Deleterious properties of certain rhizosphere bacteria on field pea
(
Pisum sativum
) under gnotobiotic and non-sterile conditions
I. Berggren
a,∗, S. Alström
b, A.M. Mårtensson
aaSoil Science Department, Swedish University of Agricultural Sciences, P.O. Box 7014, SE-750 07 Uppsala, Sweden
bEcology and Crop Production Science Department, Swedish University of Agricultural Sciences, P.O. Box 7043, SE-750 07 Uppsala, Sweden
Accepted 20 June 2000
Abstract
Three strains ofPseudomonas putida, one non-fluorescent and two fluorescent, were investigated in a series of
complemen-tary experiments to characterise their inhibitory effects on peas under different environmental conditions. Firstly, a gnotobiotic growth pouch system was developed to observe the deleterious effects of the strains on pea root development. A negative impact of the strains was observed on the development of the root morphology using these pouches which was accompanied with a subsequent reduction in root biomass. By using this method it was concluded that the deleterious effect of one of the strains was dependent on the inoculum density. Secondly, two complementary studies in non-sterile growth systems where pea seeds/seedlings were inoculated with the bacteria, showed deleterious effects on plant biomass by two of the strains. Thirdly, by using a sterile plant growth system allowing microscopic observations on root hair development, all strains were found to be able to induce root hair deformations on pea seedlings. The results showed that the mode of action for the deleterious effect differ between the strains. Based on our findings, we would like to emphasise the necessity to include a palette of different sterile and non-sterile growth systems to be able to identify characteristics of importance for deleterious rhizosphere bacteria. © 2001 Elsevier Science B.V. All rights reserved.
Keywords: Pseudomonas putida; Deleterious; Rhizosphere bacteria; DRB; Pseudomonads
1. Introduction
The rhizosphere accommodates a large number of saprophytic bacteria with stimulating, neutral or dele-terious effects on crop plants. For instance, Åström (1990) has shown that 8–26% of the bacterial strains from a rhizosphere soil had adverse effects on plants. However, although of great scientific and practical interest, more information is needed on which fac-tors regulate the deleterious effects of the bacteria
∗Corresponding author. Tel.:+46-18-67-3479;
fax:+4618-67-2795.
E-mail address:[email protected] (I. Berggren).
on the host plants. Changes in cropping practice (Alström, 1992), nutrient status and microbial pop-ulations (Fredrickson and Elliott, 1985), soil tem-perature (Davies and Whitbread, 1989) and plant genotype (Åström, 1991a; Sarathchandra et al., 1996) have for instance been shown to influence the capac-ity of certain bacterial strains to express deleterious effects. When studying deleterious rhizosphere bac-teria (DRB), it is thus important to make a series of complementary studies under different conditions before drawing any conclusions about the deleterious potential of a certain strain on a specific plant species. In most of the studies carried out to date, con-clusions regarding the deleterious effects of an
individual strain have been based upon measurements of the length and weight of the plant root and shoot (Nehl et al., 1996). Studies seldom consider the de-velopment of the root system in the presence of DRB. It is thus important to include morphological and/or structural changes in the root system when classifying DRB, since bacteria-induced alterations may finally have a great impact on plant health.
Detailed understanding of the inhibitory traits of bacteria would lead to the identification of mecha-nisms underlying the competition and survival be-tween various groups of bacteria in the rhizosphere. This information will be essential to create the con-ditions necessary to promote the legume-Rhizobium symbiosis or to favour plant growth promoting rhi-zobacteria (PGPR) in cropping practices. With this aim, we investigated whether three previously iso-lated and characterised DRB strains differed in their deleterious activity on the model plant, pea, under different environmental conditions, before studying their interrelation withRhizobiumestablishment.
2. Materials and methods
2.1. Plant material and growing conditions
A white-flowered, semi-leafless and short-stemmed field pea, Pisum sativum cv. Capella, from Svalöf Weibulls Ltd., Sweden, is a field pea widely grown in Sweden that was used as model plant. The seeds were fractionated by size and only apparently healthy seeds with a diameter between 7.0 and 7.5 mm were used.
Plants were grown in growth pouches in the growth chamber with day/night temperature of 17/15◦C, with a 16 h day length and a mean photosynthetic photon flux density of 45 klx and 70% humidity. For plants grown in soil, greenhouse conditions were 16–20◦C,
Table 1
Some characteristics of thePseudomonasbacterial strains used in this study
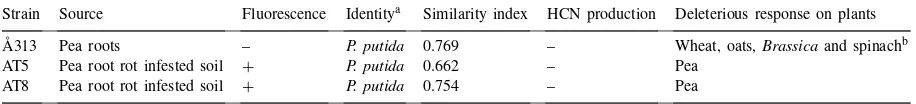
Strain Source Fluorescence Identitya Similarity index HCN production Deleterious response on plants
Å313 Pea roots – P. putida 0.769 – Wheat, oats,Brassicaand spinachb
AT5 Pea root rot infested soil + P. putida 0.662 – Pea AT8 Pea root rot infested soil + P. putida 0.754 – Pea
aFatty acid methyl ester analysis (Sasser, 1990). bÅström (1990).
light, if necessary, was supplemented with Philips HPI-T 400 W mercury lamps to give 10 klx at plant height to ensure 16 h of light period.
The soil used originated from a nearby farm, sit-uated at Ultuna, Sweden, 60◦N, 17◦E. The soil was a loamy sand, pH 7.2, with a total nitrogen content of 0.02%, an organic matter of 0.4%, slightly soluble phosphorus (Egnér et al., 1960) 9 mg/100 g soil and soluble phosphorus 49 mg/100 g soil, and with no pre-vious treatment with fungicides. The soil was moist-ened to 70% of water holding capacity before planting.
2.2. Bacterial characteristics
Three bacterial strains, one non-fluorescent (Å313) and two fluorescent (AT5 and AT8) were identified as Pseudomonas (P.)putida using fatty acid methyl ester analysis (FAME), a well-established method for identification (Sasser, 1990). Å313, previously shown to be deleterious, was used as the model organism (Åström, 1990). AT5 and AT8 were selected for their deleterious effects as shown in preliminary tests. The origin and known characteristics of the strains are presented in Table 1. Stock cultures of the bacterial strains, suspended in sterilised distilled water, were stored at−70◦C prior to use. As inoculum, the bac-terial cell suspensions were prepared by suspending 24 h old cultures grown on TSA (Tryptic soy broth agar, 3 g/l, Technical agar, 10 g/l). Cells were added in 10 mM MgSO4to OD 0.3 at 560 nm (log 7 cfu/ml) and diluted further, if necessary, in 10 mM MgSO4before use.
papers were fixed to the bottom of a Petri dish lid (diameter 90 mm) and dish was sealed with parafilm. Plates left uninoculated and plates inoculated with a known cyanide producing bacterial strain were used as controls.
2.3. Bacterial impact on early root development under gnotobiotic conditions
2.3.1. Development of roots
A initial experiment was carried out for testing whether the P. putida strains had an impact on the initial pea root development before further studies in the growth pouches. Seeds were surface-sterilised for 5 min in sodium hypochlorite (0.5%) and soaked in hydrogen peroxide (0.75%) for 30 min, followed by incubation in bacterial cell suspensions for 30 min. Seeds treated with 10 mM MgSO4 were used as con-trols. Following inoculation, the seeds were placed on large Petri dishes (diameter 190 mm) containing water agar (1.5%). One Petri dish with 15 seeds was used for each strain and incubated in dark at 22◦C. Root length was measured and root development was evaluated after 5 days.
2.3.2. Development of root hairs
Pre-germinated pea seedlings were prepared as fol-lows. A glass jar (diameter 190 mm, height 90 mm) was filled with 200 g vermiculite and 750 g distilled water, covered with a glass cover and autoclaved at 121◦C for 30 min 1 day before use. Thirty-five surface-sterilised seeds per jar, prepared as above, were placed 20 mm deep with the notch on the seeds placed in the same position, pointing down into the vermiculite and allowed to germinate in darkness at 22◦C for 4 days prior to use. Microbial contamina-tion of the seedlings was checked on tryptone yeast extract agar. Only contamination-free seedlings with straight taproots about 3–5 cm long were used in the experiment.
After pre-germination, the seedlings were inocu-lated with each bacterial suspension (log 5 cfu/ml) by soaking them for 30 min, and transferred to a Fåhraeus slide with one seedling per slide according to So-masegaran and Hoben (1994) modified so cover slips were glued to the object glasses using 2 mm glass beads as spacers instead of agar to ensure good root development. The object glasses were 75 mm×25 mm
in size and cover slips were 40 mm×24 mm. The Fåhraeus slides were placed vertically in test tubes of diameter 36 mm supplied with 35 ml Fåhraeus solu-tion. Treatments included exposure to Å313, AT5 and AT8 with six replicates each. Following inoculation, the tubes were kept in a growth chamber and scored microscopically at intervals for presence of root hair deformations.
2.3.3. Development of root structure
Seedlings, prepared as above, were inoculated with bacterial cell suspensions at three concentrations (log 3, 5 and 7 cfu/ml) by soaking them for 30 min. Cell densities were related to viable cell numbers, measured as cfu/ml of cell suspension by the standard dilution plate counting technique. Seedlings incu-bated in 10 mM MgSO4 were used as controls. Two inoculated seedlings were placed into a sterile growth pouch, prepared as follows.
Plastic growth pouches consisting of polyethylene bags of size 230 mm×150 mm×0.17 mm were
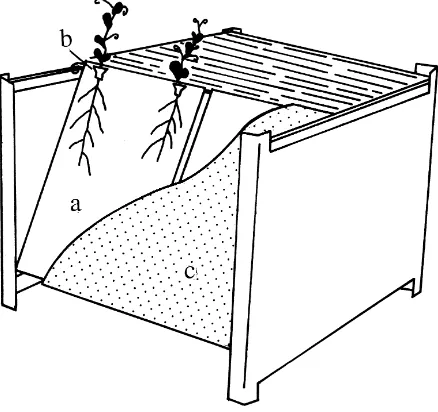
pre-pared using a sealing unit. After preparation the bags were treated at 50◦C for 3 days before use to minimise interference with contaminants. A cellulose filter, Mil-lipore (AP10) with a size of 195 mm×145 mm × 2 mm was saturated in a plant nutrient solution (Ols-son and Alström, 1996) and autoclaved at 121◦C for 20 min immediately before use, and inserted asepti-cally into each pouch. To stabilise the floppy pouch, the cellulose filter was first attached to a sterile glass plate (195 mm×145 mm×2 mm) before placing it in the pouch. At the opening of each pouch, two ster-ile polyethylene funnels were arranged to support the emerging plants. Each funnel was fitted with a ster-ile cellulose filter (Munktell), and a sterster-ile wick of cotton to retain moisture for the emerging seedlings (Fig. 1).
Fig. 1. Growth pouch system used to study bacterial im-pact on pea root development: (a) growth pouch of polyethy-lene 230 mm × 150 mm × 0.17 mm in size containing a sterilised cellulose filter, Millipore (AP10) with a size of 195 mm×145 mm×2 mm, saturated with a plant nutrient solu-tion; (b) sterile polyethylene funnel to support the emerging plant, containing a sterile cellulose filter (Munktell) and a sterile wick of cotton; (c) paper sheet to protect the roots from illumination.
taproot was marked on the pouches at days 0, 4, 11 and 21.
The plants were harvested after 21 days of growth and the plant (shoots and roots) were dried at 60◦C and weighed. At harvest, one pouch with two root systems from each treatment was randomly removed and homogenised in sterile 10 mM MgSO4in order to determine degree of bacterial colonisation and growth. Serial dilution’s were plated on TSA and incubated over night at 28◦C. These plants were not included in the statistical analyses. The experiment was set up in a random design with nine replicates.
2.4. Bacterial impact on early plant development under non-sterile conditions
When carrying out the greenhouse study, the method used was based on the experimental set up prepared by Åström and Gerhardson (1988).
Pea seeds were rinsed and soaked in 10 mM MgSO4 at 10◦C, for 1 h. The seeds were then inoculated
with bacterial suspensions by agitating them for 2 h. Seeds agitated in 10 mM MgSO4 only were used as controls. Treated seeds were sown on soil surface in pots (90 mm width, 110 mm deep) containing moist-ened field soil (one seed per pot and n = 10 per strain), covered with a thin layer of soil which was slightly moistened with sterile distilled water. Pots were placed in a greenhouse and covered with plastic film for the first 2 days to prevent evaporative losses. Plants were watered as necessary with sterile distilled water to maintain water holding capacity. Emergence and shoot development were recorded at regular in-tervals. Shoots and roots were harvested after 21 days of growth, dried separately at 60◦C and weighed.
Experiment was repeated but with some modifica-tions, pea seeds were soaked in sterile 10 mM MgSO4 overnight at 22◦C followed by thorough rinsing with sterile 10 mM MgSO4 and left for germination on sterile moist filter papers for 3 days. Three seedlings, each having 2–3 cm taproots, were placed in each pot of same size as mentioned above, containing moistened soil. Following planting, 5 ml of bacterial suspension were poured over each seedling, five repli-cates per bacterial treatment. Corresponding control plants were treated with a similar amount of sterile 10 mM MgSO4. The seedlings were then covered with a thin layer of soil and the surface was slightly moist-ened with sterile tap water. Rest of the procedure was same as above.
2.5. Statistical analyses
The data from all experiments were analysed using one way ANOVA (Newbold, 1991).
3. Results
The three strains used in this study were identified as P. putida according to FAME analysis (Table 1). None of the strains was producing cyanide (Table 1).
3.1. Bacterial impact on early root development under gnotobiotic conditions
Table 2
The deleterious impact ofPseudomonas putidastrains on development of peas (Pisum sativumcv. Capella) 3 weeks after inoculation in gnotobiotic growth pouchesa
Plant part harvested Bacterial treatment (cfu/ml)
Å313 AT5 AT8 None
log 3 log 5 log 7 log 3 log 5 log 7 log 3 log 5 log 7
Shoot 120.5 80.3b 62.7b 126.3 72.0b 106.4b 132.7 123.2 73.0b 127.9
Root 161.0b 108.5b 84.7b 155.3b 112.0b 164.8b 171.2b 184.7 93.4b 193.9 aValues represent mean dry weight (mg per plant),n=8.
bSignificant atP≤0.05 compared to none bacterial treatment.
resulted in significant (P ≤ 0.05) retardation of root
growth when compared to the control. Average root length (mm per plant) for control was 25.1, Å313 was 7.9, AT5 was 21.2 and for AT8 it was 19.7. Å313 had the strongest deleterious impact expressed as short, crooked and discoloured roots. In comparison, when exposed to AT8, in 60% of cases the roots were short and crooked with no discoloration while AT5 caused no observable negative effect on the roots compared to the non-inoculated controls.
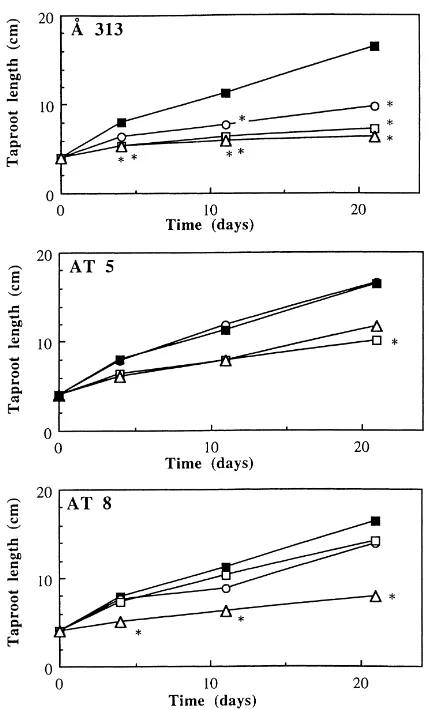
In growth pouches, treatment with AT5 and Å313 lowered the shoot dry weight at the two highest cell densities used and reduced root dry weight at all three cell densities compared to the control (Table 2). In contrast, strain AT8 reduced shoot and root dry weight only at the highest cell density, log 7 cfu/ml. Length measurements were found correlated with two of the root dry weight as a result of Å313 and AT8 treat-ments, whereas AT5 showed only a growth reduction of the taproots after 21 days at log 5 cfu/ml (Fig. 2). Treatment with AT5 showed an apparently healthy root system but with visibly thinner and sparser roots. AT8 at a density of log 7 cfu/ml, induced shortened taproots, poor lateral root development and occasional discoloration of the taproot tip. Treatment with Å313 altered root morphology irrespective of cell densities. At a high cell density, root length was drastically re-duced, also lateral root formation was either inhibited or absent. Most taproots, and even the lateral roots if present, were discoloured, shrunken and dry. It was possible to re-isolate all three strains from the roots on TSA at the end of the experiment.
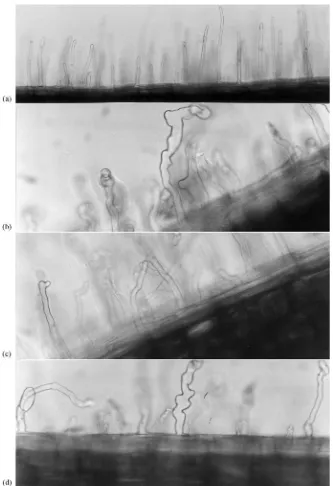
Bacteria-induced root hair deformations were ob-served on root hairs within 3 days after inoculation (Fig. 3). Å313 showed the strongest effect on root hair morphology.
Fig. 2. The deleterious impact of bacterial inoculum densities on root development of peas (Pisum sativumcv. Capella) in gnoto-biotic growth pouches 4, 11 and 21 days after inoculation with different cell densities ofPseudomonas putidastrains, Å313, AT5
Table 3
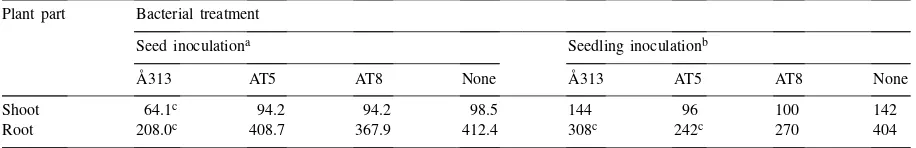
The deleterious impact ofPseudomonas putidaon pea growth in soil 3 weeks after inoculation with log 7 cfu/ml Plant part Bacterial treatment
Seed inoculationa Seedling inoculationb
Å313 AT5 AT8 None Å313 AT5 AT8 None
Shoot 64.1c 94.2 94.2 98.5 144 96 100 142
Root 208.0c 408.7 367.9 412.4 308c 242c 270 404
aValues represent mean dry weight (mg per plant); one seed per pot, (n=10). bValues represent mean dry weight (mg per pot); three seeds per pot, (n=5). cSignificant atP≤0.05 compared none bacterial treatment.
3.2. Bacterial impact on early plant development under non-sterile conditions
Germination was monitored 5 days after the seed inoculation, showing a delay in germination compared to control (90%) whereas the same was 70% for Å313 and 50% for AT5 or AT8. After 2 weeks however, per-cent germination reached to 90% in all pots irrespec-tive of treatment. Table 3 shows that only the treatment with Å313 resulted into a significant lower dry matter and root length than uninoculated control plants.
As further shown in Table 3, pre-germinated seeds treated with AT5 or AT8 had negative effects on shoot and root dry weights though not always significant compared to control plants. However, Å313 showed its deleterious effect only on roots.
4. Discussion
In the present study, we developed a gnotobiotic growth pouch system which allowed us to follow the root development in an easily and non-destructive manner. With this method, an observed alteration on the morphology of the root system caused by the bac-teria was in accordance with a subsequent dry weight reduction of the root biomass. A similar system has been used by Mia et al. (1996) when studying vari-ations in root system structure of some food legume species. In our system we used shoot and root dry weight and length of taproot to determine the extent of deleterious effects of the three bacterial strains studied.
Using the developed gnotobiotic system, a delete-rious effect of strain Å313 was observed on pea roots (Fig. 2), which could be continuously recorded over
the 3 week duration of the experiment. Å313, which was previously shown to have deleterious effects on a range of crop plants under various environmental con-ditions (Åström, 1990), induced strong reductions in the shoot and root biomass (Table 2). Inoculation with Å313 also caused discoloration and shrinkage of roots at all three cell densities tested which indicates that observations on developing root systems is one suit-able basis for testing reactions of deleterious bacterial strains in growth pouches.
Deleterious effect of AT8 was similar but, however, dose dependent compared to Å313. On the contrary, inoculation with AT5 resulted in a different relation-ship between the development of the root system and its subsequent dry weight. In this case, the low biomass weight was not due to direct damage of the root system rather due to thinner and sparser roots in an apparently healthy root system with development of lateral roots. These observations are in agree-ment with the results from the study on agar which showed that the bacterial effects on pea roots fol-lowed the same pattern as was observed in the growth pouches.
induced release of plant hormone-like substances, in analogy with Rhizobium-legume associations (Over-holt et al., 1996). Åström et al. (1993), showed that a cellfree filtrate from Å313 of a non-volatile nature inhibited elongation of wheat roots. Further, the fil-trate showed a hydrophobic character with a broad pH tolerance and a high temperature stability. Other metabolites that may cause growth inhibition in plants are phytohormones (Schippers et al., 1987; Loper and Schroth, 1986) and 2,4-diacetylphloroglucinol (Mauhofer et al., 1994) in concentrations high enough to cause phytotoxicity. If the root hair deformations in our studies are caused by phytohormones of bacterial origin would need further investigation.
Complementary, we found that the strains AT5 and Å313 gave consistent root inhibition irrespective of the bacterial inoculum cell density used. Regarding AT8, only the highest bacterial inoculum cell density had a deleterious impact on the pea root development (Tables 2 and 3 and Fig. 2). We speculate that these re-sults reflect the differences in root colonisation capac-ity of the three strains. All strains could be re-isolated from the root systems at end of the growth period which show that the populations have survived dur-ing the experiment. The number of bacteria re-isolated however, does not per se indicate the capacity of each strain to establish and colonise on the root system. Bolton and Elliott (1989) have, for instance, found that the toxin production by a rhizobacterialPseudomonas sp. is constitutive and therefore the quantity of the toxin is associated to the recovery of the cells and the capacity of the population to undergo many genera-tions. Based from these findings it can be assumed that strain AT5 and Å313 have a better capacity to estab-lish and multiply in the rhizosphere while a successful colonisation by strain AT8 require a higher inoculum cell density to give a measurable toxic effect.
Our greenhouse studies showed that Å313 was still harmful to pea plants in non-sterile field soil. AT5 was deleterious only when inoculated on seedlings but AT8 was harmless despite a high cell density (Table 3). A high cell density of the introduced bacteria had earlier been shown to be important in producing a deleterious effect in non-sterile systems with or without addition of bacterial nutrients (Fredrickson and Elliott, 1985; Alström, 1987). In our greenhouse experiments there was no addition of bacterial nutrient substrate to the field soil which shows that Å313 and AT5 are good
competitors with the native microbes. Our studies also showed that none of the strains seems to induce a cer-tain negative impact on seed germination and seedling growth. To what extent it is of ecological importance needs further investigations.
In conclusion, Å313 was demonstrated to be strongly deleterious in all the testing environments, while the effect of AT5 and AT8 were dependent on several factors including competition from the sur-rounding microflora. Interestingly, all three strains tested were able to induce root hair deformations. This demonstrates the need to use a palette of differ-ent systems to determine the deleterious effects of an strain. Selected methods should therefore be used so that characteristics of importance for DRB are iden-tified. Such tests should include growth in sterile as well as non-sterile root environments combined with rapid, reliable and simple tests on the ability of the strains to produce plant growth-altering substances in pure culture.
Dr. Paula Persson at Department of Ecology and Crop Production Sciences, SUAS, is acknowledged for advice and help with the fatty acid analyses. Financial support from Swedish Council for Agri-culture and Forestry Research (SJFR) is thankfully acknowledged.
References
Alström, S., 1987. Factors associated with detrimental effects of rhizobacteria on plant growth. Plant Soil 102, 3–9.
Åström, B., 1990. Interactions between plants and deleterious rhizosphere bacteria. Importance of plant genotype and possible mechanisms involved. Dissertation, Swedish University of Agricultural Sciences, Uppsala, Sweden, ISSN 0348–3428. Åström, B., 1991a. Intra- and interspecific variation in
plant response to inoculation with deleterious rhizosphere pseudomonads. J. Phytopathol. 131, 184–192.
Åström, B., 1991b. Role of bacterial cyanide production in differential reaction of plant cultivars to deleterious rhizosphere pseudomonads. Plant Soil 133, 93–100.
Alström, S., 1992. Saprophytic soil microflora in relation to yield reductions in soil repeatedly cropped with barley (Hordeum vulgareL.). Biol. Fertil. Soils 14, 145–150.
Alström, S., Burns, R.G., 1989. Cyanide production by rhizobacteria as a possible mechanism of plant growth inhibition. Biol. Fertil. Soils 7, 232–238.
Åström, B., Gustafsson, A., Gerhardson, B., 1993. Characteristics of a plant deleterious rhizosphere pseudomonad and its inhibitory metabolite(s). J. Appl. Bact. 74, 20–28.
Bakker, A.W., Schippers, B., 1987. Microbial cyanide production in the rhizosphere in relation to potato yield reductions and
Pseudomonasspp-mediated plant growth stimulation. Soil Biol.
Biochem. 19, 451–457.
Bolton, H., Elliott, L.F., 1989. Toxin production by a rhizobacterial
Pseudomonas sp. that inhibits wheat root growth. Plant Soil
114, 269–278.
Davies, K.G., Whitbread, R., 1989. Factors affecting the colonisa-tion of a root system by fluorescent pseudomonads: the effects of water, temperature and soil microflora. Plant Soil 116, 247– 256.
Egnér, H., Riehn, H., Domingo, W.R., 1960. Untersuchungen über die chemische Bodenanalyse als Grundlage für Beurtei-lung des Nährstoffzustandes der Böden II. Chemische Extraktionsmethoden zur Phosphor-und Kaliumbestimmung. Kungliga Lantbrukshögskolans Annaler 26, 199–215. Fredrickson, J.K., Elliott, L.F., 1985. Colonization of winter wheat
roots by inhibitory rhizobacteria. Soil Sci. Soc. Am. J. 49, 1172–1177.
Loper, J.E., Schroth, M.N., 1986. Influence of bacterial sources of indole-3-acetic acid on root elongation of sugar beet. Phytopathology 76, 386–389.
Lorck, H., 1948. Production of hydrocyanic acid by bacteria. Physiol. Plant 1, 142–146.
Mauhofer, M., Sacherer, P., Keel, C., Haas, D., Défago, G., 1994. Role of some metabolites produced by Pseudomonas fluorescens strain CHA0 in the suppression of different plant diseases. In: Ryder, M.H., Stephens, P.M., Bowen, G.D. (Eds.), Improving Plant Productivity with Rhizosphere Bacteria. CSIRO, Australia.
Mia, W., Yamauchi, A., Kono, Y., 1996. Root system structure of six food legume species: inter- and intraspecific variations. Jpn. J. Crop Sci. 65, 131–140.
Nehl, D.B., Allen, S.J., Brown, J.F., 1996. Deleterious rhizosphere bacteria: an integrating perspective. Appl. Soil Ecol. 5, 1–20. Newbold, P., 1991. Statistics for Business and Economics.
Prentice-Hall, Englewood Cliffs, NJ, 930 pp.
Olsson, S., Alström, S., 1996. Plant-affecting streptomycin-sensitive micro-organisms in barley monoculture soils. New Phytol. 133, 245–252.
Overholt, E., Engqvist, G., Lindblad, P., Mårtensson, A., Rydberg, I., Zagal, E., 1996. Pea-rhizobial and mycorrhizal symbiotic systems: a review of their commonalities with other plant-microbe systems. Symbiosis 21, 95–113.
Sarathchandra, S.U., Brown, J.A., Cox, N.R., 1996. Rhizobacteria harmful to seedling growth in white clover (Trifolium repens
L.) and perennial ryegrass (Lolium perenneL.). N. Z. J. Agric.
Res. 39, 129–136.
Sasser, M., 1990. Identification of bacteria through fatty acid analysis. In: Clement, Z., Rudolph, K., Sands, D.C. (Eds.), Methods in Phytobacteriology. Akademiai Kiado, Budapest, pp. 199–204.
Schippers, B., Bakker, A.W., Bakker, P.A.H.M., 1987. Interactions of deleterious and beneficial rhizosphere microorganisms and the effect of cropping practices. Annu. Rev. Phytopathol. 25, 339–358.
Somasegaran, P., Hoben, H.J., 1994. Handbook for Rhizobia. Methods in Legume-Rhizobium Technology. Springer, New York, Berlin, Heidelberg, 450 pp.