Molecular Microbiology (2004) 54(5), 1151–1160 doi:10.1111/j.1365-2958.2004.04356.x
Accepted 13 August, 2004. *For correspondence. E-mail Christian. Lesterlin@ibcg.biotoul.fr; Tel. (+33) 561 33 59 85; Fax (+33) 561 33 58 86.
MicroReview
Genetic recombination and the cell cycle: what we have
learned from chromosome dimers
Christian Lesterlin,* François-Xavier Barre and François Cornet
Laboratoire de Microbiologie et de Génétique Moléculaire, 118, route de Narbonne, F-31062 Toulouse Cedex, France.
Summary
Genetic recombination is central to DNA metabolism. It promotes sequence diversity and maintains genome integrity in all organisms. However, it can have perverse effects and profoundly influence the cell cycle. In bacteria harbouring circular chromo-somes, recombination frequently has an unwanted outcome, the formation of chromosome dimers. Dimers form by homologous recombination between sister chromosomes and are eventually resolved by the action of two site-specific recombinases, XerC
and XerD, at their target site, dif, located in the
repli-cation terminus of the chromosome. Studies of the Xer system and of the modalities of dimer formation and resolution have yielded important knowledge on how both homologous and site-specific recombina-tion are controlled and integrated in the cell cycle. Here, we briefly review these advances and highlight the important questions they raise.
Formation of dimers and control of crossing over
Several sets of experiments indicate that 10–15% of the growing Escherichia coli cells require Xer recombination to resolve chromosome dimers and allow monomer prod-ucts to segregate correctly (Cornet et al., 1996; Steiner and Kuempel, 1998a; Perals et al., 2000). This require-ment for Xer recombination is not observed with recA
strains, no doubt because the vast majority of dimers form by homologous recombination (Steiner and Kuempel, 1998a; Perals et al., 2000). The concentration of sister chromatids, which varies with the growth rate, has a
mod-est effect on dimerization. The apparent frequency of dimerization may be increased after treatment with DNA-damaging agents or in hyper-recombinant mutants (Steiner and Kuempel, 1998a). Inactivation of RecA also suppresses defective segregation that results from inacti-vation of the Xer system in Vibrio cholerae (Huber and Waldor, 2002).
Homologous recombination may be required for the completion of replication (Fig. 1A). For instance, the pro-cessing of replication forks that halt at a DNA lesion may create DNA ends that need recombination to be resealed so that replication can restart (Kuzminov, 1999). The recombination process can either exchange the flanking sequences (referred to as ‘Sister Chromatid Exchange’ or ‘Crossing Over’) to produce a dimer, or not, leaving mono-meric chromosomes. Thus, the rate of dimer formation depends both on the frequency of recombination between sister chromosomes and on the frequency at which recombination events lead to sister chromatid exchange. Two major RecA-dependent recombination pathways exist
in E. coli, referred to as the RecBC- and the
from endonucleolytic cleavage may be repaired by a Ruv-independent pathway that involves the RecG helicase (Meddows et al., 2004).
Both RecBC- and RecF-dependent recombination path-ways may produce chromosome dimers during normal growth (i.e. in the absence of DNA damaging agent or recombination inducing mutations), at least when the other pathway is inactivated (Steiner and Kuempel, 1998b; Perals et al., 2001). RecBC-dependent dimer for-mation can be explained by the application of the observed bias of RuvABC cleavage to ‘canonical’ models
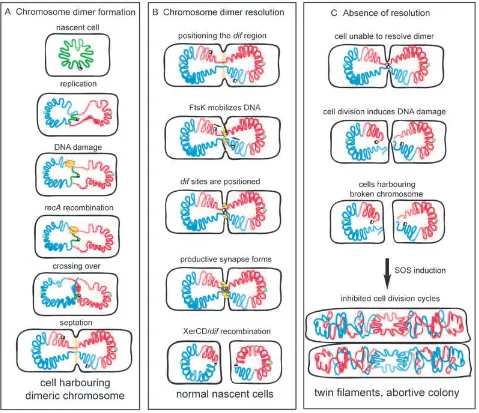
of RecBC-driven recombination (Cromie and Leach, 2000; Michel et al., 2000). However, the mechanism by which RecF-dependent recombination produces dimers during normal growth remains an open question. In addi-tion, RecBC- and RecF-dependent recombination path-ways may respond differently to induction of particular DNA damage. This has been shown in the cases of UV irradiation and of the processing of long palindromic sequences inserted on the chromosome (Cromie and Leach, 2000). In both cases, the induced dimers were shown to be only produced by RecBC-dependent recom-Fig. 1. Chromosome dimers and the cell cycle. The cartoons represent a E. coli cell with chromosomes in red and blue. The green circle represents
oriC and the black and white squares represent the dif sites.
Left. Formation of a dimer during replication. The yellow flash represents recombination repair of a double-strand break leading to a crossing over. This creates a dimer that persists until septation initiates (symbolized as the pale orange zone).
Middle. Dimer resolution. The two dif regions are thought to be loosely positioned in the septum vicinity, either on opposite sides (as represented here) or on the same side of the septum. FtsK (yellow bouquet) loads onto DNA, translocates towards dif sites and activates XerCD/dif
recombination, allowing normal cell division.
bination. In contrast, RecBC-dependent repair of a dou-ble-strand break created by endonucleolytic cleavage or ionizing radiations does not create dimers in wild-type strains (Meddows et al., 2004). This bias towards the absence of dimerization requires both RuvABC and RecG. Clearly more investigations are required to under-stand the multiple ways dimers can form and the mecha-nisms that modulate their frequency.
Cell division-induced breakage of chromosome dimers
Mutants unable to resolve chromosome dimers (because of a defect in the Xer system) display a characteristic phenotype, with increased generation time, frequent for-mation of filaments containing aberrantly segregated DNA masses and partial induction of the SOS system (Blakely
et al., 1991; Kuempel et al., 1991). Inactivation of dimer
resolution also induces high frequencies of homologous recombination between directly repeated sequences inserted in the vicinity of the resolution site, dif, located in the replication terminus. Pioneering work on this hyper-recombination phenomenon (denominated ‘terminal recombination’ to its zone of occurrence) (Louarn et al., 1991; 1994; Corre et al., 1997; 2000) and investigation of the fate of dimer-containing cells (Hendricks et al., 2000; Prikryl et al., 2001) led to the proposal that the following sequence of events occurs in a cell unable to resolve its chromosome dimer (Fig. 1C). (i) Replication and segrega-tion proceed until the bulk of the sister chromosomes are distributed to each daughter cell, but remain linked by DNA passing through the division septum (Fig. 1). The linking DNA is thought to be the dif region (see below). (ii) Cell division proceeds and the septum constricts. This process induces lesions that cause double-strand breaks in the linking DNA. The mechanism that generates these breaks (referred to as the ‘guillotine’ effect) remains unclear. It certainly does not only involve physical shear-ing of the DNA by the closshear-ing septum or by physical tension, as the force required to break a DNA duplex appears out of the range of forces involved in chromo-some segregation and cell division. An enzymatic activity may be involved, either a nuclease induced by septum closure or DNA lesions resulting from normal activities such as an attempt to replicate the entrapped DNA. This may occur, for instance, via a mechanism equivalent to the processing of stalled replication forks by RuvABC (Seigneur et al., 1998). However, no data support this hypothesis at present. (iii) The RecBCD complex loads onto the DNA ends and degrades DNA. Occasionally, RecABC-dependent recombination may reseal one of the sister chromosomes, giving rise to the terminal recombi-nation phenomenon. In the absence of repair, degradation proceeds and produces SOS-inducing signals, thus
fur-ther inhibiting cell division and leading to formation of twin filaments and cell death.
Xer: the house-keeping site-specific recombination machine
Xer recombination is catalysed by two site-specific recom-binases of the tyrosine recombinase family, XerC and XerD (Blakely et al., 1993; Azaro and Landy, 2002). Orthologues of XerC and XerD are found in most eubac-teria that harbour circular chromosomes (Recchia and Sherratt, 1999; Chalker et al., 2000) and have been shown to be required for faithful segregation of the chro-mosome in Bacillus subtilis (Sciochetti et al., 1999) and V.
cholerae (Huber and Waldor, 2002). Xer mutants
some-times display intriguing and unexplained phenotypes, indi-cating that Xer recombinases may function in processes other than chromosome dimer resolution. For instance, XerD (but not XerC) appears essential for growth in
Sta-phylococcus aureus (Chalker et al., 2000).
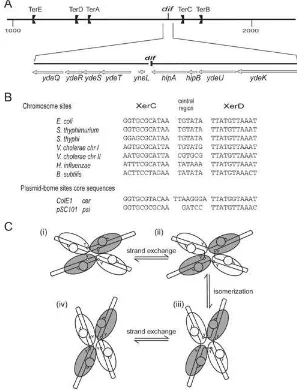
Xer recombination occurs between two ‘core’ recombi-nation sites, which are 28- to 30-bp-long DNA sequences containing one binding site for each recombinase sepa-rated by a 6- to 8-bp-long ‘central region’ (Fig. 2B). Despite the peculiarity of using two different recombi-nases, whose raison d’être remains unclear but which has proven to be very useful for mechanistic studies, the mechanism of Xer recombination is thought to conform to the tyrosine recombinase paradigm. Functional and struc-tural data obtained with the Cre/loxP system have given insight into this mechanism (Ghosh and Van Duyne, 2002; and references therein) which is illustrated in Fig. 2C. Recombination is catalysed inside a tetramer of recombi-nases bound to a pair of core sequences arranged in anti-parallel configuration. Strand exchanges (located at the edges of the central region) transit through covalent joints between the cleaved 3¢ ends of the DNA and the
con-served tyrosines of the recombinases. These covalent joints are subsequently attacked by the free 5¢-OH ends
liberated by the cleavages. Exchange of a first pair of strands is catalysed by a pair of recombinases (either XerC or XerD, see below) and leads to an intermediate containing an HJ. This complex then isomerizes to allow exchange of the second pair of strands by the second pair of recombinases.
recombination adapted to these different tasks? The out-come and the modalities of Xer recombination are influ-enced by the sequence of the recombination sites, by intrinsic properties of the recombinases and also by mod-ification of the nucleoprotein structure that are imposed by additional factors. In plasmid multimer resolution, accessory proteins and DNA sequences impose a ‘topo-logical filter’ on Xer recombination, which ensures that it occurs between sites that are directly repeated on the same molecule (Alen et al., 1997; Colloms et al., 1997). Xer recombination complexes are naturally inclined to ini-tiate recombination by XerC-mediated catalysis (Arcisze-wska and Sherratt, 1995; Arcisze(Arcisze-wska et al., 1995; Hallet
et al., 1999; Barre et al., 2000). Recombination between
plasmid-borne sites naturally follows this route although the order of strand exchange may be reversed if the ori-entation of the core relative to the accessory sequences is inverted (Bregu et al., 2002). XerD-mediated catalysis is even dispensable for recombination in certain cases
(Colloms et al., 1997). Chromosome dimer resolution fol-lows a different route: it is initiated by XerD-mediated catalysis and is controlled by features of chromosome segregation and by a DNA translocase associated with the division septum, FtsK (Aussel et al., 2002).
Coupling dimer resolution to cell division: the FtsK protein
Although a pair of dif sites is available for recombination after termination of replication, resolution of dimers is postponed until about 20 min later, at the time of constric-tion of the division septum (Steiner and Kuempel, 1998b). Moreover, inhibition of septation by drug treatment or by shifting a thermosensitive ftsZ mutant to restrictive tem-perature inactivates dimer resolution (Steiner and Kuem-pel, 1998b). In the time period between termination and septation [the G2 (D) period of the E. coli cell cycle], the two dif regions remain close to each other in vicinity of the
Fig. 2. The Xer system.
A. The drawing shows the region from 1000 kb to 2000 kb on the linear map of the E. coli
forming septum (Niki et al., 2000; Li et al., 2002; Lau
et al., 2003). During this period, the sites are thought to
be bound by XerCD and are able to synapse and form HJ intermediates (Barre et al., 2000). These HJ intermedi-ates, formed by XerC-mediated catalysis, are not con-verted to products but can be recon-verted to substrates by further XerC-mediated catalysis (Barre et al., 2000). Pro-ductive recombination depends on FtsK, a multifunctional protein associated with the division septum (Recchia
et al., 1999; Steiner et al., 1999; Aussel et al., 2002).
FtsK is an essential protein required for cell division (Begg et al., 1995). It is well conserved, even in bacteria harbouring linear chromosomes, but its role in Xer recom-bination is less conserved than the protein itself. For instance, the Haemophilus influenzae FtsK orthologue conforms to the E. coli paradigm in that it is required for Xer activation (Yates et al., 2003) but this is not the case for either of the two FtsK homologues of B. subtilis (Sci-ochetti et al., 2001). The N-terminal domain of FtsK (ª200
amino acids, harbouring four transmembrane segments) anchors the protein to the membrane and locates it at the division septum. This domain is required for cell division and is essential for viability (Draper et al., 1998; Wang and Lutkenhaus, 1998; Yu et al., 1998). The C-terminal domain, connected to the N-terminal domain by a ª
600-amino-acid linker of obscure function, is required for res-olution of dimers (Recchia et al., 1999; Steiner et al., 1999). This domain alone was shown to be sufficient for activation of Xer recombination in vivo (Barre et al., 2000) and a complete Xer recombination reaction between dif
sites could be reconstituted in vitro using a truncated form of FtsK carrying an intact C-terminal domain (FtsK50C) (Aussel et al., 2002).
FtsK does not activate Xer recombination by forcing a topological filter on the complex as it does in resolution of plasmid multimers (Aussel et al., 2002). FtsK-driven recombination may occur between dif sites in direct or inverted repetition, carried by the same molecule or not. Nevertheless, FtsK conditions the recombination reaction to yield topologically simple products, showing that it imposes a defined conformation on the recombination synapse. FtsK50C was shown to be an ATP-dependent DNA translocase, which can create domains of superhe-licity on circular and linear molecules. It was thus sug-gested that FtsK favours the encounter of recombination sites by slithering when creating plectonemic loops on DNA substrates (Ip et al., 2003). Direct evidence for loop formation by FtsK50C was recently obtained from single-molecule experiments (Saleh et al., 2004). This activity is not sufficient to induce recombination. A local action involving direct contact between the extreme C-terminal domain of FtsK and the recombination complex is required (Aussel et al., 2002; Yates et al., 2003). This activity also requires ATP hydrolysis (Massey et al., 2004). It is thought
that FtsK resets the XerCD/dif synapse to a productive complex within which XerD catalyses exchange of the first pair of strands.
Chromosome polarization controls resolution of dimers
A second crucial element in chromosome dimer resolution (and, in fact, the first discovered) is the position of the dif
site on the chromosome. In E. coli, as in most bacteria,
dif is located opposite to oriC. This position is crucial for dimer resolution (Leslie and Sherratt, 1995; Tecklenburg
et al., 1995; Cornet et al., 1996; Kuempel et al., 1996). To
be active, dif must be inserted within a narrow zone around its natural position, the DAZ (dif activity zone). This zone and the region where replication terminates, although naturally coinciding, are functionally separate (Cornet et al., 1996). The DAZ is the scene of specific recombination between dif sites that occurs only in cells that are able to form chromosome dimers (i.e. proficient for homologous recombination) (Perals et al., 2000; 2001). This strongly suggests that the formation of an active XerCD/dif–FtsK complex is restricted to cells with a dimerized chromosome.
A search for the determinants of DAZ positioning revealed an unexpected phenomenon, chromosome polarization. The sequences surrounding dif appear to be intrinsically polarized along the oriC-dif axis and their rel-ative orientation is the main determinant of DAZ position-ing. Notably, deletion of sequences surrounding dif is harmless, whereas inversion of the same sequences inhibits dimer resolution (Tecklenburg et al., 1995; Cornet
et al., 1996; Perals et al., 2000). The data suggest that the
polarization determinants are present throughout a large terminal domain (more than 200 kb around dif) and are highly repeated. The genome of bacteriophage l also
appears to be polarized by equivalent signals, as insertion of l in one orientation near dif inhibits dimer resolution
whereas insertion in the other does not (Corre et al., 2000).
These findings revealed a new aspect of chromosome organization: the first functional implication of long-range polarization of the chromosome. Identification of the polar-ization signals is complicated by the fact that inversion of one or a small number of elements does not have a detectable effect on dif activity (Perals et al., 2000). Chro-mosome sequences are oriented following the oriC/Ter
and Louarn, 2003). Among these, a family of oligomers containing the AGGG sequence appears the most likely to underlie the polarization phenomenon observed. These oligomers are over-represented in the chromosome and exhibit a strong bias along the oriC-dif axis that increases towards dif (Lobry and Louarn, 2003), and the orientation of which switches at dif. The l genome is polarized by the
same motifs. However, the role of these elements in func-tional polarization remains to be proven experimentally.
Positioning the chromosome for dimer resolution
We do not know how polarization is recognized and put to use in order to organize Xer recombination in vivo. A good candidate for positioning of the dif sites before recombination and polarization reading is the protein FtsK. In this view, septum-associated FtsK would load onto chromosomes and mobilize DNA according to its intrinsic polarization. This process would stop when encountering XerCD-bound dif sites, thereby ensuring a proper sorting of chromosomal DNA in the sister cells and synapse of the dif sites (Fig. 3). Then a physical contact between XerCD/dif complexes and septum-borne FtsK allows resolution of dimers to occur. As described in Fig. 3, we postulate that, when a dimer is present, the XerCD/dif complexes and FtsK colocalize at the division septum at the time of septation. This restricts dif recom-bination to the septum region. Localization of FtsK is ensured by its N-terminal domain and localization of the
dif sites by a chromosome polarization-dependent pro-cess. In a cell harbouring a chromosome dimer, two DNA
stretches persist between the two segregated chromo-somes and are trapped by the forming septum. The entrapped regions belong to the part of the chromosome that is the last to be segregated. The fact that terminal recombination concerns only the dif region and is maximal at the dif position is the best evidence for this model and corroborates the observed intracellular location of these regions (Niki et al., 2000; Li et al., 2002; 2003; Lau et al., 2003). Precise positioning of the dif sites is then achieved by a polarization-dependent process, which allows forma-tion of a productive synapse between dif sites.
FtsK may read chromosome polarization
The DNA translocase activity of FtsK makes it a good candidate for positioning of the dif sites before recombi-nation. In this view, septum-associated FtsK would load onto chromosomes and mobilize DNA according to its intrinsic polarization. This process would stop when encountering XerCD-bound dif sites, thereby ensuring a proper sorting of chromosomal DNA in the sister cells and synapse of the dif sites (Fig. 3). Several lines of indirect evidence support this hypothesis as they show that acti-vation of recombination is not the only role of FtsK in dimer resolution. (i) Although the Cre/loxP system recombines independently of FtsK and may functionally replace XerCD/dif, resolution of dimers by Cre/loxP depends on FtsK (Capiaux et al., 2002). (ii) Expression of the C-termi-nal domain of FtsK (lacking the N-termiC-termi-nal domain) in a strain lacking the C-terminal domain allows high fre-quency of recombination between dif sites (for instance,
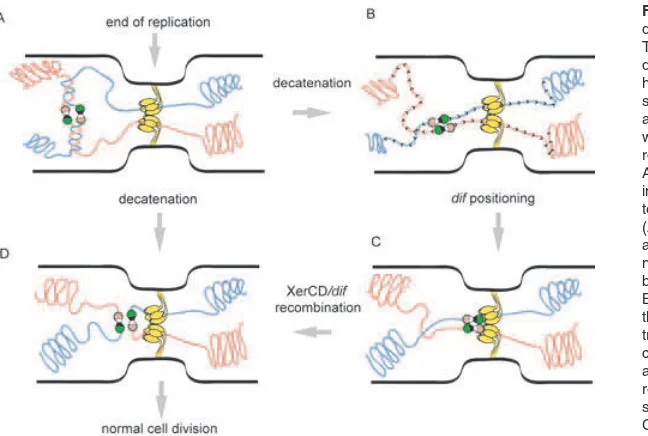
Fig. 3. Model for segregation of the Ter domains and chromosome dimer resolution. The cartoon represents the central part of a dividing cell. The yellow bouquet represents hexamers of FtsK bound at the constricting septum. The chromosomes are shown as red and yellow lanes, the dif sites as black and white dumbbells and the recombinases as the rose and green circles.
A. The two replicated dif regions are represented in a cohesion state symbolized by the persis-tence of intercatenation links between them. (AfiD) In the case of monomers, decatenation allows final separation of the chromosomes with no requirement for a contact between the recom-bination complexes and FtsK.
between two sites inserted as direct repeats on the chro-mosome or on a plasmid) but does not support resolution of dimers (Barre et al., 2000; Perals et al., 2001). (iii) In strains harbouring displaced dif sites (but unable to resolve dimers because the sites are outside of the DAZ), overexpression of wild-type FtsK activates dif recombina-tion but does not restore dimer resolurecombina-tion (Barre et al., 2000). The last two findings also suggest that only sep-tum-associated FtsK supports dimer resolution.
Most importantly, the outcome of terminal recombina-tion in ftsK mutants supports a role for FtsK in reading polarization. Frequencies of recombination between l
prophages in the terminal region depend on the orienta-tion of the l prophage with respect to dif. A simple
expla-nation is that the l genome carries polarization
determinants so that its presence may locally perturb polarization and thus dimer resolution (Corre et al., 2000). This orientation effect is abolished in ftsKC– cells (lacking the C-terminal domain) (Corre and Louarn, 2002). More-over, the distribution of DNA breaks resulting from unre-solved dimers (inferred from terminal recombination frequencies) is affected in ftsKC– cells. When compared with xer strains, DNA breaks are less precisely located in the immediate vicinity of dif and are spread more homog-enously over a large terminal domain (Corre and Louarn, 2002). This can be taken to reflect two complementary levels of sequence positioning. The first ensures approxi-mate positioning of a large Ter domain around midcell. Positioning of this domain is independent of FtsK and chromosome polarization (see below). The second involves reading of polarization elements by FtsK (and possibly other factors) to sort DNA on either side of the closing septum and thus to position the dif sites in the case of dimeric chromosomes. How can DNA polarity affect FtsK-dependent positioning of the dif sites? Moni-toring DNA translocation by FtsK50C at the single molecule level did not reveal any direct influence of the DNA sequence, suggesting that the control effected by DNA polarity on dif positioning is a complex phenomenon that implies the activities of other proteins in vivo and/or of other domains of the FtsK protein (Saleh et al., 2004). Interestingly, the C-terminal domain of H. influenzae FtsK can replace its E. coli counterpart for the in vivo process-ing of DNA polarity inside E. coli, which indicates conser-vation of the mechanism of polarity reading (Bigot et al., 2004).
Folding and segregation of the Ter domain
The DAZ is contained within a larger structural entity called the Ter macrodomain. A structural peculiarity of this region was first suggested by the fact that it contains two ‘non-divisible zones’ that are regions refractory to inver-sion (Rebollo et al., 1988; Guijo et al., 2001). This Ter
domain was defined following the observation that sequences belonging to a large part of the chromosome around the terminus display similar intracellular location, suggesting that these sequences behave as a structural unit during the cell cycle (Niki et al., 2000). In slowly growing cells, sequences of the Ter macrodomain move to the new cell centre after division and remain there until the next division. The same study revealed an equivalent 1 Mb Ori macrodomain that encompasses oriC. Macro-domain organization of the chromosome is also supported by recent genetic data on the capacity for remote sequences to collide (M. Valens et al., submitted). By this criterion, macrodomains, within which communication between sequences is frequent, appear insulated from the rest of the chromosome. The Ter macrodomain defined by both approaches extends from about 25¢ (1150 kb) to 45¢
(2050 kb) on the chromosome. It is noteworthy that sequences belonging to the Ter macrodomain display sev-eral peculiarities (Pedersen et al., 2000): they are partic-ularly poor in repeated elements (REPs, BIMEs) and show a general trend for intrinsic curvature and low flexibility. No biological significance has been yet attributed to any of these features.
Two recent series of experiments based on live fluores-cence labelling techniques provide a closer view of Ter macrodomain segregation (Li et al., 2002; 2003; Lau
et al., 2003). Whereas sequences outside Ter appear as
two separate foci in the cell soon after their replication, Ter sequences most often remain together as a single fluorescent focus during the time between termination of replication and septum constriction (the D period, about 20¢). The replicated Ter region frequently remains
and allows separation of sister chromosomes involving oriented translocation of FtsK towards dif that facilitates removal of the last catenation links and eventually induces XerCD/dif recombination in the case of a dimer.
Acknowledgements
We want to thank Bénédicte Michel, Jean-Yves Bouet, Leonara Poljak and David Lane for critical reading of this manuscript. C.L. is funded by a PhD fellowship from the French ‘Ministère de la Recherche’. F.X.B. received an ATIPE from the CNRS.
References
Alen, C., Sherratt, D.J., and Colloms, S.D. (1997) Direct interaction of aminopeptidase A with recombination site DNA in Xer site-specific recombination. EMBO J16: 5188– 5197.
Arciszewska, L.K., and Sherratt, D.J. (1995) Xer site-specific recombination in vitro. EMBO J14: 2112–2120.
Arciszewska, L., Grainge, I., and Sherratt, D. (1995) Effects of Holliday junction position on Xer-mediated recombina-tion in vitro. EMBO J14: 2651–2660.
Aussel, L., Barre, F.X., Aroyo, M., Stasiak, A., Stasiak, A.Z., and Sherratt, D. (2002) FtsK is a DNA motor protein that activates chromosome dimer resolution by switching the catalytic state of the XerC and XerD recombinases. Cell
108: 195–205.
Azaro, M.A., and Landy, A. (2002) l integrase and the l Int family. In Mobile DNA II. Craig, N.L., Craigie, R., Gellert, M., and Lambowitz, A. (eds). Washington, DC: American Society for Microbiology Press, pp. 118–148.
Barre, F.X., Aroyo, M., Colloms, S.D., Helfrich, A., Cornet, F., and Sherratt, D.J. (2000) FtsK functions in the process-ing of a Holliday junction intermediate durprocess-ing bacterial chromosome segregation. Genes Dev14: 2976–2988. Begg, K.J., Dewar, S.J., and Donachie, W.D. (1995) A new
Escherichia coli cell division gene, ftsK. J Bacteriol177: 6211–6222.
Bigot, S., Corre, J., Louarn, J.-M., Cornet, F., and Barre, F.-X. (2004) FtsK activities in Xer recombination, DNA mobilization and cell division involve overlapping and separate domains of the protein. Mol Microbiol
doi:10.1111/j.1365-2958.2004.04335.x.
Blakely, G., Colloms, S., May, G., Burke, M., and Sherratt, D. (1991) Escherichia coli XerC recombinase is required for chromosomal segregation at cell division. New Biol 3: 789–798.
Blakely, G., May, G., McCulloch, R., Arciszewska, L.K., Burke, M., Lovett, S.T., and Sherratt, D.J. (1993) Two related recombinases are required for site-specific recom-bination at dif and cer in E. coli K12. Cell75: 351–361. Blattner, F.R., Plunkett, G., 3rd, Bloch, C.A., Perna, N.T.,
Burland, V., Riley, M., et al. (1997) The complete genome sequence of Escherichia coli K-12. Science 277: 1453– 1474.
Bregu, M., Sherratt, D.J., and Colloms, S.D. (2002) Acces-sory factors determine the order of strand exchange in Xer recombination at psi. EMBO J21: 3888–3897.
Campos, J., Martinez, E., Marrero, K., Silva, Y., Rodriguez, B.L., Suzarte, E., et al. (2003a) Novel type of specialized transduction for CTX phi or its satellite phage RS1 medi-ated by filamentous phage VGJ phi in Vibrio cholerae. J Bacteriol185: 7231–7240.
Campos, J., Martinez, E., Suzarte, E., Rodriguez, B.L., Mar-rero, K., Silva, Y., et al. (2003b) VGJ phi, a novel filamen-tous phage of Vibrio cholerae, integrates into the same chromosomal site as CTX phi. J Bacteriol185: 5685–5696. Capiaux, H., Lesterlin, C., Perals, K., Louarn, J.M., and Cor-net, F. (2002) A dual role for the FtsK protein in Escherichia coli chromosome segregation. EMBO Rep3: 532–536. Chalker, A., Lupas, A., Ingraham, K., So, C., Lunsford, R., Li,
T., et al. (2000) Genetic characterization of gram-positive homologs of the XerCD site-specific recombinases. J Mol Microbiol Biotechnol2: 225–233.
Colloms, S.D., Bath, J., and Sherratt, D.J. (1997) Topological selectivity in Xer site-specific recombination. Cell88: 855– 864.
Cornet, F., Louarn, J., Patte, J., and Louarn, J.M. (1996) Restriction of the activity of the recombination site dif to a small zone of the Escherichia coli chromosome. Genes Dev10: 1152–1161.
Corre, J., and Louarn, J.M. (2002) Evidence from terminal recombination gradients that FtsK uses replichore polarity to control chromosome terminus positioning at division in
Escherichia coli. J Bacteriol184: 3801–3807.
Corre, J., Cornet, F., Patte, J., and Louarn, J.M. (1997) Unraveling a region-specific hyper-recombination phenom-enon: genetic control and modalities of terminal recombi-nation in Escherichia coli. Genetics147: 979–989. Corre, J., Patte, J., and Louarn, J.M. (2000) Prophage
lambda induces terminal recombination in Escherichia coli
by inhibiting chromosome dimer resolution. An orientation-dependent cis-effect lending support to bipolarization of the terminus. Genetics154: 39–48.
Cox, M.M., Goodman, M.F., Kreuzer, K.N., Sherratt, D.J., Sandler, S.J., and Marians, K.J. (2000) The importance of repairing stalled replication forks. Nature404: 37–41. Cromie, G.A., and Leach, D.R. (2000) Control of crossing
over. Mol Cell6: 815–826.
Draper, G.C., McLennan, N., Begg, K., Masters, M., and Donachie, W.D. (1998) Only the N-terminal domain of FtsK functions in cell division. J Bacteriol180: 4621–4627. Espeli, O., Lee, C., and Marians, K.J. (2003a) A physical and
functional interaction between Escherichia coli FtsK and topoisomerase IV. J Biol Chem278: 44639–44644. Espeli, O., Levine, C., Hassing, H., and Marians, K.J. (2003b)
Temporal regulation of topoisomerase IV activity in E. coli.
Mol Cell11: 189–201.
Ghosh, K., and Van Duyne, G. (2002) Cre-loxP biochemistry.
Methods28: 374–383.
Guijo, M.I., Patte, J., del Mar Campos, M., Louarn, J.-M., and Rebollo, J.E. (2001) Localized remodelling of the Escheri-chia coli chromosome. The patchwork of segments refrac-tory and tolerant to inversion near the replication terminus.
Genetics4: 1413–1423.
Hendricks, E.C., Szerlong, H., Hill, T., and Kuempel, P. (2000) Cell division, guillotining of dimer chromosomes and SOS induction in resolution mutants (dif, xerC
and xerD) of Escherichia coli. Mol Microbiol 36: 973– 981.
Huber, K.E., and Waldor, M.K. (2002) Filamentous phage integration requires the host recombinases XerC and XerD. Nature417: 656–659.
Ip, S.C., Bregu, M., Barre, F.X., and Sherratt, D.J. (2003) Decatenation of DNA circles by FtsK-dependent Xer site-specific recombination. EMBO J22: 6399–6407.
Kuempel, P.L., Henson, J.M., Dircks, L., Tecklenburg, M., and Lim, D.F. (1991) dif, a recA-independent recombination site in the terminus region of the chromosome of Escheri-chia coli. New Biol3: 799–811.
Kuempel, P., Hogaard, A., Nielsen, M., Nagappan, O., and Tecklenburg, M. (1996) Use of a transposon (Tndif) to obtain suppressing and nonsuppressing insertions of the
dif resolvase site of Escherichia coli. Genes Dev10: 1162– 1171.
Kuzminov, A. (1999) Recombinational repair of DNA damage in Escherichia coli and bacteriophage lambda. Microbiol Mol Biol Rev63: 751–813, table of contents.
Lau, I.F., Filipe, S.R., Soballe, B., Okstad, O.A., Barre, F.X., and Sherratt, D.J. (2003) Spatial and temporal organization of replicating Escherichia coli chromosomes. Mol Microbiol
49: 731–743.
Leslie, N.R., and Sherratt, D.J. (1995) Site-specific recom-bination in the replication terminus region of Escheri-chia coli: functional replacement of dif. EMBO J 14: 1561–1570.
Li, Y., Sergueev, K., and Austin, S. (2002) The segregation of the Escherichia coli origin and terminus of replication.
Mol Microbiol46: 985–995.
Li, Y., Youngren, B., Sergueev, K., and Austin, S. (2003) Segregation of the Escherichia coli chromosome terminus.
Mol Microbiol50: 825–834.
Lobry, J.R., and Louarn, J.M. (2003) Polarisation of prokary-otic chromosomes. Curr Opin Microbiol6: 101–108. Louarn, J.M., Louarn, J., Francois, V., and Patte, J. (1991)
Analysis and possible role of hyperrecombination in the termination region of the Escherichia coli chromosome. J Bacteriol173: 5097–5104.
Louarn, J., Cornet, F., Francois, V., Patte, J., and Louarn, J.M. (1994) Hyperrecombination in the termi-nus region of the Escherichia coli chromosome: possi-ble relation to nucleoid organization. J Bacteriol 176: 7524–7531.
Massey, T.H., Aussel, L., Barre, F.-X., and Sherratt, D.J. (2004) Asymmetric activation of Xer site-specific recombi-nation by FtsK. EMBO Rep4: 399–404.
Meddows, T.R., Savory, A.P., and Lloyd, R.G. (2004) RecG helicase promotes DNA double-strand break repair. Mol Microbiol52: 119–132.
Michel, B., Recchia, G.D., Penel-Colin, M., Ehrlich, S.D., and Sherratt, D.J. (2000) Resolution of Holliday junctions by RuvABC prevents dimer formation in rep mutants and UV irradiated cells. Mol Microbiol37: 181–191.
Niki, H., Yamaichi, Y., and Hiraga, S. (2000) Dynamic orga-nization of chromosomal DNA in Escherichia coli. Genes Dev14: 212–223.
Pedersen, A., Jensen, L., Brunak, S., Staerfeldt, H., and Ussery, D. (2000) A DNA structural atlas for Escherichia coli. J Mol Biol299: 907–930.
Perals, K., Cornet, F., Merlet, Y., Delon, I., and Louarn, J.M. (2000) Functional polarization of the Escherichia coli chro-mosome terminus: the dif site acts in chromosome dimer resolution only when located between long stretches of opposite polarity. Mol Microbiol36: 33–43.
Perals, K., Capiaux, H., Vincourt, J.B., Louarn, J.M., Sherratt, D.J., and Cornet, F. (2001) Interplay between recombina-tion, cell division and chromosome structure during chro-mosome dimer resolution in Escherichia coli. Mol Microbiol
39: 904–913.
Prikryl, J., Hendricks, E.C., and Kuempel, P.L. (2001) DNA degradation in the terminus region of resolvase mutants of
Escherichia coli, and suppression of this degradation and the Dif phenotype by recD. Biochimie83: 171–176. Rebollo, J., Francois, V., and Louarn, J. (1988) Detection and
possible role of two large nondivisible zones on the Escher-ichia coli chromosome. Proc Natl Acad Sci USA85: 9391– 9395.
Recchia, G.D., and Sherratt, D.J. (1999) Conservation of Xer site-specific recombination genes in bacteria. Mol Micro-biol34: 1146–1148.
Recchia, G.D., Aroyo, M., Wolf, D., Blakely, G., and Sher-ratt, D.J. (1999) FtsK-dependent and -independent pathways of Xer site-specific recombination. EMBO J18: 5724–5734.
Saleh, O.A., Perals, C., Barre, F.X., and Allemand, J.F. (2004) Fast, DNA-sequence independent translocation by FtsK in a single-molecule experiment. EMBO J23: 2430– 2439.
Salzberg, S.L., Salzberg, A.J., Kerlavage, A.R., and Tomb, J.F. (1998) Skewed oligomers and origins of replication.
Gene217: 57–67.
Sciochetti, S.A., Piggot, P.J., Sherratt, D.J., and Blakely, G. (1999) The ripX locus of Bacillus subtilis encodes a site-specific recombinase involved in proper chromosome par-titioning. J Bacteriol181: 6053–6062.
Sciochetti, S.A., Piggot, P.J., and Blakely, G.W. (2001) Iden-tification and characterization of the dif site from Bacillus subtilis. J Bacteriol183: 1058–1068.
Seigneur, M., Bidnenko, V., Ehrlich, S.D., and Michel, B. (1998) RuvAB acts at arrested replication forks. Cell 95: 419–430.
Steiner, W.W., and Kuempel, P.L. (1998a) Sister chromatid exchange frequencies in Escherichia coli analyzed by recombination at the dif resolvase site. J Bacteriol 180: 6269–6275.
Steiner, W.W., and Kuempel, P.L. (1998b) Cell division is required for resolution of dimer chromosomes at the dif
locus of Escherichia coli. Mol Microbiol27: 257–268. Steiner, W., Liu, G., Donachie, W.D., and Kuempel, P. (1999)
The cytoplasmic domain of FtsK protein is required for resolution of chromosome dimers. Mol Microbiol31: 579– 583.
Tecklenburg, M., Naumer, A., Nagappan, O., and Kuempel, P. (1995) The dif resolvase locus of the Escherichia coli
Van Gool, A.J., Hajibagheri, N.M., Stasiak, A., and West, S.C. (1999) Assembly of the Escherichia coli RuvABC resolva-some directs the orientation of Holliday junction resolution.
Genes Dev13: 1861–1870.
Wang, L., and Lutkenhaus, J. (1998) FtsK is an essential cell division protein that is localized to the septum and induced as part of the SOS response. Mol Microbiol29: 731–740.
Yates, J., Aroyo, M., Sherratt, D.J., and Barre, F.X. (2003) Species specificity in the activation of Xer recombination at dif by FtsK. Mol Microbiol49: 241–249.
Yu, X.C., Tran, A.H., Sun, Q., and Margolin, W. (1998) Local-ization of cell division protein FtsK to the Escherichia coli