stimulation of the SFO (Fig. 1B) were recorded receptor reflex acting on GABAA receptors of neurons from 25 neurons histologically confirmed in the region of projecting directly to the SFO in the region of the NTS. the NTS (Fig. 1C). The mean spontaneous discharge rate It has been reported that, in the rat, the cardiovascular of these neurons was 3.660.8 spikes / s. The mean latency effects of the GABA uptake inhibitor nippecoptic acid of antidromic responses was 36.766.3 ms, ranging from injected into the NTS are antagonized by phaclofen but not 22 to 58 ms. The threshold current required to evoke these by bicuculline [19]. The present electrophysiological data antidromic spikes ranged from 176 to 580 mA (389647 are not consistent with these findings indicating that
associated with vagal outflow, Brain Res. Bull. 5 (Suppl. 2) (1980) distantly located upon the dendrites, it may be difficult to
325–328. achieve adequate concentrations, especially with a low
[9] G.-B. Gu, G. Ju, The parabrachio-subfornical organ projection in the potency drug such as phaclofen. Further work using the rat, Brain Res. Bull. 38 (1995) 41–47.
potent GABA antagonists is necessary to clarify more [10] M. Iovino, L. Steardo, Vasopressin release to central and peripheral precisely the GABAergic regulatory mechanisms of the angiotensin II in rats with lesions of the subfornical organ, Brain
Res. 322 (1984) 365–368. baroreflex circuits.
[11] M.P. Kalia, Localization of aortic and carotid baroreceptor and Immunohistochemical tracing observations have
re-chemoreceptor primary afferents in the brain stem, in: J.P. Buckley, vealed that noradrenergic fibers originating in cells of the C.M. Ferrario (Eds.), Central Nervous System Mechanisms in A2 cell group in the dorsomedial medulla project directly Hypertension, Raven, New York, 1981, pp. 9–24.
to the SFO [3]. Our previous investigations with electro- [12] W. Knepel, D. Nutto, D.K. Meyer, Effect of transection of subforni-cal organ efferent projections on vasopressin release induced by physiological [22] or microdialysis [23] techniques
demon-angiotensin or isoprenaline in the rat, Brain Res. 248 (1982) 180– strated that activation of noradrenergic ascending pathways
184.
from the NTS region to the SFO following hemorrhage [13] T. Kubo, M. Kihara, Evidence for g-aminobutyric acid receptor-enhances the excitability of SFO neurons through an a- mediated modulation of the aortic baroreceptor reflex in the nucleus adrenoceptor mechanism. Thus, it seems likely that the tractus solitarii of the rat, Neurosci. Lett. 89 (1988) 156–160.
[14] R.W. Lind, A.K. Johnson, Subfornical organ-median preoptic con-GABAergic system in the NTS region may participate in
nections and drinking and pressor responses to angiotensin II, J. the regulation of the medullary noradrenergic circuits to
Neurosci. 2 (1982) 1043–1051.
the SFO. To clarify the role of GABAergic systems in the [15] M.L. Mangiapane, J.B. Simpson, Subfornical organ: forebrain site of control of the noradrenergic circuits, further studies in pressor and dipsogenic action of angiotensin II, Am. J. Physiol. 239
progress. (1980) R382–R389.
[16] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates, Academic Press, Sydney, 1986.
[17] B. Persson, A hypertensive response to baclofen in the nucleus
Acknowledgements tractus solitarii in rats, J. Pharmacol. 33 (1981) 226–231.
[18] M. Shioya, J. Tanaka, Inputs from the nucleus of the solitary tract to This research was supported in part by Grant 08671091 subfornical organ neurons projecting to the paraventricular nucleus
in the rat, Brain Res. 483 (1989) 192–195. from the Ministry of Education, Science and Culture,
[19] A.F. Sved, J.C. Sved, Endogenous GABA acts on GABA receptorsB
Japan.
in nucleus tractus solitarius to increase blood pressure, Brain Res. 526 (1990) 235–240.
[20] J.C. Sved, A.F. Sved, Cardiovascular responses elicited byg
-amino-References butyric acid in the nucleus tractus solitarius: evidence for action at
the GABAB receptor, Neuropharmacology 28 (1989) 515–520. [21] J. Tanaka, K. Seto, Neurons in the nucleus of the solitary tract with [1] W.W. Blessing, W.H. Oertel, J.O. Willoughby, Glutamic acid
de-ascending projections to the subfornical organ in the rat, Neurosci. carboxylase immunoreactivity is present in perikarya of neurons in
Lett. 89 (1988) 152–155. the nucleus tractus solitarius of rat, Brain Res. 322 (1984) 346–350.
[22] J. Tanaka, Y. Hayashi, S. Shimamune, M. Nomura, Ascending [2] P. Bousqet, J. Feldman, R. Bloh, J. Schwartz, Evidence for a
pathways from the nucleus of the solitary tract to the subfornical neuromodulatory role of GABA at the first synapse of the
baro-organ in the rat, Brain Res. 777 (1997) 237–241. receptor reflex pathway. Effects of GABA derivative injected into
[23] J. Tanaka, Y. Hayashi, T. Watai, K. Hori, M. Nomura, Noradrenaline the NTS, Naunyn-Schmiedeberg’s Arch. Pharmacol. 319 (1982)
release in the rat subfornical organ area to blood pressure changes, 168–171.
Exp. Neurol. 152 (1998) 303–306. [3] J. Ciriello, M.P. Rosas-Arellano, P. Solano-Flores, Direct projections
[24] J. Tanaka, H. Kaba, H. Saito, K. Seto, Subfornical organ efferents to subfornical organ from catecholaminergic neurons in the caudal
influence the activity of median preoptic neurons projecting to the nucleus of the solitary tract, Brain Res. 726 (1996) 227–232.
hypothalamic paraventricular nucleus in the rat, Exp. Neurol. 93 [4] R. Eng, R.R. Miselis, Polydipsia and abolition of
angiotensin-(1986) 647–651. induced drinking after transections of subfornical organ efferent
[25] J. Tanaka, K. Nojima, Y. Yamamuro, H. Saito, M. Nomura, projections in the rat, Brain Res. 255 (1981) 200–206.
Responses of subfornical organ neurons projecting to the hypo-[5] A.V. Ferguson, N.W. Kasting, Electrical stimulation in the
subforni-thalamic paraventricular nucleus to hemorrhage, Brain Res. 608 cal organ increases plasma vasopressin concentrations in the
con-(1993) 141–144. scious rat, Am. J. Physiol. 251 (1986) R425–R428.
[26] J. Tanaka, H. Saito, H. Kaba, Subfornical organ and hypothalamic [6] A.V. Ferguson, L.P. Renaud, Hypothalamic paraventricular nucleus
paraventricular nucleus connections with median preoptic nucleus lesions decrease pressor responses to subfornical organ stimulation,
neurons: an electrophysiological study in the rat, Exp. Brain Res. 68 Brain Res. 305 (1984) 361–364.
(1987) 579–585. [7] A. Forentino, K. Varga, G. Kunos, Mechanism of the cardiovascular
[27] A.M. Zardetto-Smith, C. Watson, A direct neural projection from the effects of GABA receptor activation in the nucleus tractus solitariiB
nucleus of the solitary tract to the subfornical organ in the rat, of the rat, Brain Res. 535 (1990) 264–270.
Neurosci. Lett. 80 (1987) 163–166. [8] K. Gale, B.L. Hamilton, S.C. Brown, W.P. Norman, J.D. Souza, R.A.