A
NTIDEPRESSANT
A
CTION
Regulation of Hippocampal Neurogenesis in Adulthood
Elizabeth Gould, Patima Tanapat, Tracy Rydel, and Nicholas Hastings
A substantial number of new granule neurons are pro-duced in the dentate gyrus in adulthood in a variety of mammalian species, including humans. Numerous studies have demonstrated that the production and survival of new hippocampal neurons can be enhanced or diminished by hormones and experience. Steroid hormones of the ovaries and adrenal glands have been shown to modulate the production of immature neurons by affecting the prolifer-ation of granule cell precursors. Aversive experiences have been demonstrated to decrease the production of immature granule cells, whereas enriching experiences, including learning, have been shown to enhance the survival of new hippocampal cells. These studies indicate that adult-generated neurons represent a unique form of structural plasticity that can be regulated by the environ-ment, and furthermore suggest that new neurons play an important role in hippocampal function. Biol Psychiatry
2000;48:715–720 © 2000 Society of Biological
Psychiatry
Key Words: Neurogenesis, dentate gyrus, hippocampus,
stress, hormones
Adult Neurogenesis in the Dentate Gyrus
A
dult neurogenesis in the dentate gyrus was first reported over 30 years ago by Altman and colleagues (Altman and Das 1965). With [3H]thymidine autoradiog-raphy to label proliferating cells and their progeny, it was shown that new cells are produced in the dentate gyrus of the adult rat. These new cells were the daughter cells of progenitors located in the dentate gyrus, primarily on the border of the granule cell layer (GCL) and hilus (a region called the subgranular zone [SGZ]). The new cells become incorporated into the GCL and develop the morphological characteristics of mature granule neurons (Altman and Das 1965). Sporadic studies over the ensuing three decades reported additional evidence that new cells become neu-rons, and the evidence is now compelling. It has been shown that adult-generated cells produced in the dentate gyrus of the rat receive synaptic input, extend axons into the mossy fiber pathway, and express a number of markersof mature neurons (Cameron et al 1993a, 1993b; Gould and Tanapat 1997; Gould et al 1999b; Hastings and Gould 1999; Kaplan and Bell 1983; Kaplan and Hinds 1977; Markakis and Gage 1999; Stanfield and Trice 1988; Figure 1). The majority of these earlier studies were carried out in rats. Over the past few years, however, reports of neurogenesis in adult tree shrews (Tupaia
belangeri), marmosets (Callithrix jacchus), macaques
(Macaca fascicularis and Macaca mulatta), and humans have demonstrated that adult-generated neurons are a feature common to the mammalian hippocampus (Eriks-son et al 1998; Gould et al 1997, 1998, 1999a; Kornack and Rakic 1999).
The prolonged period of granule cell genesis in the dentate gyrus makes it unusually susceptible to experi-ence-dependent structural changes. The proliferation of progenitor cells and the subsequent developmental events such as dendritic growth, axon elongation, and synapse formation represent ongoing structural plasticity that may be altered by experience. The continual addition of imma-ture neurons could allow restructuring of this area accord-ing to the current environment, thus providaccord-ing important adaptive plasticity. On the other hand, these ongoing structural changes might render the dentate gyrus partic-ularly sensitive to environmental perturbations that may impair hippocampal structure and function.
Studies conducted over the past several years have identified endocrine, neural, and experiential factors that regulate the production and survival of late-generated neurons in this brain region. Although most studies exam-ining the regulation of adult neurogenesis have been carried out in rats, the limited data available from other species suggest that the production of new neurons is affected by the same factors in rodents and primates.
Positive Regulators of Adult-Generated
Neurons
Estrogen
The ovarian hormone estrogen has been shown to stimulate the production of new granule cells in the adult female rat. Removal of circulating estrogen by ovariectomy results in a significant decrease in the proliferation of granule cell pre-cursors. This decrease can be prevented by replacement of
From the Department of Psychology, Princeton University, Princeton, New Jersey. Address reprint requests to Elizabeth Gould, Princeton University, Department of
Psychology, Green Hall, Princeton NJ 08544.
Received March 21, 2000; revised July 7, 2000; accepted July 28, 2000.
estrogen to ovariectomized rats. Moreover, a natural fluctu-ation in cell proliferfluctu-ation is observed across the estrous cycle in the adult rat. During proestrus, a time when estrogen levels are high, the number of proliferating cells in the dentate gyrus is maximal, as compared with estrus and diestrus, when ovarian hormone levels are lower (Tanapat et al 1999). This natural increase in cell proliferation is most likely responsible for the gender difference in the proliferation of granule cell precursors observed in adult rats. Adult female rats produce more new cells with the characteristics of immature neurons in the dentate gyrus than do adult male rats; however, many of these new cells eventually degenerate, such that by several weeks after mitosis the gender difference favoring females is no longer detectable (Tanapat et al 1999). These findings suggest that estrogen induces a transient increase in the number of immature neurons; however, because the survival of adult-generated cells appears to be dependent on experi-ence (see below), the persistexperi-ence of a gender differexperi-ence in new neurons for longer periods of time in animals exposed to nondeprived conditions cannot be ruled out.
The extent to which other gonadal hormones, such as progesterone and testosterone, affect adult neurogenesis remains unknown. Moreover, no previous studies have explored the possibility that periods of dramatic or chronic changes in gonadal hormones such as puberty, pregnancy, or aging result in altered granule cell genesis.
Environmental Complexity and Learning
Several studies have shown that environmental complexity can enhance the number of new neurons in the hippocam-pus. Barnea and Nottebohm (1994) have demonstrated that black-capped chickadees that live in the wild maintain more new hippocampal neurons than those living in
captivity. A similar enhancement in the number of new hippocampal neurons has been observed in the dentate gyrus of adult mice and rats living in an enriched envi-ronment, as compared with those living under standard laboratory control conditions (Kempermann et al 1997; Nilsson et al 1999). There are many variables that differ between living in the wild and living in captivity and between living in an enriched environment and under control conditions in the laboratory, such as social inter-action, nutrition, stress, activity levels, and learning op-portunities.
Evidence suggests that both learning and physical ac-tivity enhance the number of new granule neurons, appar-ently through different mechanisms. Observations in black-capped chickadees suggest that certain types of learning may increase the survival of new cells. For example, seasonal differences exist in the number of new hippocampal neurons that correlate with changes in seed storage and retrieval, behaviors that involve spatial navi-gation learning (Barnea and Nottebohm 1994). Similarly, it has been demonstrated that training on a task that requires the hippocampal formation for acquisition results in an increase in the number of adult-generated granule cells (Gould et al 1999b). In untrained laboratory animals, most adult-generated cells appear to degenerate within 2 weeks of production (Cameron et al 1993a, 1993b; Gould et al 1999b); however, training on either of two hippocam-pal-dependent tasks, spatial learning during Morris water maze training and trace eyeblink conditioning, rescues a significant proportion of these cells (Ambrogini et al 2000; Gould et al 1999b). In the Morris water maze task, rats use extramaze cues to locate a platform submerged in a pool of water. Rats that learned the location of the hidden platform
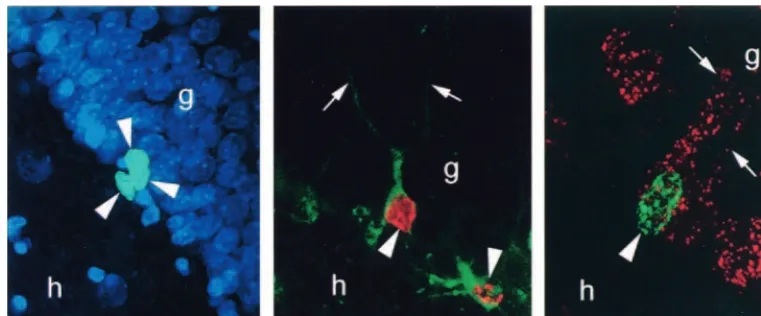
Figure 1. Bromodeoxyuridine (BrdU)–labeled cells in the dentate gyrus of the adult rat. (Left) BrdU-labeled progenitor cells in the subgranular zone of the adult rat (arrowheads). (Middle) BrdU-labeled cell in the granule cell layer (GCL) of the adult rat (arrowhead). This cell is colabeled with TOAD-64, a marker of immature neurons, and has the morphological characteristics of a granule cell.
(Right) BrdU-labeled cell (arrowhead) in the GCL of an adult rat that is colabeled with the retrograde tracer fluoro-ruby injected into
exhibited an increase in the survival of adult-generated cells, as compared with animals that remained in a pool of water in the absence of a platform and as compared with control subjects. A similar increase in the number of new cells has also been observed using the trace eyeblink conditioning task. In this paradigm, rats learn to associate an unconditioned stimulus (US; periorbital shock) and a conditioned stimulus (CS; white noise) that are separated in time. Again, as little as 4 days of training resulted in a greater than twofold increase in the number of new granule neurons, as compared with rats that were pre-sented with the US and CS in an unpaired condition. This increase is most likely the result of enhanced cell survival, as opposed to cell proliferation, for the following three reasons: 1) learning appears to be effective at increasing the number of new neurons only when it occurs during the time of maximal death of newly born cells in laboratory control subjects (Gould et al 1999b; compare with Greenough et al 1999; van Praag et al 1999b); 2) the number of pyknotic or degenerating cells in the SGZ is diminished following hippocampal-dependent learning, and bromodeoxyuridine (BrdU)–labeled pyknotic cells that are observed between 1 and 2 weeks after labeling in control subjects are completely absent after learning; and 3) the number of BrdU-labeled cells does not increase when BrdU is administered during the training phase, after all animals have reached criteria. Although these studies have shown that certain types of learning increase the number of newly generated cells by enhancing their survival, the life span of these cells is not known. Because the number of cells in the brain does not increase through-out adulthood, these cells, or some comparable population, must eventually degenerate. The death of newly generated cells has been observed in the dentate gyrus of laboratory control animals (Gould et al 1999b); however, given that cell survival is enhanced by living in a more complex environment and by learning experiences, these data are not likely to accurately reflect the timing or magnitude of such events in animals living in natural conditions.
Another aspect of the enriched environment that has been shown to enhance the number of new granule cells is increased physical activity. Providing female mice access to a running wheel enhances the number of new granule cells by stimulating the proliferation of granule cell precursors (van Praag et al 1999a, 1999b).
Negative Regulators of Adult-Generated
Neurons
Deprivation
Whereas they provide important information about the specific experiences necessary for the production and survival of new cells, studies demonstrating that
environ-mental enrichment, running, and learning enhance the number of adult-generated cells as compared with control subjects (Gould et al 1999b; Kempermann et al 1997; Nilsson et al 1999; van Praag et al 1999a, 1999b) may also be a reflection of the abnormal state of laboratory control animals. Rodents living in the wild experience a more complex environment, including enhanced activity and learning opportunities, compared with that of laboratory animals used in controlled studies. Because standard laboratory conditions for rodents severely restrict natural behavior and experience, these animals should be thought of as living in relative deprivation. The complexity of natural living may increase the production and extended survival of more new neurons than what is typically reported for deprived laboratory control animals. This possibility should also be considered with regard to adult neurogenesis in primates. Adult neurogenesis studies car-ried out in nonhuman primates thus far have only focused on animals living in laboratory settings (Gould et al 1998, 1999a; Kornack and Rakic 1999). Such conditions involve dramatic cognitive, motor, and social deprivation relative to those produced by living in natural settings. The extent to which laboratory-enriched environments mimic natural living, in terms of effects on neurogenesis, remains unknown.
Adrenal Steroids
suggests that the effects of adrenal steroids on cell prolif-eration occur indirectly through another factor.
Excitatory Input
The proliferation of granule cell precursors in the dentate gyrus is also affected by N-methyl-D-aspartate (NMDA) receptor–mediated excitatory input. In adulthood, treat-ment with either competitive or noncompetitive NMDA receptor antagonists increases the number of proliferating cells in the rodent and tree shrew dentate gyri (Cameron et al 1995; Gould et al 1997). Conversely, activation of NMDA receptors inhibits cell proliferation in the dentate gyrus during adulthood (Cameron et al 1995). The granule cell population receives a major excitatory input from the entorhinal cortex via the perforant path. Transmission across this pathway can involve NMDA receptor activa-tion (Kelsey et al 2000), suggesting that perforant path input may participate in the suppression of cell prolifera-tion in the dentate gyrus. Consistent with this possibility, lesion of the entorhinal cortex has an effect on cell proliferation similar to that of the blockade of NMDA receptors, to enhance cell proliferation in the dentate gyrus in adulthood (Cameron et al 1995). These results may appear to be at variance with studies demonstrating en-hancing effects of seizures on hippocampal neurogenesis (Nakagawa et al 2000; Parent et al 1997); however, most experimentally induced seizures result in cell death, a condition known to stimulate granule cell genesis in the dentate gyrus (Gould and Tanapat 1997). Thus, it is likely that seizure-induced changes in neurogenesis are the result of a complex cascade of events, and not merely of changes in excitatory input. Available evidence suggests that ad-renal steroids suppress cell proliferation in the dentate gyrus by acting through an NMDA receptor–mediated excitatory pathway (Cameron et al 1998).
Stressful Experience
The suppressive effects of glucocorticoids and NMDA receptor activation on granule cell genesis in the intact dentate gyrus suggest that exposure to stress, which elevates glucocorticoid levels and stimulates hippocam-pal glutamate release in adulthood (Moghaddam et al 1994; Stein-Behrens et al 1999), naturally inhibits cell proliferation.
Acute exposure to stress decreases the proliferation of granule cell precursors in the dentate gyri of adult rats (Tanapat et al, submitted), tree shrews (Gould et al 1997), and marmosets (Gould et al 1998). Exposure of adult rats to predator odor elicits a stress response characterized by increased adrenal steroid levels (Heale et al 1994; Sgoifo et al 1996) and a characteristic electrophysiologic re-sponse in the dentate gyrus (Heale et al 1994). A single
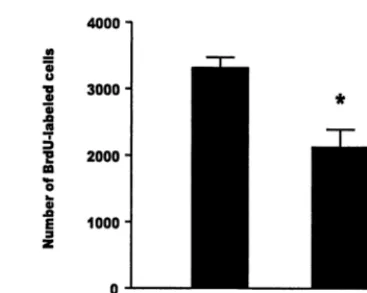
brief exposure to trimethyl thiazoline, a component of fox feces, rapidly suppresses cell proliferation in the dentate gyrus (Tanapat et al, submitted). This effect is likely to be the result of stress-induced increases in glucocorticoids because rendering animals incapable of an increase in glucocorticoids (by removing the adrenal glands and replacing with low-dose corticosterone) prevents the effect (Tanapat et al, submitted). Similarly, a suppressive effect of stress on the number of BrdU-labeled cells has been observed in the dentate gyri of adult rats living as subordinates in a visible burrow system (Figure 2). Con-sistent with these findings, nonrodent mammalian species also show a suppressive effect of stress on hippocampal cell proliferation. In adult tree shrews, a single exposure to subordination stress results in a rapid decrease in the number of new cells produced in the dentate gyri of stressed animals, as compared with control subjects (Gould et al 1997). Likewise, studies of marmosets in a resident intruder paradigm have shown that a single exposure to social stress results in a rapid decrease in the number of new cells produced in the dentate gyrus, as compared with control subjects (Gould et al 1998). The extent to which other types of stressors have a similar effect on cell proliferation remains to be determined.
in cortisol levels in adult tree shrews (Fuchs et al 1995) and a decrease in new cell production in the dentate gyrus, as well as a decrease in the total GCL volume (Fuchs et al 1997). It is likely that chronic suppression of granule cell production is at least partially responsible for the changes in GCL volume, although a causal relationship between these two changes has yet to be established.
Functional Consequences of Changes in
Adult-Generated Neurons
Although the functional significance of adult-generated neurons is not known, two lines of evidence suggest that these new cells play an important role in learning. First, many new neurons are added to the hippocampus, a brain region involved in certain types of learning and memory. Second, regulators of hippocampal neurogenesis in adult-hood also alter performance on hippocampal-dependent learning tasks in a manner that is consistent with a link between new cells and learning. It is important to note that functional changes in the number of adult-generated neu-rons are not likely to be immediately evident. New neurons require time to differentiate and become inte-grated into functional circuitry. Thus, it is the period following changes in cell production that is most likely to be functionally relevant; however, the fact that adult-generated cells in the dentate gyrus extend axons into the CA3 region of the hippocampal formation as early as 4 –10 days following division (Hastings and Gould 1999) sug-gests that the impact of changes in cell production may be functionally significant as early as 4 days later. In addi-tion, it is important to note that acute changes in cell production may not alter hippocampal function. Thus, the impact of chronic changes in neuron production is more likely to be of interest because such effects are likely to be additive.
Chronic exposure to positive regulators of adult neuro-genesis, such as estrogen, enriched-environment living, and running, has been associated with enhanced perfor-mance on hippocampal-dependent learning tasks in ro-dents (Kempermann et al 1997; Luine et al 1997; van Praag et al 1999a) and other cognitive tasks in humans (McEwen et al 1997). Conversely, chronic exposure to negative regulators of adult neurogenesis, such as adrenal steroids and stress, has been associated with impaired performance on such tasks (Bodnoff et al 1995; Endo et al 1996; Krugers et al 1997; Luine et al 1994). These correlations are consistent with the view that adult-gener-ated neurons participate in hippocampal-dependent learn-ing; however, these neuroendocrine and experiential fac-tors also alter other aspects of brain structure and function that cannot be ruled out as alternative mechanisms for the changes in hippocampal-dependent learning. Studies
de-signed to examine the behavioral consequences of specific depletion of new neurons will provide a more direct approach to this question.
References
Altman J, Das GD (1965): Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124:319 –335.
Ambrogini P, Cuppini R, Cuppini C, Ciaroni S, Cecchini T, Ferri P, et al (2000): Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neurosci Lett 286:21–24. Barnea A, Nottebohm F (1994): Seasonal recruitment of hip-pocampal neurons in adult free-ranging black-capped chick-adees. Proc Natl Acad Sci U S A 91:11217–11221. Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen
B, Sakai RR (1995): Visible burrow system as a model of chronic social stress: Behavioral and neuroendocrine corre-lates. Psychoneuroendocrinology 20:117–134.
Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ (1995): Enduring effects of chronic corti-costerone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci 15:61– 69.
Cameron HA, Gould E (1994): Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 61: 203–209.
Cameron HA, McEwen BS, Gould E (1995): Regulation of adult neurogenesis by excitatory input and NMDA receptor activa-tion in the dentate gyrus. J Neurosci 15:4687– 4692. Cameron HA, McKay RD (1999): Restoring production of
hippocampal neurons in old age. Nat Neurosci 2:894 – 897. Cameron HA, Tanapat P, Gould E (1998): Adrenal steroids and
N-methyl-D-aspartate receptor activation regulate neurogen-esis in the dentate gyrus of adult rats through a common pathway. Neuroscience 82:349 –354.
Cameron HA, Woolley CS, Gould E (1993a): Adrenal steroid receptor immunoreactivity in cells born in the adult rat dentate gyrus. Brain Res 611:342–346.
Cameron HA, Woolley CS, McEwen BS, Gould E (1993b): Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience 56:337–344.
Endo Y, Nishimura JI, Kimura F (1996): Impairment of maze learning in rats following long-term glucocorticoid treat-ments. Neurosci Lett 203:199 –202.
Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nor-dborg C, Peterson DA, Gage FH (1998): Neurogenesis in the adult human hippocampus. Nat Med 4:1313–1317.
Fuchs E, Flugge G, McEwen BS, Tanapat P, Gould E (1997): Chronic subordination stress inhibits neurogenesis and de-creases the volume of the granule cell layer. Soc Neurosci 23:317.
Fuchs E, Uno H, Flugge G (1995): Chronic psychosocial stress induces morphological alterations in hippocampal pyramidal neurons of the tree shrew. Brain Res 673:275–282. Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS
Gould E, McEwen BS, Tanapat P, Galea LAM, Fuchs E (1997): Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activa-tion. J Neurosci 17:2492–2498.
Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E (1999a): Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci U S A 96:5263–5267. Gould E, Tanapat P (1997): Lesion-induced proliferation of
neuronal progenitors in the dentate gyrus of the adult rat. Neuroscience 80:427– 436.
Gould E, Tanapat P, Beylin A, Reeves AJ, Shors TJ (1999b): Hippocampal-dependent learning enhances the survival of granule neurons generated in the dentate gyrus of adult rats. Nat Neurosci 2:260 –265.
Gould E, Tanapat P, Hastings NB, Shors TJ (1999c): Neurogen-esis in adulthood: A possible role in learning. Trends Cogn Sci 3:186 –192.
Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E (1998): Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A 95:3168 –3171.
Gould E, Woolley CS, Cameron HA, Daniels DC, McEwen BS (1991): Adrenal steroids regulate postnatal development of the rat dentate gyrus: II. Effects of glucocorticoids and mineralocorticoids on cell birth. J Comp Neurol 313:486 – 493.
Greenough WT, Cohen NJ, Juraska JM (1999): New neurons in old brains: Learning to survive? Nat Neurosci 2:203–205. Hastings NB, Gould E (1999): Rapid extension of axons into the
CA3 region by adult-generated granule cells. J Comp Neurol 413:146 –154.
Heale VR, Vanderwolf CH, Kavaliers M (1994): Components of weasel and fox odors elicit fast wave bursts in the dentate gyrus of rats. Behav Brain Res 63:159 –165.
Kaplan MS, Bell DH (1983): Neuronal proliferation in the 9-month-old rodent-radioautographic study of granule cells in the hippocampus. Exp Brain Res 52:1–5.
Kaplan MS, Hinds JW (1977): Neurogenesis in the adult rat: Electron microscopic analysis of light radioautographs. Sci-ence 197:1092–1094.
Kelsey JE, Sanderson KL, Frye CA (2000): Perforant path stimulation in rats produces seizures, loss of hippocampal neurons, and a deficit in spatial mapping which are reduced by prior MK-801. Behav Brain Res 107:59 – 69.
Kempermann G, Kuhn HG, Gage FH (1997): More hippocampal neurons in adult mice living in an enriched environment. Nature 386:493– 495.
Kornack DR, Rakic P (1999): Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A 96:5768 –7573.
Krugers HJ, Douma BR, Andringa G, Bohus B, Korf J, Luiten PG (1997): Exposure to chronic psychosocial stress and corticosterone in the rat: Effects on spatial discrimination learning and hippocampal protein kinase Cgamma immuno-reactivity. Hippocampus 7:427– 436.
Kuhn HG, Dickinson-Anson H, Gage FH (1996): Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci 16:2027–2033. Luine V, Villegas M, Martinez C, McEwen BS (1994): Repeated
stress causes reversible impairments of spatial memory per-formance. Brain Res 639:167–170.
Luine VN, Richards ST, Wu VY, Beck KD (1998): Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav 34:149 –162.
Markakis EA, Gage FH (1999): Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol 406:449 – 460.
McEwen BS, Alves SE, Bulloch K, Weiland NG (1997): Ovarian steroids and the brain: Implications for cognition and aging. Neurology 48:S8 –S15.
Moghaddam B, Bolinao ML, Stein-Behrens B, Sapolsky R (1994): Glucocorticoids mediate the stress-induced extracel-lular accumulation of glutamate. Brain Res 655:251–254. Nakagawa E, Aimi Y, Yasuhara O, Tooyama I, Shimada M,
McGeer PL, Kimura H (2000): Enhancement of progenitor cell division in the dentate gyrus triggered by initial limbic seizures in rat models of epilepsy. Epilepsia 41:10 –18. Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS
(1999): Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neu-robiol 39:569 –578.
Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH (1997): Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci 17:3727–3738.
Sapolsky RM. (1992): Do glucocorticoid concentrations rise with age in the rat? Neurobiol Aging 13:171–174.
Sgoifo A, de Boer SF, Haller J, Koolhaas JM (1996): Individual differences in plasma catecholamine and corticosterone stress responses of wild-type rats: Relationship with aggression. Physiol Behav 60:1403–1407.
Stanfield BB, Trice JE (1988): Evidence that granule cells generated in the dentate gyrus of adult rats extend axonal projections. Exp Brain Res 72:399 – 406.
Stein-Behrens BA, Lin WJ, Sapolsky RM (1999): Physiological elevations of glucocorticoids potentiate glutamate accumula-tion in the hippocampus. J Neurochem 63:596 – 602.
Tanapat P, Galea LA, Gould E (1998): Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Int J Dev Neurosci 16:235–239.
Tanapat P, Hastings N, Reeves AJ, Gould E (1999): Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci 19:5792–5801.
Tanapat P, Hastings NB, Rydel T, Gould E (submitted): Expo-sure to predator odor inhibits hippocampal cell proliferation via adrenal steroids.
van Praag H, Christie BR, Sejnowski TJ, Gage FH (1999a): Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A 96:13427– 13431.