Insect Biochemistry and Molecular Biology 30 (2000) 947–952
www.elsevier.com/locate/ibmb
Isolation and identification of cell hypertrophy-inducing substances
in the gall-forming aphid
Colopha moriokaensis
Shinji Ohta
a,*, Naoki Kajino
b, Hiroto Hashimoto
b, Toshifumi Hirata
baInstrument Center for Chemical Analysis, Hiroshima University, 1-3-1 Kagamiyama, Higashi-Hiroshima 739-8526, Japan bDepartment of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, 1-3-1 Kagamiyama, Higashi-Hiroshima
739-8526, Japan
Received 22 June 1999; received in revised form 6 March 2000; accepted 9 March 2000
Abstract
The insect galls on the leaves of Zelkova serratawere formed by the aphid Colopha moriokaensis. The crude extracts of the gall-forming aphid showed hypertrophy-inducing activity against the cultured cells ofZ. serrata. Bioassay-guided fractionation of the extracts led to isolation of three triacylglycerides containing (E,E,E)-octa-2,4,6-trienoic acid.2000 Elsevier Science Ltd. All rights reserved.
Keywords:Insect gall; Cell hypertrophic agent; Octatrienoic acid-containing triacylglycerides; Hypertrophy-inducing activity; Gall-forming aphid;
Colopha moriokaensis
1. Introduction
Aphids have the distinctive potential of reproduction, genetic adaptability, and feeding methods. Because of these properties, aphids have become one of the most successful insects in the temperate and tropical regions of the Earth and are classified among the most destruc-tive of agricultural pests. A number of aphids have been investigated chemically regarding their alkanes (Dixon et al., 1965; Cameron and Drake, 1976; Stransky et al., 1976), carotenes (Brown et al., 1969; Banks et al., 1973; Jenkins et al., 1999), steroids (Campbell and Nes, 1983; Douglas, 1988; Polgar et al., 1996), aphid glycosides (Duewell et al., 1948), cardenolides, pheromones, and triacylglycerides (Brown, 1975). Of these chemical con-stituents, triacylglycerides are major components and amount to as much as one-third of the weight of aphids. Aphid triacylglycerides are known to be esterified at the
sn-1,3 position of glycerol mainly with myristic acid (14:0), and also to a lesser extent with lauric and palmitic acid (12:0 and 16:0). These triacylglycerides have been
* Corresponding author. Tel.:+81-824-24-7487; fax:+ 81-824-24-7486.
E-mail address:[email protected] (S. Ohta).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 6 7 - 9
shown to contain a short chain fatty acid such as hexa-noic and sorbic acid (6:0 and 6:2) at the sn-2 position (Bowie and Cameron, 1965). Hexanoic and sorbic acid are restricted to the triacylglyceride fraction. No hexa-noic acid- or sorbic acid-containing phospholipids appear to be contained in aphids (Cameron and Drake, 1976; Ryan et al., 1982; Febvay et al., 1992). Within the whole animal kingdom, the sorbic acid-containing triacylglycerides were found only in Aphididae. The sor-bic acid-containing triacylglycerides have been shown to be present in cornicle secretions of various species of aphids (Callow et al., 1973; Greenway and Griffiths, 1973) and have been found to accumulate in cornicle cells of the aphidAcyrthosiphon pisumat very high lev-els (Rahbe et al., 1994). A significant use of the sorbic acid-containing triacylglyceride by the aphids has been described as in defense (Callow et al., 1973; Greenway and Griffiths, 1973). When disturbed or attacked, aphids produce from their cornicles a supercooled liquid which rapidly becomes a sticky solid upon contact with any surface. The supercooled liquid appears to be almost tot-ally composed of a low-melting triacylglyceride mixture, sometimes similar to but rarely identical with that in the total body lipids (Brown, 1975).
in the spring (Moritsu, 1983, p. 455; Akimoto, 1985; Yeo et al., 1997). Every gall is founded by one female (a stem mother) emerging from a fertilized egg. The aphid propagates in the gall by asexual reproduction. The size of cells in the insect galls increases with the growing stage of the gall (Kaneko et al., 1994). Generally, insect galls of higher plants are thought to be caused by the introduction of chemical substances produced by the causative insect (Bayer et al., 1994). Several workers have induced abnormal plant growths by the application of extracts of gall-forming insects. A number of specific inorganic chemicals have been reported to produce gall-like plant growths. Several amino acids as well as aden-ine containing compounds have also been implicated in insect gall formation (Miles, 1968; Schaller, 1968). Although a gall-forming insect did not contain auxins, extracts from galls have been reported to contain higher levels of phyto hormones than extracts from normal plant leaves (Tandon and Arya, 1980).
During the course of our search of aphid components concerned in the gall-forming process, we found that the hexane-soluble fraction of the EtOH extract of the aphid,
C. moriokaensis, caused the cultured cells ofZ. serrata
to enlarge 60-fold in volume greater than normal. Bioas-say guided fractionation led to the isolation of three tria-cylglycerides having octatrienoic acid. This paper deals with the isolation, identification, and biological activities of the hypertrophy-inducing substances.
2. Materials and methods
2.1. Insect
The aphids,C. moriokaensis(2.9 g, wet weight), were collected in the field of Hiroshima Prefecture, Japan, in the middle of May 1998.
2.2. Plant cells
The cultured cells ofZ. serratawere prepared in 300-ml conical flasks, each containing 100 300-ml of Murashige and Skoog’s medium and used for the preparation of single cells. Seeds of Asparagus officinalis L. were planted on moist sterile soil and kept in a growth room at 25°C under a daily 16-h light period. The cladodes of 30- to 60-day-old spears were used for the preparation of single cells of A. officinalis L. Preparation of single cells was performed according to the methods described by Matsubayashi and Sakagami (1996) as follows. The cultured cells of Z. serrata or the sterilized cladodes of
A. officinalis L. were homogenized in a glass homogen-izer containing sterile water. The homogenate was fil-tered through a 37-µm stainless mesh, and the filtrate was centrifuged at 100 g for 3 min. The precipitated
single cells were washed with sterile water three times and used in the bioassay.
2.3. Bioassay
Murashige and Skoog medium was used for cell cul-ture. Dispersed single cells ofZ. serrataorA. officinalis
L. were suspended in sterilized water, adjusted to twice the final cell density with the hemacytometer, and dis-pensed into 24-well microplates at a volume of 250 µl per well. Culture medium (125µl) prepared at four-fold concentration, and various 5% ethanol solutions (125µl) of sample were added to the cell suspension in each well. The initial cell density was 1.0×105 cells/ml. The plates
were sealed with Parafilm to avoid evaporation of the medium and then incubated in the dark at 25°C with continuous rotary shaking at 120 rpm. Hypertrophy-inducing activity for each well was calculated by divid-ing the number of hypertrophic cells by the total number of cells. Although the hypertrophy-inducing activity of compounds 1–3 and the hexane-soluble fraction of the EtOH extract of the aphid increased gradually with incu-bation time, from the fifth day, dead cells increased gradually. So, the hypertrophy-inducing activity was cal-culated on the fourth day of culture. Experiments were performed in triplicate on different days.
2.4. Isolation of hypertrophy-inducing substances
The ethanolic extract (0.7 g) of the aphid was par-titioned between hexane and water. The hexane extract (0.5 g) was subjected to chromatography on a silica gel column using 0–100% EtOAc in hexane as the eluent. The bioactive fraction (20–40% EtOAc in hexane) was fractionated by HPLC on ODS (10% hexane in MeOH) to produce three bioactive compounds 1 (0.35 mg), 2
(0.82 mg), 3 (0.33 mg), as amorphous solids.
2.5. Analytical instruments
1H NMR spectra were recorded on a JEOL GSX500
spectrometer (500 MHz). NMR chemical shifts were ref-erenced to solvent peaks:dH7.26 (residual CHCl3).
Elec-tron impact mass spectra (EIMS) and high resolution (HR) EIMS were measured on a JEOL SX102A spec-trometer. UV and IR spectra were recorded on a Shim-adzu UV-160A and a JASCO FT/IR-5300 spec-trometer, respectively.
2.6. Chemical structures of hypertrophy-inducing substances
The 1H NMR chemical shifts of1were in agreement
with those reported by Addae-Mensah and Cameron (1978) and Rahbe et al. (1994).
1:1H NMR (500 MHz, CDCl
S. Ohta et al. / Insect Biochemistry and Molecular Biology 30 (2000) 947–952
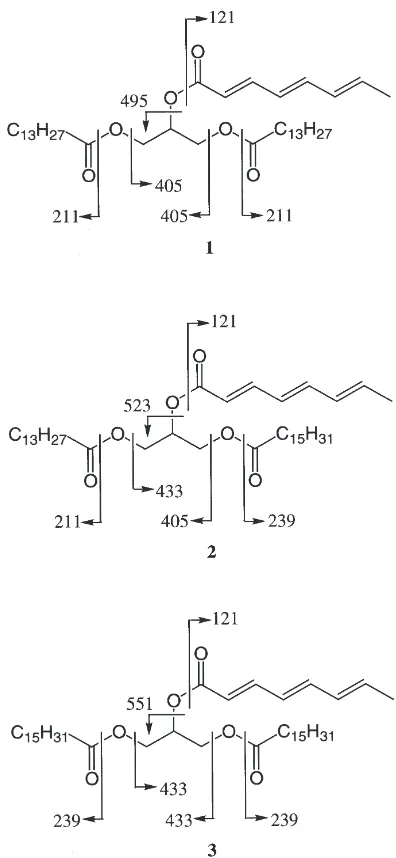
(relative intensity) 632 (M+; 18), 495 (100), 405 (10), 211 (11), 121 (90); HREIMS m/z 632.5027 (calculated for C39H68O6, 632.5016). (6), 239 (10), 211 (9), 121 (60); HREIMSm/z660.5316 (calculated for C41H72O6, 660.5329).
(relative intensity) 688 (M+; 25), 551 (100), 433 (20), 432 (22), 121 (100); HREIMSm/z688.5627 (calculated for C43H76O6, 688.5642).
2.7. Chemicals
1,3-Dimyristin and 1,3-dipalmitin were purchased from Sigma Chemical Co., St Louis, USA. Methyl tri-phenylphosphoranylideneacetate, 2,4-hexadienal, N,N9 -dicyclohexylcarbodiimide, 4-dimethylaminopyridine were purchased from Tokyo Chemical Industry Co. Ltd, Tokyo, Japan.
2.8. Synthesis of 1
To a solution of methyl triphenylphosphoranylidene-acetate (5.0 g) in CHCl3(16 ml), a solution of
2,4-hexad-ienal (0.96 g) in MeOH (16 ml) was added. The mixture was stirred at room temperature for 2 h. After removal of the solvent, a solution of NaOH (0.8 g) in 50% t -BuOH (20 ml) was added to the residue. The mixture was stirred at room temperature for 1 h. The reaction mixture was acidified by the addition of 2 M HCl and then extracted with EtOAc. The EtOAc extract, after
dryness over dry Na2SO4, was crystallized from EtOH
to afford (E,E,E)-octa-2,4,6-trienoic acid (450 mg, yield 33%).
To a solution of (E,E,E)-octa-2,4,6-trienoic acid (7 mg), N,N9-dicyclohexylcarbodiimide (11 mg) and 4-dimethylaminopyridine (5 mg) in toluene (1 ml), a sol-ution of 1,3-dimyristin (20 mg) in toluene (0.5 ml) was added. The mixture was stirred at room temperature for 15 h. After removal of the precipitate by filtration, the filtrate was evaporated to dryness and subjected to a pre-parative TLC to afford synthetic 1 (13 mg, yield 43% starting from 1,3-dimyristin): 1H NMR (500 MHz,
CDCl3):dH0.88 (6H, t,J=7.3 Hz), 1.25 (40H, m), 1.60
632.5012 (calculated for C39H68O6, 632.5016).
2.9. Synthesis of 3
Following a method similar to that used for the syn-thesis of 1, 1,3-dipalmitin (20 mg) was condensed with (E,E,E)-octa-2,4,6-trienoic acid (7 mg) in the presence of N,N9-dicyclohexylcarbodiimide (11 mg) and 4-dime-thylaminopyridine (5 mg) to afford synthetic 3(13 mg, yield 36% starting from 1,3-dipalmitin): 1H NMR (500
MHz, CDCl3):dH0.88 (6H, t,J=7.3 Hz), 1.25 (48H, m),
551 (40), 239 (100), 121 (43); HREIMS m/z 688.5663 (calculated for C43H76O6, 688.5642).
3. Results and discussion
chromato-graphy, followed by ODS HPLC to afford three bioac-tive compounds 1, 2, and 3.
Compound 1 had a molecular formula of C39H68O6,
as determined by HREI mass spectrometry (m/z
632.5027, M+,D +1.1 mmu). The1H NMR and1H–1H
COSY spectra indicate the presence of a glycerol moiety [dH4.22 (2H), 4.29 (2H), and 5.33 (1H)], long-chain acyl
groups [dH0.88 (Me×2), 1.25 (CH2×20), 1.60 (CH2×2),
and 2.31 (CH2×2)], and an (E,E,E)-octa-2,4,6-trienoyl
group [dH 1.84 (Me), 5.83, 5.97, 6.17, 6.20, 6.55, and
7.31 (=CH×6)]. Further, EIMS data (Fig. 1) revealed that
1 is 1,3-dimyristoyl-2-[(E,E,E )-octa-2,4,6-trienoyl]gly-cerol.
Compounds 2 and 3 had a molecular formula of C41H7206 (m/z 660.5316, M+, D 21.3 mmu) and
C43H76O6 (m/z 688.5627, M+, D 21.5 mmu),
respect-ively, as determined by HREI mass spectrometry. The
Fig. 1. Structures and EIMS fragment ions of1–3.
1H NMR, IR, UV, and EIMS (Fig. 1) spectral data
revealed that 2 and 3 are 1-myristoyl-2-[(E,E,E )-octa-2,4,6-trienoyl]-3-palmitoylglycerol and 2-[(E,E,E )-octa-2,4,6-trienoyl]-1,3-dipalmitoylglycerol, respectively. We have treated the mesophyll cells of A. officinalisL. with compounds1,2, and3to investigate the effects of these compounds on cell hypertrophy. As given in Table 1, compounds 1, 2, and 3 showed hypertrophy-inducing activity against 0.9–1.2% of the mesophyll cells of A. officinalis L., whereas no hypertrophic growth of cells was observed in the control experiments.
Compounds1and3were synthesized by the conden-sation of 1,3-dimyristin and 1,3-dipalmitin, respectively, with (E,E,E)-octa-2,4,6-trienoic acid. IR,1H NMR, and
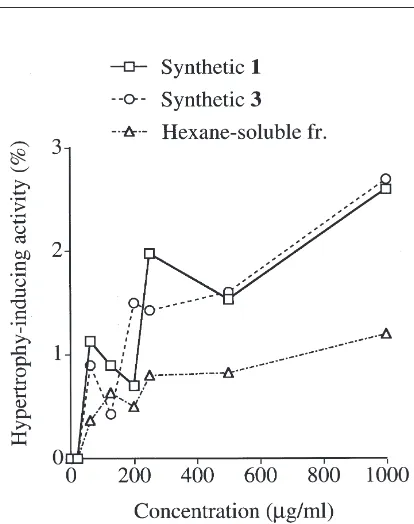
EIMS spectra of synthetic 1and synthetic3were ident-ical to those of the corresponding natural compounds. The synthetic compounds showed hypertrophy-inducing activity against the cultured cells of Z. serrata and the mesophyll cells of A. officinalisL., as given in Table 1. The dose dependence of the response was examined with synthetic 1, synthetic 3, and the hexane-soluble fraction of the EtOH extract of C. moriokaensis (Fig. 2). Octatrienoic acid-containing triacylglycerides 1 and
3induced hypertrophy in 0.5–3% of the mesophyll cells of A. officinalis L. at a concentration of 63 µg/ml or greater. The low proportion (,3%) may be caused by a poor permeability of the compounds into the plant cells and/or an instability (Addae-Mensah and Cameron, 1978) of the unsaturated compounds. However, the hypertrophy-inducing activity of the octatrienoic acid-containing triacylglycerides is biologically meaningful enough because any hypertrophy of plant cells was not induced in the control experiments or in the treatment of the EtOAc- or 1-BuOH-soluble fraction of the EtOH extract of the aphid. On the other hand, the response of the hexane-soluble fraction of the EtOH extract of the aphid was less than those of the octatrienoic acid-con-taining triacylglycerides1and3due to interference from the other components. The hexane-soluble fraction was found to contain carotenes, fatty acids, monoacylglycer-ides, aphid glycosides (protoaphins), galactocerebros-ides, phosphatidylethanolamines, and phosphatidylchol-ines, besides octatrienoic acid-containing triacylglycerides1–3by preliminary MS and TLC analy-ses. The fractions containing these components, other than compounds 1–3, obtained by chromatography on a column of silica gel showed no or negligible hypertro-phy-inducing activity.
S. Ohta et al. / Insect Biochemistry and Molecular Biology 30 (2000) 947–952
Table 1
Hypertrophy-inducing effects of1–3, synthetic1, and synthetic3against the cultured cells ofZ. serrataor the mesophyll cells ofA. officinalisL.
Compound Concentration (µg/ml) Plant cells used Hypertrophy-inducing activity (%)
1 240 A. officinalisL. 1.0
2 240 A. officinalisL. 1.2
3 240 A. officinalisL. 0.9
Synthetic1 250 A. officinalisL. 2.0
Synthetic1 250 Z. serrata 0.8
Synthetic3 250 A. officinalisL. 1.4
Synthetic3 250 Z. serrata 1.0
Control 0 A. officinalisL. 0.0
Control 0 Z. serrata 0.0
Fig. 2. Hypertrophy-inducing effects of synthetic1, synthetic3, and the hexane-soluble fraction of the EtOH extract of the aphid, C. moriokaensis, against the mesophyll cells ofA. officinalisL.
was reported to be defensive (Dixon, 1958; Edwards, 1966; Strong, 1967; Kislow and Edwards, 1972). The sorbic acid-containing triacylglycerides were reported to be a defensive material because of their relatively low melting points (Greenway and Griffiths, 1973). The melting point of 2-sorboyl-1,3-dimyristin is 54–54.5°C (Bowie and Cameron, 1965) and that of 2-sorboyl-1,3-dipalmitin is 62–63°C (Brown, 1975). The sorbic acid-containing triacylglycerides do not solidify in the corni-cle and remain liquid until in contact with the predator. When in contact with the predator, aphids secrete the supercooled liquid of the sorbic acid-containing triacyl-glycerides from their cornicles. The liquid rapidly becomes a sticky solid upon contact with any surface (Greenway and Griffiths, 1973; Brown, 1975). On the other hand, the role of the octatrienoic acid-containing triacylglycerides has not been mentioned yet. We have
now found that the octatrienoic acid-containing triacyl-glycerides 1–3, isolated from the gall-forming aphidC. moriokaensis, showed hypertrophy-inducing activity against plant cells. These findings imply that the aphids which live in protected situations such as insect galls utilize the triacylglycerides that have highly unsaturated short-chain fatty acids for cell hypertrophic agents rather than for defensive materials.
Acknowledgements
The authors thank Mr Kimio Nishikawa, Higashi-Hiroshima City, for his assistance in the collection of the aphids, Ms Mihoko Yanai and Ms Emi Ohta for the MS measurements, Mr Hitoshi Fujitaka for the NMR measurements, and Dr Shunsuke Izumi for helpful dis-cussions.
References
Addae-Mensah, I., Cameron, D.W., 1978. Colouring matters of the Aphidoidea. XLIV. A survey of long-chain acid derivatives from aphid lipids compared with those of related insects. Triacylglycer-ides of octa-2,4,6-trienoic acid. Aust. J. Chem. 31, 2085–2090. Akimoto, S., 1985. Taxonomic study on gall aphids Colopha,
Paracolo-pha and Kaltenbachiella, Aphidoidea, Pemphigidae in East Asia with special reference to their origins and distributional patterns. Insecta Matsumurana. Series Entomology. New Series 31, 1–80. Banks, H.J., Cameron, D.W., 1973. Phenolic glycosides and pterins
from the Homoptera. Insect Biochem. 3, 139–162.
Bayer, M.H., Kaiser, H.E., Micozzi, M.S., 1994. Abnormal growth processes in plants and animals: a comparison. In Vivo 8, 3–16. Bowie, J.H., Cameron, D.W., 1965. Colouring matters of the
Aphidi-dae. Part XXV. A comparison of aphid constituents with those of their host plants. A triacylglyceride of sorbic acid. J. Chem. Soc. 5651–5657.
Brown, K.S. Jr., 1975. The chemistry of aphids and scale insects. Chem. Soc. Rev. 4, 263–288.
Brown Jr., K.S., Cameron, D.W., Weiss, U., 1969. Chemical constitu-ents of the bright orange aphid,Aphis meriifonscolombe I. Neria-phin and 6-hydroxymusizin 8-O-β-d-glucoside. Tetrahedron Lett.
471–476.
Apido-idea. XL1. The phospholipids ofEriosoma lanigerum(Hemiptera: Insecta). Aust. J. Chem. 29, 2723–2725.
Campbell, B.C., Nes, W.D., 1983. A reappraisal of sterol biosynthesis and metabolism in aphids. J. Insect Physiol. 29, 149–156. Callow, R.K., Greenway, A.R., Griffiths, D.C., 1973. Chemistry of the
secretion from the cornicles of various species of aphids. J. Insect Physiol. 19, 737–748.
Douglas, A.E., 1988. On the source of sterols in the green peach aphid,
Myzus persicae, reared on holodic diets. J. Insect Physiol. 34, 403–408.
Dixon, A.F.G., 1958. Protective function of siphunculi of the nettle aphidMicrolophium evansi(Theob.) (Hem. Aphididae). Entomol. Mon. Mag. 94, 8.
Dixon, A.F.G., Martin-Smith, M., Subramanian, G., 1965. Constituents ofMegoura viciaeBuckton. J. Chem. Soc. 1562–1564.
Duewell, H., Human, J.P.E., Johnson, A.W., MacDonald, S.F., Todd, A.R., 1948. Colouring matters of the Aphididae. Nature 162, 759–761.
Edwards, J.S., 1966. Defence by smear: supercooling in the cornicle wax of aphids. Nature 211, 73–74.
Febvay, G., Pageaux, J.F., Bonnot, G., 1992. Lipid composition of the pea aphid,Acyrthosiphon pisum(Harris) (Homoptera: Aphididae), reared on host plant and on artificial media. Arch. Insect. Biochem. Physiol. 21, 103–118.
Jenkins, R.L., Loxdale, H.D., Brookes, C.P., Dixon, A.F.G., 1999. The major carotenoid pigments of the grain aphid,Sitobion avenae(F.) (Hemiptera: Aphididae). Physiol. Entomol. 24, 171–178. Greenway, A.R., Griffiths, D.C., 1973. A comparison of
triacylglycer-ides from aphids and their cornicle secretions. J. Insect Physiol. 19, 1649–1655.
Kaneko, K., Matsumoto, A., Kurashiki, N., Ishikawa, K., 1994. Mor-phological studies on the insect galls of Zelkova serrata. Bull. Fukuoka Univ. Edu. 75–80.
Kislow, C.J., Edwards, L.J., 1972. Repellent odour in aphids. Nature 235, 108–109.
Matsubayashi, Y., Sakagami, Y., 1996. Phytosulfokine, sulfated pep-tides that induce the proliferation of single mesophyll cells of
Asparagus officinalis L. Proc. Natl. Acad. Sci. USA 93, 7623– 7627.
Miles, P.W., 1968. Studies on the salivary physiology of plant-bugs: experimental induction of galls. J. Insect Physiol. 14, 97–106. Moritsu, M., 1983. Aphids of Japan in Colors. Zenkoku Noson
Kyo-iku Kyokai, Tokyo, Japan (ISBN 4-88137-017-0).
Polgar, L.A., Darvas, B., Volkl, W., Porcheron, P., Szekacs, A., Sze-linger, S., 1996. Comparison of ecdysteroid concentration in differ-ent morphs of aphids. Comp. Biochem. Physiol. 115C, 179–183. Rahbe, Y., Delobel, B., Febvay, G., Chantegrel, B., 1994.
Aphid-spe-cific triacylglycerides in symbiotic and aposymbiotic Acyrthosi-phon pisum. Insect Biochem. Molec. Biol. 24, 95–101.
Ryan, R.O., De Renobales, M., Dillwith, J.W., Heisler, C.R., Blomqu-ist, G.J., 1982. Biosynthesis of myristate in an aphid: involvement of a specific acylthioesterase. Arch. Biochem. Biophys. 213, 26–36. Schaller, G., 1968. Untersuchungen zur erzeugung kunstlicher
pflan-zengallen. Marcellia 35, 131–153.
Stransky, K., Trka, A., Kohoutova, J., Streibl, M., 1976. Chemical composition of compounds produced by the pea aphid Acyrthosi-phon pisum(Harris): pentane and chloroform-methanolic extracts of body lipids. Collection Czechoslov. Chem. Commun. 41, 1799–1804.
Strong, F.E., 1967. Observation on aphid cornicle secretions. Ann. Ent. Soc. Am. 60, 668–673.
Tandon, P., Arya, H.C., 1980. Auxin autotrophy and hyperauxinity of eriophyes induced zizyphus stem galls in culture. Biochem. Phy-siol. Pflanz. 175, 537–541.
Yeo, U.D., Chae, Y.K., So, S.S., Lee, W.K., Sakurai, N., 1997. Devel-opmental changes of sugar contents in the gall on the leaf of elm (Zelkowa serrata Makino) formed by Paracolopha morrisoni