www.elsevier.com / locate / bres
Short communication
A melatonin agonist facilitates circadian resynchronization in old
hamsters after abrupt shifts in the light–dark cycle
a a b a ,
*
L. Weibel , F.W. Turek , E. Mocaer , O. Van Reeth
a
ˆ ´
Centre d’Etudes des Rythmes Biologiques, School of Medicine, Hopital Erasme, Universite Libre de Bruxelles, Route de Lennik, 808, 1070 Brussels, Belgium
b
Institut de Recherches Internationales SERVIER, Courbevoie, France
Accepted 2 August 2000
Abstract
Age-related changes in the mammalian circadian system may be associated with a decline in circulating melatonin levels. Using ‘jet lag’ paradigms involving abrupt shifts in the light–dark cycle, we showed that a melatonin agonist, S-20098, accelerated by |25% resynchronization of the circadian activity rhythm in old hamsters to the new light–dark cycle. It suggests the usefulness of melatonin-related compounds to treat circadian disorders associated with aging. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neural basis of behaviour
Topic: Biological rhythms and sleep
Keywords: Circadian clock; Aging; Jet lag; Melatonin; Agonist; Re-entrainment
In mammals, the circadian system is organized around a (LD) cycle [24] may be due in part to this decrease in central pacemaker located in the suprachiasmatic nuclei melatonin secretion.
(SCN) of the hypothalamus. The circadian system can be Recently, a naphthalenic analogue of melatonin, S-entrained by periodic exposure to photic (i.e. light–dark 20098, has demonstrated potent and specific agonist prop-cycles) or non-photic (i.e. sleep–wake, activity–rest, social erties at the melatonin receptor level, both in vitro and in schedules) stimuli [10]. Aging is associated with various vivo [5,12,13,21]. There is now evidence to indicate that alterations in biological time-keeping [6], including re- exogenous melatonin or its agonists can be effective duced responsiveness of the circadian system to the treatments for adapting to phase shifts in both rodents synchronizing effects of both photic and non-photic inputs [12,13,18,19] and humans [1,2]. Behavioural studies have [14,16,17]. Those alterations interfere with the internal shown that repeated administration of S-20098 can acceler-temporal organization of the senescent organism and its ate the rate of re-entrainment of the activity rhythm after adaptation to the environment [6,24]. an advance shift in the LD cycle, both in nocturnal [13]
Little is known about the mechanisms by which aging and diurnal rodents [19].
alters the response of the circadian system to its primary We have recently shown that chronic treatment with synchronizer, light. The pineal hormone melatonin has S-20098 in old hamsters can reversibly enhance the phase been implicated in circadian synchronization processes, shifting effects of non-photic stimuli [20], demonstrating likely through a feedback effect on the SCN [1,8]. Aging is that S-20098 was able to strengthen the response of the associated with a decrease in circulating melatonin levels, aging circadian clock to these stimuli. In the present study possibly secondary to an age-associated decrease in pineal we tested the hypothesis that the melatonin agonist S-function [4,9,15] An altered rate of resynchronization in 20098 could be used to facilitate resynchronization of the senescent organisms after phase shifts in the light–dark activity rhythm in old hamsters subjected to abrupt phase shifts in the LD cycle (i.e. a simulated ‘jet lag’ paradigm). Male golden hamsters (Mesocricetus auratus) purchased
*Corresponding author. Tel.:132-2-555-6427; fax:132-2-555-3569.
E-mail address: [email protected] (O. Van Reeth). from Charles River Lakeview (Newfield, NJ, USA) were
hamsters were placed in light-tight animal chambers (lights-on: 6.00 h–20.00 h) equipped with continuously operating ventilating fans, and fed with regular powdered food.
After 10 days of adaptation to the cage with the running wheel, hamsters were separated into two groups: one group (N512) remained on regular powdered food, while the other group (N512) was switched to powdered food containing S-20098 (2000 parts per million, ppm). Based on the average weight of the animals and their daily food consumption, 2000 ppm of S-20098 correspond to an average daily dose of 20 mg / kg, a dose that was previous-ly shown to induce significant phase shifts in the circadian clock of free running hamsters [18]. After the switch, hamsters were left undisturbed for 14 days before being subjected to an abrupt 8 h advance shift in the LD cycle. On the day of the shift, lights were turned-off at the usual time and were turned-on 2 h later, and the new LD cycle (lights on: 22.00 h–12.00 h) was maintained thereafter. The procedure was repeated 1 month later with an abrupt 8 h delay shift in the LD cycle. On the day of the shift, lights were turned-off at the usual time (i.e. 12.00 h) and were turned-on 18 h later to obtain the new LD cycle (lights-on: 6.00 h–20.00 h). Five hamsters (two vehicle-fed and three S-20098-fed) died during the course of the experiment: data collected from those animals were not included.
The time taken for re-entrainment to the new LD cycle was individually assessed by an observer blind to the treatment given to the animals, using the ‘onset’ of activity criterion [19]. This criterion was defined as the smallest number of days required for the shifted activity onset to occur within 30 min of lights-off and to be stable for .3 days under the new LD cycle. Rates of re-entrainment for activity onsets for each animal were calculated, and
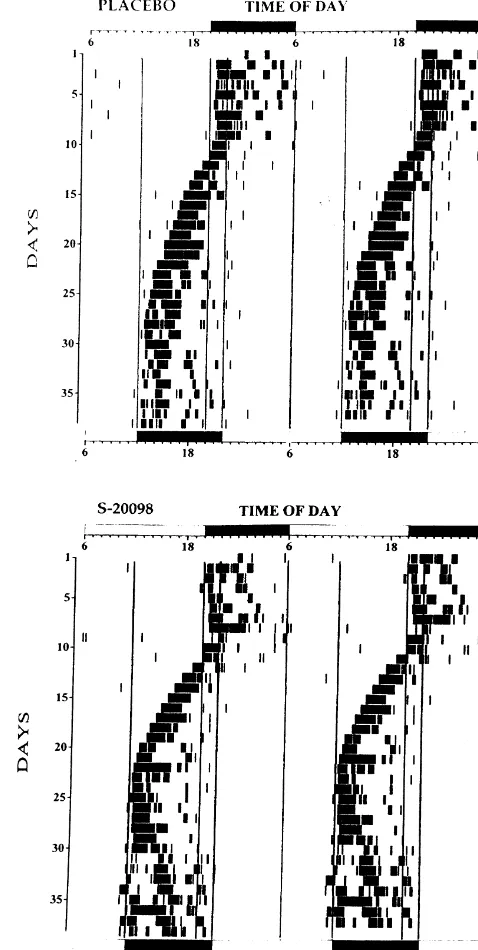
Fig. 1. Representative activity records from two old hamsters fed with
differences between control and treated animals were placebo powder (top) or S-20098 containing powder (bottom) and evaluated using an unpaired Student’s t-test. All parame- subjected to an 8 h phase advance in the light–dark (LD) cycle. The activity plots have been double mounted to facilitate visual inspection of
ters are given as mean6S.E.M.
the records. Each line represents 48 h of the animal’s life, and those
Under the 14 / 10 LD cycle, activity onset in old
successive time intervals are plotted from top to bottom. The first LD
hamsters occurred before or simultaneously with lights-off
cycle is shown at the top of each panel and the new LD cycle at the
in all animals (mean: 19.50 h66 min). After the imposed 8 bottom. On day 8 of this record, the LD cycle was advanced by 8 h. h advance shift in the LD cycle, all animals readjusted
their activity to the new LD cycle by daily phase advances.
The activity rhythm of one hamster (S-20098-fed) became mals and in 16.861.8 days in S-20098-treated animals too erratic and this animal was excluded from analysis. (P,0.006, unpaired Student t-test; Fig. 2 top). Mean daily Representative activity records from two hamsters (one activity did not significantly differ between the vehicle-vehicle-fed and one S-20098-fed) subjected to the 8 h treated animals and the S-20098-treated animals during the advance shift in the LD cycle are shown in Fig. 1. After phase advance paradigm (26226382 vs. 25706905 wheel the 8 h advance shift in the LD cycle, activity onset revolutions / day, unpaired Student’s t-test, NS).
Fig. 2. (Top) Mean (6S.E.M.) number of days for the circadian rhythm of locomotor activity in old hamsters to become resynchronized after an abrupt 8 h advance shift in the LD cycle (*P,0.006). (Bottom) Mean (6S.E.M.) number of days required for the circadian rhythm of locomotor activity to become resynchronized after an abrupt 8 h delay shift in the LD cycle (*P,0.02).
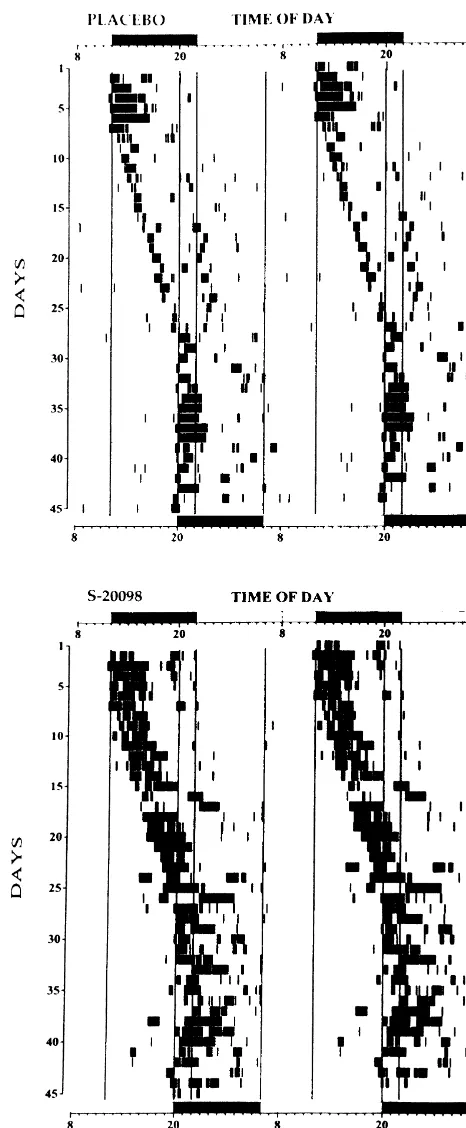
Fig. 3. Representative activity records from two old hamsters fed with placebo powder (top) or S-20098 containing powder (bottom) and
readjusted their activity rhythm to the new LD cycle by subjected to an 8 h phase delay in their LD cycle (on day 6 of this record). See Fig. 1 for further details.
daily phase delays. The activity rhythm in two animals (one placebo-fed and one S-20098-fed) became too erratic
advance shift in the LD cycle, and by 27% after an 8 h not really mimicking the circadian rhythm of plasma delay shift. Despite existing reports of an age-related melatonin [1]. Indeed, we measured S-20098 plasma decline in the density of SCN melatonin receptors and in concentrations at four different times of day in a group of circulating melatonin levels [4,15], the present data indi- 14 old hamsters kept in running wheel cages under a cate that aging does not block the responsiveness of the regular 14 / 10 LD cycle and fed with 2000 ppm S-20098 circadian clock to melatonin-related compounds. for 3 weeks: mean (6S.E.M.) plasma concentrations Aging is associated with an alteration in the rate of averaged 6.360.9 ng / ml, with no significant diurnal resynchronization of the circadian system after abrupt variation. In view of recent data showing the influence of shifts in the LD cycle [22,24]. Such an alteration may be timing / duration of S-20098 administration on circadian related to a decrease in sensory input(s) to the circadian entrainment in rodents [12], future protocols designed to clock, to deficits in its output pathway(s) and / or to changes provide high night-time plasma levels of melatonin agon-in circadian clock function itself. Zhang and co-workers ists and low daytime levels should be tested to determine have shown that old hamsters were approximately 20 times whether mimicking physiological profiles of endogenous less sensitive to the phase-shifting effects of light on the melatonin [3] will strengthen the action of chronobiotic activity rhythm, and that the photic irradiance threshold for compounds on synchronization processes. Of particular Fos-like immunoreactivity induction in the SCN was relevance for clinical applications is the fact that melatonin elevated when compared to young animals [22,23]. Aging agonists retain their ability to synchronize the aging was also associated with a deficit in cyclic-AMP response circadian system, supporting the clinical usefulness of such element-binding protein phosphorylation by light [22]. compounds for treatment of circadian disruption in the They also reported that neither age-related changes in lens elderly.
transmittance nor in the retino-hypothalamic tract could entirely account for the dysfunction of the circadian system
in old rodents, supporting the hypothesis that the observed Acknowledgements decrease in sensitivity to light in the aged circadian system
occurs within the SCN itself and / or retino-hypothalamic This work was supported by the Belgian National Fund tract photoreceptors [23]. for Scientific Research (O.V.R.) and by National Institute
In the present study we found that the time taken to on Aging Grant AG-11412 (F.W.T.). re-entrain the circadian clock to phase advances in the LD
cycle was shorter than to re-entrain it to phase delays. This
directional asymmetry is opposite to the one found in References young hamsters, in which resynchronization after delay
shifts in the LD cycle is shorter than after advance shifts. [1] J. Arendt (Ed.), Melatonin and the Pineal Gland, Chapman and Hall, Taken together with our previous data [24], those direc- London, 1995.
tion-dependent effects of age on circadian re-entrainment [2] J. Arendt, D.J. Skene, B. Middleton, S.W. Lockley, S. Deacon, Efficacy of melatonin treatment in jet lag, shift work and blindness,
suggest an underlying dysfunction of the circadian
pace-J. Biol. Rhythms 12 (1997) 604–617.
maker. Indeed these differences between young and old
[3] L. Benes, B. Claustrat, F. Horriere, M. Geoffriau, J. Konsil, K.A.
hamsters may be related to a shortening of the free running Parrott, G. De Grande, R.L. McQuinn, J.W. Ayres, Transmucosal, period of locomotor activity rhythm in old hamsters, as oral controlled-release, and transdermal drug administration in
reported by some [11] but not all [7] investigators. Also human subjects: a crossover study with melatonin, J. Pharmac. Sci. 86 (1997) 1115–1119.
consistent with the shortening of the endogenous period in
[4] S. Benloucif, M.I. Masana, M.L. Dubocovich, Responsiveness to
aging is our observation of an onset of activity rhythm
melatonin and its receptor expression in the aging circadian clock of
before time of lights-off during entrainment to the 14:10 mice, Am. J. Physiol. 273 (1997) R1855–R1860.
LD cycle in old hamsters [24]. Such shortening of the free [5] C. Bonnefond, L. Martinet, D. Lesieu, G. Adam, B. Guardiola-ˆ
running period, an intrinsic property of the SCN, supports Lema ıtre, Characterization of S-20098, a new melatonin analog, in: Y. Touitou, J. Arendt, P. Pevet (Eds.), Melatonin and the Pineal
the idea that aging may interfere with the structural and
Gland, Elsevier, Amsterdam, 1993, pp. 123–126.
functional integrity of the circadian pacemaker itself.
[6] M.A. Brock, Chronobiology and aging, J. Am. Geriat. Soc. 39
The present study indicates that S-20098 is beneficial for (1991) 74–91.
facilitating resynchronization of the aging circadian clock [7] F.C. Davis, N. Viswanathan, Stability of circadian timing with age in
[8] M.L. Dubocovich, S. Benloucif, M.I. Masana, Melatonin receptors on the entraining properties of activity-inducing stimuli on the in the mammalian suprachiasmatic nucleus, Behav. Brain Res. 73 circadian clock, Brain Res. 607 (1992) 286–292.
(1996) 141–147. [18] O. Van Reeth, E. Olivarez, Y. Zhang, P.C. Zee, E. Mocaer, R. ´
[9] W. Humbert, P. Pevet, The pineal gland of the aging rat: calcium Defrance, F.W. Turek, Comparative effects of a melatonin agonist on localization and variation in the number of pinealocytes, J. Pineal the circadian system in mice and Syrian hamsters, Brain Res. 762
Res. 18 (1995) 32–40. (1997) 185–194.
[10] J.D. Miller, L.P. Morin, W.J. Schwartz, R. Moore, New insights into [19] O. Van Reeth, E. Olivarez, F.W. Turek, L. Granjon, E. Mocaer, In a the mammalian circadian clock, Sleep 19 (1996) 641–667. diurnal rodent, adaptation of circadian rhythmicity to a shift in the [11] L.P. Morin, Age-related changes in hamster circadian period, light–dark cycle can be accelerated by a melatonin-agonist,
Neuro-entrainment and rhythm splitting, J Biol. Rhythms 3 (1988) 237– Report 9 (1998) 1901–1905.
248. [20] O. Van Reeth, O.E. Olivarez, E. Mocaer, P.C. Zee, F.W. Turek, A
´
[12] B. Pitrosky, R. Kirsch, A. Malan, E. Mocaer, P. Pevet, Organization melatonin agonist can reverse age-related changes in circadian clock of rat circadian rhythms during daily infusion of melatonin or response to an environmental stimulus, Am. J. Physiol. in press. S-20098, a melatonin agonist, Am. J. Physiol. 277 (1999) R812– [21] S.W. Ying, B. Rusak, P. Delagrange, E. Mocaer, P. Renard, B.
ˆ
R828. Guardiola-Lemaı tre, Melatonin analogues as agonist and antagonists
ˆ
[13] J.R. Redman, B. Guardiola-Lemaıtre, M. Brown, P. Delagrange, in the circadian system and other brain areas, Eur. J. Pharmacol. 296 S.M. Armstrong, Dose-dependent effects of S-20098, a melatonin (1996) 33–42.
agonist, on direction and reentrainment of rat circadian rhythms, [22] Y. Zhang, J.M. Kornhauser, P.C. Zee, K.E. Mayo, J.S. Takahashi, Psychopharmacology 118 (1995) 385–390. F.W. Turek, Effects of aging and light-induced phase shifting of [14] F.W. Turek, P. Penev, Y. Zhang, O. Van Reeth, P.C. Zee, Effects of circadian behavioral rhythms, fos expression and CREB phosphoryl-age on the circadian system, Neurosci. Biobehav. Rev. 19 (1995) ation in the hamster suprachiasmatic nucleus, Neuroscience 70
53–58. (1996) 951–961.
[15] A. Van Coevoorden, J. Mockel, E. Laurent, M. Kerkhofs, M. [23] Y. Zhang, G.C. Brainard, P.C. Zee, L.H. Pinto, J.S. Takahashi, F.W. ´
L’Hermite-Baleriaux, C. Decoster, P. Neve, E. Van Cauter, Neuroen- Turek, Effects of aging on lens transmittance and retinal input to the docrine rhythms and sleep in aging men, Am. J. Physiol. 260 (1991) suprachiasmatic nucleus in golden hamsters, Neurosci. Lett. 258
E651–E661. (1998) 167–170.
[16] O. Van Reeth, Y. Zhang, P.C. Zee, F.W. Turek, Aging alters the [24] P.C. Zee, R.S. Rosenberg, F.W. Turek, Effects of aging on entrain-feedback effects of the activity–rest cycle on the circadian clock, ment and rate of resynchronization of circadian locomotor activity, Am. J. Physiol. 263 (1992) R981–R986. Am. J. Physiol. 263 (1992) R1099–R1103.