Summary The expression of acrotony (i.e., the increasing size of comparable lateral shoots toward the apex of the main shoot) was similar among first-order shoots borne along pre-vious-year leaders of young Picea glauca (Moench) Voss, Picea mariana (Mill.) B.S.P. and Picea rubens Sarg. trees. The position and nature (i.e., whether cones, second-order shoots or non-flushed buds) of lateral axes borne along the first-order shoots were investigated. Seed cones occurred in proximal to distal positions, but not in terminal positions, on upper shoots, in medial to terminal positions on middle shoots, and in distal to terminal positions on lower shoots. Most non-flushed buds occurred in proximal positions on middle and lower shoots. In P. rubens, rate, duration and amount of elongation of first-order shoots increased acropetally. Thus lower shoots stopped elon-gating before upper shoots. Evidence of bud differentiation in P.rubens, as indicated by the presence of initiating leaf primor-dia, was seen first in sections of terminal buds of upper shoots. Differentiation of buds then proceeded basipetally along shoots and among shoots down the crown. Differentiation of buds in positions where cones are commonly borne became evident soon after shoot elongation was completed.
Keywords: bud differentiation, Picea glauca, Picea mariana, Picea rubens, seed cones, shoots, shoot elongation.
Introduction
It is well established that application of gibberellin A4/7 en-hances cone production in Picea (Marquard and Hanover 1984a, 1984b, Cecich 1985, Philipson 1985, 1987, Ross 1985, Bonnet-Masimbert 1987, Hall 1988, Ho 1988, Greenwood et al. 1991, Owens et al. 1992). However, the underlying physi-ological bases of the GA4/7-induced enhancement of cone production remains obscure (e.g., Ross and Pharis 1985, Pharis et al. 1987), partly because the intricate complexity of tree crowns has not been considered when designing treatments to enhance cone production or in interpreting the results of cone induction experiments. Thus studies have focused on whole crowns rather than on morphogenetic patterns (i.e., shoot ori-gin, placement, elongation and size, and seed-cone and pollen-cone placements) among shoots within crowns.
Shoot patterns within a crown are genus specific (e.g.,
De-bezac 1965, Powell 1977a, 1988, 1991), and even within a genus, such as Picea, differences in expression of basic char-acteristics and responses occur (Greenwood et al. 1991). The distinctive morphogenetic pattern of shoots in young Picea trees is strongly acrotonic. In Picea, which exhibits preformed growth, acrotony is expressed as an increase in size of lateral structures toward the shoot apex (cf. Champagnat 1978) and is clearly displayed in the lateral buds along a tree’s leading shoot. Numbers of bud scales and leaf primordia per lateral bud increase acropetally (Baxter and Cannell 1978), and this in-crease, in turn, leads to an acropetal increase in the lengths of the first-order shoots when the preformed elements grow out from the buds. All of the buds arise similarly in leaf axils along a shoot, and so, except for the acrotoneous arrangement, they are of equal status.
It is on the first-order shoots arising from lateral buds along a tree leader of the previous year that seed cones commonly differentiate at first bearing (Marquard and Hanover 1984a, 1984b, Caron and Powell 1990, 1992, 1993). Similar shoots from lateral buds on subsequent tree leaders bear many cones in following years (Caron and Powell 1993). In any year, these first-order shoots represent a population of similar origin and age (axis age), but of different lengths. Thus the population provides a basis for inter-shoot comparison free from variables such as differing branching order or age of parent structure. With decreasing shoot length, cones are positioned progres-sively more distally and are terminal on many of the short shoots (e.g., Powell 1983, Caron 1987, Caron and Powell 1993). Marquard and Hanover (1984b) found that seed cones of Picea glauca (Moench) Voss were medially situated on the most-distal, first-order shoots, but underwent a slight acropetal shift in response to GA4/7 treatment.
In Picea, the lower, shorter shoots cease elongation earlier than the upper, longer shoots (e.g., Fraser 1966, Bonnet-Masimbert 1987, Ford et al. 1987). If lateral buds differentiate when shoots cease elongating (cf. Owens and Molder 1976a, 1976b, 1977, Owens et al. 1977, Harrison and Owens 1983, Maquard and Hanover 1984b), then buds on shorter shoots would differentiate before those on longer shoots. Alterna-tively, because elongation along a shoot is completed first proximally and last distally, differentiation of lateral buds may occur progressively along individual shoots. If this is the case,
The role of acrotony in reproductive development in
Picea
G. R. POWELL
Faculty of Forestry and Environmental Management, University of New Brunswick, P.O. Box 44555, Fredericton, New Brunswick E3B 6C2, Canada
Received May 31, 1994
distal buds on shorter shoots may differentiate at the same time as more proximally situated buds on longer shoots. Timing may be important in terms of when particular buds are respon-sive to imposed treatment and endogenous biochemical gradi-ents.
The interrelationships among elongation, timing and posi-tioning, all occasioned by acrotony, have not been elucidated. In this study, the acrotonal pattern among first-order shoots undergoing their first elongation was used as a basis to inves-tigate positional aspects of cone production and temporal as-pects of bud differentiation under natural conditions. Three Picea species were studied, P. glauca, P. mariana (Mill.) B.S.P. and P. rubens Sarg.
Materials and methods
The 1992 crop of seed cones on Picea species growing in central New Brunswick was better than average. In May 1993, 20 trees of each species, P. glauca, P. mariana and P. rubens, were selected in the University of New Brunswick (UNB) Forest, Fredericton, N.B., or in the Acadia Forest Experiment Station, 20 km to the east. Selection criteria were: trees 0.7 to 3.7 m tall in 1990, single tree leader in 1990, normal branch development in 1991 and 1992, and presence of cones that had matured in 1992 on first-order shoots of 1991 (i.e., shoots that had extended in 1991 from the 1990 leader).
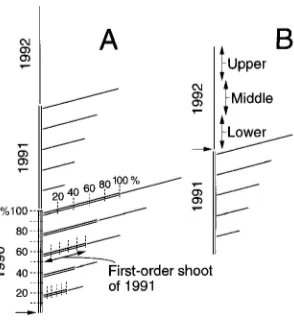
Each first-order shoot of 1991 was examined in acropetal sequence (Figure 1A). Recorded were the distance of the shoot from the base of the 1990 leader and the distance along each shoot of each non-flushed bud, shoot, seed cone or pollen cone. The distance data were expressed as percentages of total lengths of the respective leaders or first-order shoots to facili-tate analysis on a comparative basis. The percentages were
grouped acropetally in 10% classes along the parent leaders and 20% classes along the first-order shoots (Figure 1A).
Separately, 20 P. rubens between 1 and 2 m tall were se-lected in the UNB Forest. Each had a single 1992 leader (Figure 1B) between 20 and 40 cm long and was free from local competition from other trees. In mid-May 1993, in acropetal sequence, the length of each bud on each leader was measured with vernier calipers (nearest 0.1 mm). The bud’s distance from the base of the leader and its position around the leader (north, east, south or west quadrant) were recorded. From among the buds, three lower, three middle and three upper lateral buds (Figure 1B), and the terminal bud were selected and marked. To minimize variability, the lateral buds were selected preferentially from the southern exposure, with numbers made up as necessary from closest buds on first the western and then the eastern exposure.
Elongation of marked buds and subsequently the first-order shoots produced from them was measured each Monday, Wednesday and Friday. To facilitate tracking of shoot elonga-tion, and particularly of its compleelonga-tion, each measurement was entered into a computer file on the day it was made and used to generate growth curves. When elongation of the lower shoots was deemed from their growth curves to be 95% com-pleted (Day 190), two lower, two middle and two upper shoots were harvested on each of three additional, randomly chosen trees. The harvested shoots were labeled and transported in a cooler (< 10 °C) to the laboratory. Similar collections were made from other trees when elongation of first, middle (Day 195) and upper shoots (Day 200) on the measured trees was deemed to be 95% completed. When elongation of the tree leaders was deemed to be 95% completed (Day 207), shoots, including tree leaders, were collected from additional trees. Further collections were made on Days 215, 221 and 229.
For each collected shoot, the distance from the base of the shoot to each bud (numbered in acropetal sequence) was re-corded. Each bud was then carefully excised, placed in for-malin/acetic acid/alcohol (FAA) and subjected to reduced pressure, and then left for a minimum of 2 days. Each labeled bud was placed in a container and passed through an n-butanol series into Paraplast in a Histomatic processor. Each Paraplast-embedded bud was arranged in molten Paraplast in a boat and cooled. The resultant blocked buds were mounted on wooden stubs and serially longitudinally sectioned at 10 µm on a rotary microtome. The sections were mounted on microscope slides, stained with safranin and fast green, and covered for sub-sequent microscopic examination.
To facilitate comparative examination, the medial section (or most nearly medial section in obliquely sectioned buds) was photographed at a magnification of 20.5× using a Wild M400 Macroscrope with Kodak 100 Gold film and a daylight (blue) filter. A paper print at 72× magnification was made of each selected section (over 200), and sets of sections of buds were compared for the successive collection dates.
On Day 221 (August 9), first-order shoots were collected from two extra trees. Samples of buds from a range of positions along the shoots were dissected, and flanks of the apical meristems were examined directly.
Figure 1. Stylized diagrams of upper sections of one side of young
Results
Shoot lengths and distribution of lateral axes along shoots
The seed-cone-bearing P. glauca, P. mariana and P. rubens trees averaged (mean ± SE) 2.31 ± 0.17, 1.54 ± 0.11 and 1.75 ± 0.12 m in height in 1990, respectively. Leading shoots of 1990 were 254 ± 22, 182 ± 23 and 216 ± 27 mm long, but when adjusted with tree height in 1989 as a covariate, the means were estimated at 204 ± 21, 218 ± 20 and 228 ± 19 mm, respectively, and were not significantly different (P = 0.71). The lateral shoots that elongated in 1991 from buds along the 1990 leading shoots showed distinct acrotony (Figure 2). The pattern among the shoots was least uniform in P. rubens, which also had the fewest shoots (from Figure 2). In each species, shoots were least numerous in the 70 to 90% region along the leader and near its base, although in P. glauca, there were many shoots in the 70--80% and 0--10% regions of the leaders (Fig-ure 2). Lengths of shoots on north-, east-, south- and west-fac-ing surfaces were not different for any species (P = 0.75, 0.41 and 0.69 for P. glauca, P. mariana and P. rubens, respectively, with leader lengths in 1990 as covariates).
Because number of lateral axes borne per shoot was corre-lated with shoot length (r = 0.941, 0.901 and 0.906 for P. glauca, P. mariana and P. rubens, respectively), it followed a similar pattern to that of lateral shoot length along the leading shoot (Figure 3). Picea glauca bore more lateral axes per unit length of shoots originating between 30 and 90% of the dis-tance along the leader than did the other species (cf. Figures 2 and 3). Overall, P. glauca bore 0.77 ± 0.01 lateral axis per cm of shoot, P. mariana 0.75 ± 0.02, and P. rubens 0.66 ± 0.01.
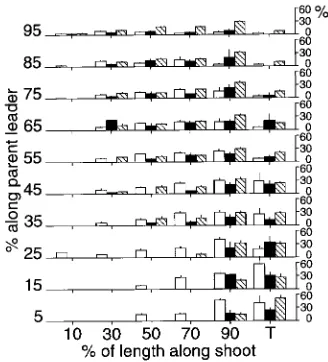
When expressed in terms of position along the bearing shoot, lateral axes (non-flushed buds, second-order shoots or cones) occurred all along the upper shoots, but only along the distal halves of the lower shoots (Figure 4). Proportions of non-flushed buds increased downward in the crown among the shoots. The most proximal buds on lower and some upper
shoots did not flush, but non-flushed buds also occurred at all locations where cones or lateral shoots occurred. Frequency of occurrence of lateral shoots in mid-shoot positions decreased downward. Most lateral shoots on upper parent shoots were situated distally. On lower parent shoots, most new shoots were terminal.
Cones occurred laterally all along the uppermost shoots, but not terminally (Figure 4), whereas on lower shoots, cones were most frequently terminal. Overall, cone positions tended to become more distal toward the base of the crown. The pattern of cone occurrence was consistent for all three species (Fig-ure 5); however, the few very short shoots that occurred near the bases of the leaders of P. mariana and P. rubens (Figure 2) did not bear cones (Figure 5).
Figure 3. Mean numbers of lateral axes borne per shoot at different heights (10% distance classes) along 1990 leaders of P. glauca,
P. mariana and P. rubens. Details as in Figure 2.
Figure 2. Mean lengths of 1991 shoots borne on 1990 leaders of
P. glauca, P. mariana and P. rubens expressed as percentages of current (1991) leader lengths at different heights (10% distance classes) along the 1990 leaders. Standard errors are shown as project-ing lines from the bars, and numbers on bars indicate numbers of shoots included in the respective classes (means).
Elongation of shoots borne along leaders
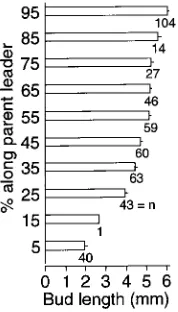
In P. rubens, lengths of lateral buds formed in 1992 increased acropetally along leaders (Figure 6). Terminal buds on leaders were not as long as distal lateral buds (5.07 ± 0.14 versus 5.98 ± 0.07 mm for buds in the 95% distance-class).
Bud elongation became detectable (increase of 0.05 mm) between Days 141 and 165 (May 21 and June 14, 1993). Bud burst occurred between Days 162 and 174 (June 11 and 23). It was achieved for all buds on single leaders in 2 to 7 days. Mean days by which bud burst was achieved (assessed at 2- and 3-day intervals) on lower, middle and upper lateral buds, and terminal buds were 169.6a, 170.0a, 169.0a and 168.0b, respec-tively (the same letter after the means indicates no significant difference at the 0.05 level), indicating a slight basipetal trend.
The rate and duration of post-bud-burst, first-order shoot elongation increased acropetally (Figure 7). Mean days by which shoot elongation ceased were 204.0a (July 23), 204.9a, 208.4 and 211.3 for lower, middle, upper and terminal shoots, respectively. Overall, lower shoots completed 95% of their elongation 2 days before middle shoots, middle shoots 2 days before upper shoots, and upper shoots 7 days before terminal shoots (mean days of 192.0 ± 0.4, 194.1 ± 0.2, 196.2 ± 0.3 and 203.1 ± 1.0 for lower, middle, upper and terminal shoots, respectively). On individual trees, achievement of 95% of final length of first-order shoots alone and of first-order and termi-nal shoots together spanned 2 to 12 days and 4 to 18 days, respectively.
Total post-bud-burst elongation took 34.4 ± 0.7, 34.9 ± 0.7, 39.3 ± 0.7 and 43.3 ± 1.3 days for lower, middle and upper first-order shoots, and terminal shoots, respectively. The differ-ence between means for lower and middle first-order shoots was not significant at the 0.05 level.
Differentiation of buds along shoots
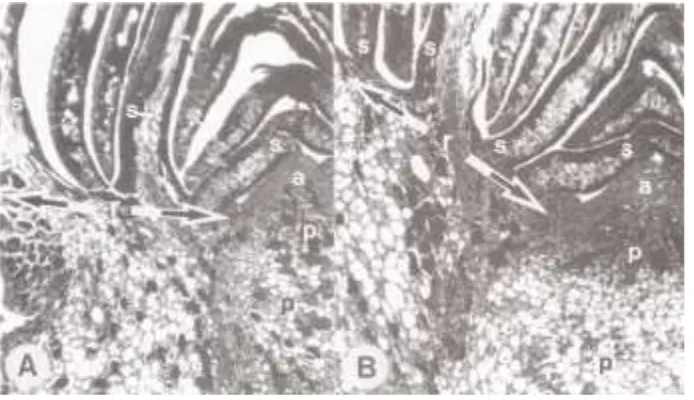
Sections of buds collected on Day 190 (July 9) showed no evidence that differentiation (presence of leaf primordia on the lower flanks of apical meristems) had occurred. The apical meristems (apices) were rounded domes to rounded cones with inner bud scales generally close to the apex surfaces (Figure 8). Apices of lateral buds were situated at the level of the recepta-cle bearing the older bud scales, whereas apices of terminal buds were situated well below the level of the receptacle bearing older bud scales (e.g., Figures 8A and 8B). This mas-sive receptacle development in terminal buds was most appar-ent in buds terminating upper shoots. These buds had the broadest apices. The receptacle was only moderately raised above the level of the bases of the narrower apices in terminal buds on lower shoots. Most lateral buds on lower shoots, some on middle shoots and occasional buds on upper shoots lagged in development. Their apices were small, rounded domes, which essentially lacked pith development in their lower parts and in the tissues beneath. These buds were categorized as Figure 5. Percentages of seed cones borne at successive positions
(20% distance classes and terminally, T) along shoots at different heights (10% distance classes) along the leaders of P. glauca (open),
P. mariana (solid) and P. rubens (cross-hatched) (n = 20).
Figure 6. Mean lengths of lateral buds borne at different heights (10% distance classes) on 1992 leaders of 20 P. rubens trees. Standard errors are shown as projecting lines from the bars, and numbers on bars indicate the numbers of buds included in the respective classes (means).
latent. The pith areas of better-developed buds were distinc-tive (Figure 8), and the portions in and just below the lower reaches of the apex stained darkly. The proportion of darkly stained pith tissues tended to increase acropetally in buds along individual shoots and upward among shoots.
Collections on Days 195 and 200 (July 14 and 19) showed increases in proportions of more conical apices among lateral
buds. No indications of initiation of leaf primordia were ob-served on these apices (Figure 9). The outer flanks of the broad apices of terminal buds of upper shoots provided the first evidence of initiation of leaf primordia (Figure 10) as in-dicated by suggestive staining of surficial and associated subsurface cells (Figure 10A), and as slight surficial mounding among such cells (Figure 10B).
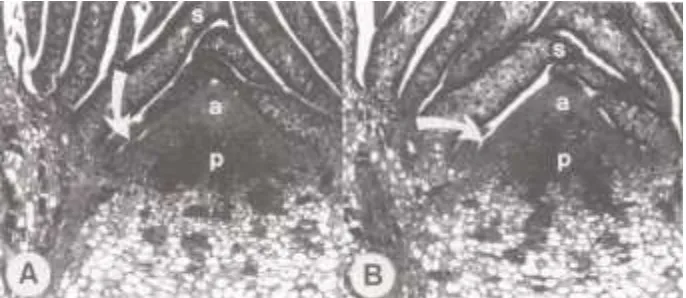
Figure 8. Median longitudinal sec-tions of comparable parts of buds from an upper shoot of onePicea rubenstree, Day 190, showing over half of the apical meristem (a), pith (p) and receptacle (r and arrows) bearing bud scales (s) (72×). (A) The 6th of 10 lateral buds positioned at 76% of the distance along the shoot, and (B) the terminal bud.
Figure 10. Median longitudinal sec-tions of comparable parts of terminal buds from upper shoots of twoPicea rubenstrees (72×). (A) Cellular pat-terns (arrows) suggestive of leaf-pri-mordium initiation on Day 195, and (B) bulging tissues and cellular pat-terns (arrows) indicating leaf primor-dium initiation on Day 200. Other symbols as in Figure 8.
Collections on Day 207 (July 26) showed that leaf primordia had initiated in terminal buds of upper shoots. Leaf primordia were also evident in terminal buds of some middle ure 11A) and lower shoots, and in distal lateral buds (Fig-ure 11B) on upper shoots. By Day 207, dark staining of pith in the lower reaches of and just below the apex was pronounced, especially in the terminal and distal lateral buds. Outside the pith zone, the peripheral flanks of the apex, where leaf primordia were forming, were three to five cells thick.
By Day 215 (August 3), apices of terminal buds of upper shoots were developing mammillary tips. Leaf primordia were evident on lower flanks of apices of buds situated more basipetally on shoots at all levels. On Day 221 (August 9), proximally situated buds with well-developed apices (hence, apparently not latent) showed little evidence of initiation of leaf primordia (Figure 12A), whereas there was an acropetal trend in the development of leaf primordia in buds along single shoots (Figure 12B to 12F). Associated with this development of leaf primordia, there were increases in apex size, density of staining in the pith and relative height above the base of the apex of the bud-scale-bearing receptacle (Figure 12). Buds collected and dissected on Day 221 showed similar acropetal trends in development of leaf primordia and in apex size and shape.
Discussion
Acrotony among directly comparable shoots of similar origin was expressed similarly inP. glauca,P. marianaandP. rub-ens. Shoot length was associated with bud size (Figure 6) and with preformed bud composition (Baxter and Cannell 1978). Shoot length was also associated with the propensity of the lat-eral axis to differentiate and develop into a cone or shoot, or remain as a non-flushed bud. Although the cause of non-flush-ing of buds was not examined, their distribution (Figure 4) suggests that most were latent buds. Sections of developing
P. rubensbuds confirmed that many buds in more proximal positions had small apices that appeared inactive. Latent buds, which have been described in otherPicea(e.g., Owens et al. 1977, 1992), represent a reserve of lateral axes that can be acti-vated if more distally situated vegetative structures are de-stroyed (cf. Powell 1982). Also, in some genera of Pinaceae, cone buds tend more frequently to take the place of latent buds than of vegetative buds (Owens 1969, Powell 1977a). The oc-currence of the three kinds of buds inPiceawarrants further investigation in relation to position on the shoot and to years of varying cone abundance (cf. Powell 1977b).
The acrotoneous arrangement of shoots was also associated with rate and duration of shoot elongation that led to an acropetal increase in timing of cessation of shoot elongation. The upper, longer shoots, which started to elongate slightly earlier, exhibited a much greater rate of elongation and stopped elongating 4 days later than the lower, shorter shoots. This
Figure 11. Median longitudinal sec-tions of comparable parts of buds from shoots of twoPicea rubenstrees on Day 207 (72×). (A) Terminal bud from a middle shoot, and (B) the 6th of 7 lateral buds at 96% of the dis-tance along an upper shoot. Arrows indicate leaf primordia; other sym-bols as in Figure 8.
pattern is consistent with that reported for shoots extending principal branches of the crowns of large Picea trees (e.g. Fraser 1966, Ford et al. 1987).
Morphological-anatomical evidence of differentiation of buds on the acrotoneously arranged shoots followed neither the upward (shorter to longer shoots) pattern of timing, sug-gested by the notion that differentiation occurs when shoot elongation ceases, nor the outward (acropetal along shoots) pattern of timing, suggested by the progressive completion of elongation along a shoot (see Introduction). The evidence indicates an opposite or basipetal pattern of timing of bud differentiation. Terminal buds of upper shoots showed signs of differentiation first. Differentiation was evident next in termi-nal buds of middle and some lower shoots, and in distal lateral buds of upper shoots. Evidence of differentiation then moved to medial lateral buds of upper shoots and distal lateral buds of middle shoots, and then to more proximal buds on all shoots. Mammillary tips to the apices, as described in some differen-tiating apices in other Picea (Owens and Molder 1976a, Owens et al. 1977, 1992, Marquard and Hanover 1984b), were observed only in terminal and distal lateral buds of the upper and middle shoots, indicating that they are associated with more vigorous development.
The times when evidence of differentiation became apparent were associated with cessation of shoot elongation, but not in a consistent manner. On upper shoots, differentiation of termi-nal buds became evident when shoots were completing elon-gation (95% completed). On middle and lower shoots, differentiation of terminal buds became evident just after com-pletion of shoot elongation. Thus the time when evidence of differentiation became apparent was dependent on the relative position of the parent shoot and the relative position of the bud along the parent shoot.
The consistent pattern of cone distribution in the three Picea species (Figure 5), coupled with the time when differentiation became evident, indicates that the buds most likely to differen-tiate as cones should all show evidence of differentiation at about the same time, and after shoot elongation has been completed. Thus measures aimed at enhancing cone differen-tiation must take place before shoot elongation ends (cf. Mar-quard and Hanover 1984b, Ross 1985, Ho 1988). Moreover, because visible evidence of differentiation must follow precur-sor events within the tissues of the buds or shoots, time must be allowed for such events to occur after any given cone-en-hancing treatment. However, the reaction times to stimuli of differentiation appear to vary among shoots or among buds along shoots. Thus the patterns of shoot elongation, of non-dif-ferentiation (latency) and difnon-dif-ferentiation, of sizes of bud apices along shoots, and of density of staining in the pith in buds along shoots provide an indication that response times may be slower on shorter shoots and in proximal positions on longer shoots. The corollary to this is that the rate of development appears to be fastest in terminal buds of upper shoots. This, and the finding that such buds normally do not differentiate as cones, can be interpreted as a mechanism to ensure continued vegetative development of the most vigorous shoots, and thus expansion of the crown by means of the most distal branches
of any one year.
This study has demonstrated the complexity yet orderliness of morphogenetic patterning in populations of similar shoots in a region of the crown of young Picea trees and has provided information on where and when changes associated with, or precursive to, cone differentiation occur. The information should be of value in designing treatments to enhance cone production and in interpreting results of experiments on cone induction.
Acknowledgments
This research was made possible through research grants from the Natural Science and Engineering Research Council of Canada and the University of New Brunswick Research Fund. The author thanks J.G. Floyd, D.W. Gimby, M.H. Hancox and P. Pobihushchy for technical assistance in the field and laboratory, and W.L. Staples for photo-graphic processing.
References
Baxter, S.M. and M.G.R. Cannell. 1978. Branch development on leaders of Picea sitchensis. Can. J. For. Res. 8:121--128.
Bonnet-Masimbert, M. 1987. Preliminary results on gibberellin induc-tion of flowering of seedlings and cuttings of Norway spruce indi-cate some carry-over effects. For. Ecol. Manage. 19:163--171. Caron, G.E. 1987. Development of branch patterns and seed
produc-tion in young black spruce (Picea mariana (Mill.) B.S.P.). Ph.D. Thesis. University of New Brunswick, Fredericton, N.B., Canada, 330 p.
Caron, G.E. and G.R. Powell. 1990. Morphological variation, fre-quency, and distribution of bisporangiate strobili in Picea mariana. Can. J. Bot. 68:1826--1930.
Caron, G.E. and G.R. Powell. 1992. Patterns of cone distribution in crowns of young Picea mariana. I. Effect of tree age on seed cones. Can. J. For. Res. 22:46--55.
Caron, G.E. and G.R. Powell. 1993. Patterns of on-shoot positioning of seed cones in relation to shoot length and position in the crowns of young Picea mariana. Trees 7:182--188.
Cecich, R.A. 1985. White spruce (Picea glauca) flowering in response to spray application of gibberellin A4/7. Can. J. For. Res. 15:170--174.
Champagnat, P. 1978. Formation of the trunk in woody plants. In
Tropical Trees as Living Systems. Eds. P.B. Tomlinson and M.H. Zimmermann. Cambridge University Press, Cambridge, U.K., pp 401--422.
Debezac, E.F. 1965. Morphogénèse et sexualité chez les Pinacées. Bull. Acad. Soc. Lorraines Sci. 5:212--228.
Ford, E.D., J.D. Deans and R. Milne. 1987. Shoot extension in Picea sitchensis. I. Seasonal variation within a forest canopy. Ann. Bot. 60:531--542.
Fraser, D.A. 1966. Vegetative and reproductive growth of black spruce (Picea mariana (Mill.) B.S.P.) at Chalk River, Ontario, Canada. Can. J. Bot. 44:567--580.
Greenwood, M.S., G.W. Adams and M. Gillespie. 1991. Stimulation of flowering by grafted black spruce and white spruce: a compara-tive study of the effects of gibberellin A4/7, cultural treatments, and environment. Can. J. For. Res. 21:395--400.
Harrison, D.L.S. and J.N. Owens. 1983. Bud development in Picea engelmannii. I. Vegetative bud development, differentiation, and early development of reproductive buds. Can. J. Bot. 61:2291--2301.
Ho, R.H. 1988. Promotion of cone production on white spruce grafts by gibberellin A4/7 application. For. Ecol. Manage. 23:39--46. Marquard, R.D. and J.W. Hanover. 1984a. Sexual zonation in the
crown of Picea glauca and flowering response to exogenous GA4/7. Can. J. For. Res. 14:27--30.
Marquard, R.D. and J.W. Hanover. 1984b. Relationship between gib-berellin A4/7 concentration, time of treatment, and crown position on flowering of Picea glauca. Can. J. For. Res. 14:547--553. Owens, J.N. 1969. The relative importance of initiation and early
development on cone production in Douglas-fir. Can. J. Bot. 47:1039--1049.
Owens, J.N. and M. Molder. 1976a. Bud development in Sitka spruce. I. Annual growth cycle of vegetative buds and shoots. Can. J. Bot. 54:313--325.
Owens, J.N. and M. Molder. 1976b. Bud development in Sitka spruce. II. Cone differentiation and early development. Can J. Bot. 54:766--779.
Owens, J.N. and M. Molder. 1977. Bud development in Picea glauca. II. Cone differentiation and early development. Can. J. Bot. 55:2746--2760.
Owens, J.N., M. Molder and H. Langer. 1977. Bud development in
Picea glauca. I. Annual growth cycle of vegetative buds and shoot elongation as they relate to date and temperature sums. Can. J. Bot. 55:2728--2745.
Owens, J.B., J.J. Philipson and D.L.S. Harrison. 1992. The effects of the duration and timing of drought plus heat plus gibberellin A4/7 on apical meristem development and coning in Sitka spruce (Picea sitchensis (Bong.) Carr.). New Phytol. 122:515--528.
Pharis, R.P., J.E. Webber and S.D. Ross. 1987. The promotion of flowering in forest trees by gibberellin A4/7 and cultural treatments: a review of possible mechanisms. For. Ecol. Manage. 19:65--84.
Philipson, J.J. 1985. The effect of top pruning, girdling, and gibberel-lin A4/7 application on the production and distribution of pollen and seed cones in Sitka spruce. Can. J. For. Res. 15:1125--1128. Philipson, J.J. 1987. Promotion of cone and seed production by
gib-berellin A4/7 and distribution of pollen and seed cones on Sitka spruce in a clone bank. For. Ecol. Manage. 19:147--154.
Powell, G.R. 1977a. Patterns of development in Abies balsamea
crowns and effects of megastrobilus production on shoots and buds. Can. J. For. Res. 7:498--509.
Powell, G.R. 1977b. Biennial strobilus production in balsam fir: a review of its morphogenesis and a discussion of its apparent physi-ological basis. Can. J. For. Res. 7:547--555.
Powell, G.R. 1982. Shoot and bud development in balsam fir: implica-tions for pruning of Christmas trees. For. Chron. 58:168--172. Powell, G.R. 1983. Red spruce, Picea rubens Sarg. In Reproduction
of Conifers: a Handbook for Cone Crop Assessment. Can. For. Serv., Environ. Can., For. Tech. Rep. 31, pp 35--36.
Powell, G.R. 1988. Shoot elongation, leaf demography and bud forma-tion in relaforma-tion to branch posiforma-tion on Larix laricina saplings. Trees 2:150--164.
Powell, G.R. 1991. Preformed and neoformed extension of shoots and sylleptic branching in relation to shoot length in Tsuga canadensis. Trees 5:107--116.
Ross, S.D. 1985. Promotion of flowering in Picea engelmannii (Parry) grafts: effects of heat, drought, gibberellin A4/7 and their timing. Can. J. For. Res. 15:618--624.